Open Access Article
Open Access ArticleSolvated-electron production using cyanocuprates is compatible with the UV-environment on a Hadean–Archaean Earth†‡
Zoe R.
Todd
 *ab,
Albert C.
Fahrenbach
bc,
Christopher J.
Magnani
*ab,
Albert C.
Fahrenbach
bc,
Christopher J.
Magnani
 ab,
Sukrit
Ranjan
ab,
Sukrit
Ranjan
 a,
Anders
Björkbom
a,
Anders
Björkbom
 bd,
Jack W.
Szostak
bd,
Jack W.
Szostak
 bc and
Dimitar D.
Sasselov
a
bc and
Dimitar D.
Sasselov
a
aDepartment of Astronomy, Harvard-Smithsonian Center for Astrophysics, 60 Garden Street, Cambridge, MA 02138, USA. E-mail: zoe.todd@cfa.harvard.edu
bHoward Hughes Medical Institute, Department of Molecular Biology and Center for Computational and Integrative Biology, Massachusetts General Hospital, 185 Cambridge Street, Boston, MA 02114, USA
cEarth-Life Science Institute, Tokyo Institute of Technology, 2-12-1-IE-1 Ookayama, Meguro-ku, Tokyo, 152-8550, Japan
dDepartment of Biosciences, Åbo Akademi University, Åbo FI-20520, Finland
First published on 15th January 2018
Abstract
UV-driven photoredox processing of cyanocuprates can generate simple sugars necessary for prebiotic synthesis. We investigate the wavelength dependence of this process from 215 to 295 nm and generally observe faster rates at shorter wavelengths. The most efficient wavelengths are accessible to a range of potential prebiotic atmospheres, supporting the potential role of cyanocuprate photochemistry in prebiotic synthesis on the early Earth.
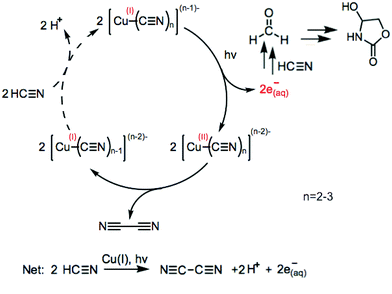
Many prebiotic syntheses of simple biomolecules, including ribonucleotides, amino acids, and lipid precursors,1 require simple 2- and 3-carbon sugar feedstock molecules, glycolaldehyde and glyceraldehyde. Past suggestions for the synthesis of simple sugars include the formose reaction,2 atmospheric photochemical production and subsequent transport to the surface,3 and delivery from space.4–6 However, unspecific products2 and large threshold concentrations7–9 (formose reaction), and low yields3 (atmospheric production) are all drawbacks. Alternatively, Ritson and Sutherland (2012)10 demonstrated that hydrogen cyanide can be converted to the glycolaldehyde and glyceraldehyde oxazolidinone derivatives via a Kiliani–Fischer homologation mechanism using cyanocuprate photoredox chemistry. This mechanism uses UV light to photooxidize the cyanocuprates, producing aqueous (solvated) electrons, which are capable of reducing HCN and 2-hydroxynitriles to imines, which can then hydrolyze to give rise to formaldehyde and α-hydroxy aldehydes in a stepwise fashion.
Glyceraldehyde is the simplest sugar that contains a chiral center and can thus influence the stereochemistry in downstream synthesis. Glycolaldehyde and formaldehyde can form enantiomerically enriched glyceraldehyde under chiral amino acid catalysts.11–13 Similarly, stereoselective tetrose14,15 and pentose16 sugars were demonstrated under chiral amino acid catalysts and plausible prebiotic conditions. Steer et al. (2017)17 showed the selective synthesis of 2-deoxy-D-ribose from glycolaldehyde and formaldehyde under the influence of proteinogenic amino esters and amino nitriles. Plausible syntheses of glycolaldehyde and glyceraldehyde may thus be intimately connected with the issue of homochirality.
In the Ritson and Sutherland system, UV light photooxidizes the cyanocuprates, producing aqueous electrons, which are key for putting into motion the reaction network capable of producing glycolaldehyde and glyceraldehyde. (See Fig. 5 of Ritson and Sutherland 201210 for a detailed mechanism.) Additional work by Ritson and Sutherland (2013)18 modified this cycle to include hydrogen sulfide as the stoichiometric reductant and produced free sugars upon 254 nm irradiation. The UV-wavelength dependence of the system with the addition of sulfide may not necessarily be the same as for the cyanocuprate cycle alone. Further work1 expanded upon the cyanosulfidic chemistry in Ritson and Sutherland (2012)10 and found that sugars, amino acids, ribonucleotides, and lipid precursors can be generated under UV irradiation at 254 nm. In this system, copper is not strictly necessary, but can increase the overall efficiency.
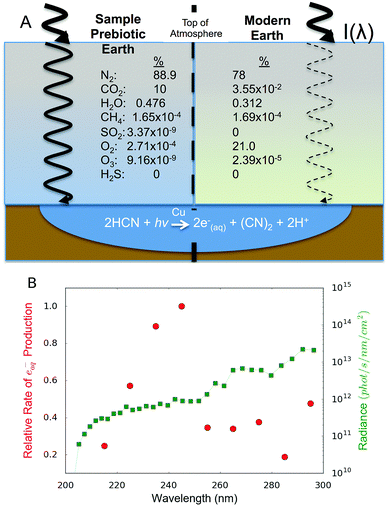
Cyanocuprate photochemistry has only been studied at limited wavelengths (254 nm10,18–20 and 266 nm21), which are poor approximations of the spectral radiance illuminating the surface of the prebiotic Earth.22 The young Sun is thought to have been on average about 20–30% dimmer than today, but with a larger fraction of its radiation in the UV range.23,24 The early atmosphere (typically considered to be 1 bar of N2 and CO225) was anoxic,26,27 providing ample radiation at wavelengths >200 nm, which are not screened out by the likely prebiotic atmospheric constituents.28
UV light is a potentially important source of energy to drive prebiotic reactions.29–31 Since photochemical reactions are generally wavelength-dependent,32 it is necessary to study the suggested prebiotic photochemistry at multiple wavelengths and fluxes more relevant to the prebiotic Earth. The flux from our experimental apparatus is consistent with the flux expected on the surface of the early Earth in the wavelength range of 235–255 nm to within an order of magnitude; our lamp provides roughly 6–9 times as much flux in this wavelength interval. Here, we investigate the wavelength dependence of the cyanocuprate photochemical process (Scheme 1) and assess if this reaction is plausible on the early Earth; other systems, such as the cyanosulfidic chemistry, will be addressed in future work. Studying the cyanocuprate photoprocess can act as a valuable test case for more complex chemical systems, such as those described in Ritson and Sutherland (2013)18 and Patel et al. (2015).1
We began by testing how the relative rates and quantum yields of the photoprocess depend on irradiation wavelengths from 215 to 295 nm (10 nm intervals, 10 nm bandwidths). These wavelengths of light (roughly 200–300 nm, or mid-range UV) were selected according to conditions expected on the surface of the early Earth. Aqueous solutions of dilute cyanocuprate complexes (63 μM copper(I) with three equivalents of cyanide) were prepared at neutral pH anaerobically. The absorption spectra of these solutions (Fig. 1A) display peaks at 210 and 234 nm, indicating that the solution is primarily composed of dicyanocuprate complexes. The tricyanocuprate species has absorption maxima at 205 nm and 239 nm. As the number of equivalents of cyanide per copper center is increased, the absorption spectrum changes from that characteristic of the dicyanocuprate to that of the tricyanocuprate (Fig. S5, ESI‡). Irradiation of the solutions resulted over time in a decrease in the absorption intensity across the spectrum, the rate of which depended on the irradiation wavelength.
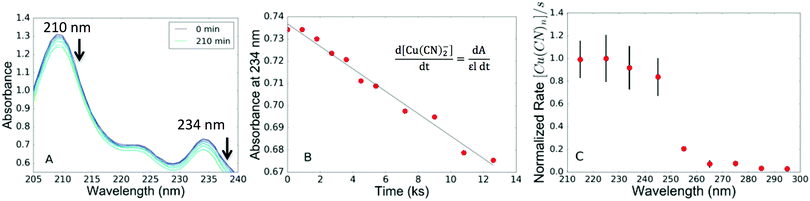 | ||
| Fig. 1 (A) Absorption spectra of an aqueous solution of cyanocuprates (63 μM CuCN, 125 μM KCN) during UV-irradiation. Arrows indicate the two absorption maxima at 210 and 234 nm decreasing as a function of irradiation time. (B) Absorbance at 234 nm as a function of irradiation time. The slope of the trendline measures the rate. (C) Action spectrum with the maximum value set to 1. Each wavelength was tested in triplicate, with the values representing the averages and errors of the standard deviation. The rates are faster at wavelengths below roughly 250 nm. The numerical values (and errors) for that data presented here are given in the ESI‡ (Section VII). | ||
We attribute the decreases in absorbance to the following mechanism: photoexcitation by UV light of cyanocuprates releases solvated electrons, a fraction of which are scavenged by HCN to eventually yield sugar products. The cyanide ligands of the oxidized copper(II) cyanide complexes undergo reductive elimination, forming cyanogen and regenerating the copper(I) state. Each turn of the cyanocuprate photoprocess removes multiple HCN molecules per copper complex, causing the cyanocuprate distribution to favor complexes with lower coordination numbers and the observed decrease in absorbance. The relative rates of the reaction at different wavelengths were also monitored with a cyanide-selective electrode (Fig. S7, ESI‡) to confirm the overall trend from the rates determined via absorbance measurements. LC-MS studies also confirmed the production of an oxazolidinone end product at all wavelengths tested (ESI,‡ Scheme S1). This detection indicates that the production of simple sugars can occur even at more prebiotically relevant sub-millimolar concentrations of copper and cyanide, while previous experiments used higher initial concentrations (200 mM KCN and 10 mM CuCN).10
The decrease in absorbance of the cyanocuprate solution with irradiation time allows us to determine an apparent rate (Fig. 1B) for the overall photochemical process, which serves as a proxy for the relative rate of solvated electron production at each irradiation wavelength studied. In order to compare reaction rates at different irradiation wavelengths, we normalize the apparent rates by incident photon fluxes, to give the action spectrum34 (Fig. 1C; see the ESI‡ for details). The normalized rate is larger at irradiation wavelengths <250 nm.
We calculated the relative quantum yields (number of reactions per absorbed photon) at the various irradiation wavelengths tested and normalized all quantum yields such that the maximum yield (at 245 nm irradiation, near the absorption maxima of the cyanocuprates) was set to 1 (Fig. 2). Two different mechanisms have been proposed for this process: (1) the Ritson–Sutherland mechanism10 postulates a photoionization as the underlying photoprocess, suggesting that any photon with energy greater than the activation energy of the process should be sufficient, and (2) Banerjee et al. 201433 use theoretical calculations of electronic structures and a mechanism involving dipole-bound forms of HCN to predict a Gaussian efficiency centered at 265 nm. Our results do not support this mechanism, given that the relative quantum yield at 265 nm is roughly a tenth of the maximum value at 245 nm.
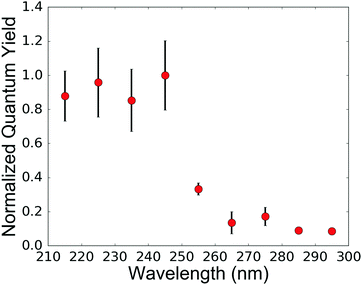 | ||
| Fig. 2 Relative quantum yield (normalized such that the maximum is 1) as a function of irradiation wavelength. The quantum yield is the highest at a wavelength of 245 nm. The numerical values (and errors) for the data presented here are given in the ESI‡ (Section VII). Wavelength-dependent experiments employed solutions of 63 μM CuCN and 125 μM KCN prepared from concentrated stocks. The pH value was adjusted to 7.4 using HCl. All preparations were done anaerobically, inside a glove box filled with an inert gas mixture (98% N2 and 2% H2). The final samples were placed inside gas-tight screw-top Spectrosil cuvettes (Starna cells part number 9-Q-10-GL14-C) to ensure anaerobic conditions. For each experiment, 0.7 mL of cyanocuprate solution was irradiated. Every 15 minutes for the first two hours, and every 30 minutes thereafter (210 minutes total), UV-Vis absorption spectra were recorded in order to monitor the reaction and determine the rate. | ||
We next studied how the wavelength-dependent reaction rates compare to the spectral irradiation on the modern and prebiotic Earth. The relative rates determined herein suggest that the process proceeds more efficiently at wavelengths below 250 nm, but shorter wavelengths are less accessible on the surface of a planet due to shielding by atmospheric gases and decreased solar output. We compute the intensity of light reaching the surface of the Earth as a function of wavelength by convolving the solar flux with the spectral screening by atmospheric gases (ESI,‡ Section VI). We compute the relative rate of solvated-electron production as a function of wavelength by multiplying the action spectrum and integrated surface radiance at each wavelength.34 The resulting curve is shown in Fig. 3B (red dots); the area under it is commonly referred to as the relative dose rate.22
The modern surface of the Earth receives low levels of UV light (due to UV-shielding O2 and O3) leading to a UV flux inadequate to drive this photochemistry. Even when including the effects of the atmospheric attenuation and stellar spectral slope, we find that the most productive wavelength of irradiation remains 245 nm for the early Earth. Energy at the relevant wavelengths should be accessible on the early Earth for a range of plausible atmospheres, suggesting that this mechanism for simple sugar generation is viable. The peak wavelength does not precisely correspond with the 254 nm light that is typically used in prebiotic experiments, exemplifying the importance of wavelength dependence considerations. Our study, though carried out at specific individual wavelengths that do not mimic the spectrum of the Sun, when combined with information about the spectral flux on the early Earth, can provide insight into the plausibility of the process in the overall context of the UV environment on the early Earth.
In summary, we performed a wavelength-dependent analysis to assess the plausibility of the synthesis of 2- and 3-carbon sugar building blocks (necessary for simple biomolecule synthesis) from photoredox processing of cyanocuprates. Past studies of the cyanocuprate photoprocess used monochromatic emission at 254 nm, which is a poor proxy of the surface irradiation on the early Earth. In order to assess the prebiotic plausibility of this pathway, we measured the wavelength dependence and found that the process is more efficient at wavelengths <250 nm. The wavelength with the highest quantum yield (245 nm) should be accessible not only in the atmosphere we tested here, but also in other plausible early Earth atmospheres. Thus, we argue that the photochemical cycling of cyanocuprates is a prebiotically plausible source of 2- and 3-carbon sugar building blocks on the early Earth, across a diversity of possible atmospheric states. This process should also work on early Mars (unless high levels of dust are present25,28) or exoplanets analogous to the young Earth orbiting stars with UV emission similar to the Sun.
We thank C. P. Tam and F. Ng for experimental and laboratory assistance. We thank R. Szabla and D. Bucher for helpful comments and discussions. We thank S. Trauger and others at the Harvard Small Molecule Mass Spectrometry Facility. This work was supported in part by grants from the Simons Foundation (290363 to J. W. S. and 290360 to D. D. S.) and we acknowledge the Harvard Origins of Life Initiative. J. W. S. is an investigator of the Howard Hughes Medical Institute. A. B. was supported by a grant from the Academy of Finland. A. B. is currently at Statens Serum Institut, Artillerivej 5, DK-2300 Copenhagen S, Denmark. C. J. M. is currently at the School of Medicine, Stanford University, 291 Campus Drive, Palo Alto CA, 94305.
Conflicts of interest
There are no conflicts to declare.Notes and references
- B. H. Patel, C. Percivalle, D. J. Ritson, C. D. Duffy and J. D. Sutherland, Nat. Chem., 2015, 7, 301 CrossRef CAS PubMed
.
- A. Butlerov, Liebigs Ann. Chem., 1861, 120, 295 CrossRef
.
- C. E. Harman, J. F. Kasting and E. T. Wolf, Origins Life Evol. Biospheres, 2013, 43, 77 CrossRef CAS PubMed
.
- C. F. Chyba, C. Sagan, L. Brookshaw and P. J. Thomas, Origins Life Evol. Biospheres, 1989, 19, 467 CrossRef
.
- C. F. Chyba, P. J. Thomas, L. Brookshaw and C. Sagan, Science, 1990, 249, 366 CAS
.
- M. Maurette, J. Duprat, C. Engrand, M. Gounelle, G. Kurat, G. Matrajt and A. Toppani, Planet. Space Sci., 2000, 48, 1117 CrossRef CAS
.
- C. Reid and L. Orgel, Nature, 1967, 216, 455 CrossRef CAS PubMed
.
- A. W. Schwartz and R. M. De Graaf, J. Mol. Evol., 1993, 36, 101 CrossRef CAS
.
- N. Gabel and C. Ponnamperuma, Nature, 1967, 216, 453 CrossRef CAS PubMed
.
- D. Ritson and J. D. Sutherland, Nat. Chem., 2012, 4, 895 CrossRef CAS PubMed
.
- R. Breslow, V. Ramalingam and C. Appayee, Origins Life Evol. Biospheres, 2013, 43, 323 CrossRef PubMed
.
- R. Breslow and Z.-L. Cheng, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 5723 CrossRef CAS PubMed
.
- J. E. Hein, E. Tse and D. G. Blackmond, Nat. Chem., 2011, 3, 704 CrossRef CAS PubMed
.
- S. Pizzarello and A. L. Weber, Science, 2004, 303, 1151 CrossRef CAS PubMed
.
- A. L. Weber and S. Pizzarello, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12713 CrossRef CAS PubMed
.
- S. Pizzarello and A. L. Weber, Origins Life Evol. Biospheres, 2010, 40, 3 CrossRef CAS PubMed
.
- A. M. Steer, N. Bia, D. K. Smith and P. A. Clarke, Chem. Commun., 2017, 53, 10362 RSC
.
- D. J. Ritson and J. D. Sutherland, Angew. Chem., Int. Ed., 2013, 52, 5845 CrossRef CAS PubMed
.
- A. Horvath, S. Papp and Z. Decsy, J. Photochem., 1984, 24, 331 CrossRef CAS
.
- A. Horvath, Z. Zsilak and S. Papp, J. Photochem. Photobiol., A, 1989, 50, 129 CrossRef CAS
.
- K. L. Stevenson and J. H. Jarboe, J. Photochem. Photobiol., A, 2002, 150, 49 CrossRef CAS
.
- S. Ranjan and D. D. Sasselov, Astrobiology, 2016, 16, 68 CrossRef CAS PubMed
.
- I. Ribas, G. F. Porto de Mello, L. D. Ferreira, E. Hebrard, F. Selsis, S. Catalan, A. Garces, J. D. do Nascimento Jr. and J. R. de Medeiros, Astrophys. J., 2010, 714, 384 CrossRef CAS
.
- M. W. Claire, J. Sheets, M. Cohen, I. Ribas, V. S. Meadows and D. C. Catling, Astrophys. J., 2012, 757, 95 CrossRef
.
- S. Rugheimer, A. Segura, L. Kaltenegger and D. Sasselov, Astrophys. J., 2015, 806, 137 CrossRef
.
- J. Farquhar, J. Savarino, S. Airieau and M. H. Thiemens, J. Geophys. Res., 2001, 106, 32829 CrossRef CAS
.
- A. A. Pavlov and J. F. Kasting, Astrobiology, 2002, 2, 27 CrossRef CAS PubMed
.
- S. Ranjan and D. D. Sasselov, Astrobiology, 2017, 17, 169 CrossRef CAS PubMed
.
- C. Sagan and B. N. Khare, Science, 1971, 173, 417 CAS
.
- C. Chyba and C. Sagan, Nature, 1992, 355, 125 CrossRef CAS PubMed
.
- O. Pestunova, A. Simonov, V. Snytnikov, V. Stoyanovsky and V. Parmon, Adv. Space Res., 2005, 36, 214 CrossRef CAS
.
- T. Matsunaga, K. Hieda and O. Nikaido, Photochem. Photobiol., 1991, 54, 403 CrossRef CAS PubMed
.
- A. Banerjee, G. Ganguly, R. Tripathi, N. N. Nair and A. Paul, Chem. – Eur. J., 2014, 20, 6348 CrossRef CAS PubMed
.
- An action spectrum is the rate of a photochemical process as a function of wavelength, normalized by flux. The action-spectral weighted surface radiance is the product of the action spectrum (Fig. 1C) and the surface radiance at each wavelength.
Footnotes |
| † In memory of Dr Alexei Trifonov. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cc07748c |
| This journal is © The Royal Society of Chemistry 2018 |