Highly-luminescent Eu,Sm,Mn-doped CaS up/down conversion nano-particles: application to ultra-sensitive latent fingerprint detection and in vivo bioimaging†
Abstract
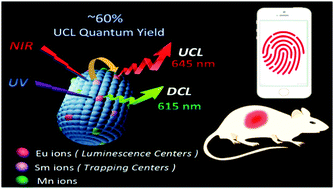
Due to their unique properties, rare-earth doped upconversion luminescence (UCL) nanomaterials are of considerable scientific interest. Meanwhile, alkaline-earth sulfide materials based on a completely different electron trapping (ET) mechanism demonstrate extremely high UCL efficiencies, which are several dozens of times more than those of conventional fluoride UCL nanomaterials. However, the large particle size, easy hydrolysis, and difficulty in achieving uniform dispersion have precluded bioassay applications. Herein, we have synthesized super-bright Eu,Sm,Mn-doped CaS nanoparticles of ∼30 nm average particle size using a reverse microemulsion technique. The UCL quantum yield was up to nearly 60%. Modification of the nanoparticles with an organic layer allows their stable dispersion throughout aqueous solutions without significant loss of the fluorescence intensity. We demonstrate the application of the novel UCL materials to latent fingerprint detection, deep tissue imaging, and in vivo bioimaging.


 Please wait while we load your content...
Please wait while we load your content...