Forged and fashioned for faithfulness—ruthenium olefin metathesis catalysts bearing ammonium tags
Abstract
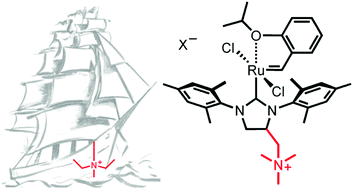
In this article, the synthesis and applications of selected ammonium tagged Ru-alkylidene metathesis catalysts were described. Because of the straightforward synthesis, the first generation of onium-tagged catalysts have the ammonium group installed in the benzylidene ligand. Such catalysts usually give relatively pure metathesis products, and are used in polar solvents and water, or immobilised on various supports. Later, catalysts tagged in the N-heterocyclic carbene ligand (NHC) were developed to offer higher stability and even lower metal contamination levels. Due to minimal leaching, the non-dissociating ligand tagged systems were successfully immobilised on various supports, including zeolites and Metal Organic Frameworks (MOFs) and used in batch and in continuous flow conditions.


 Please wait while we load your content...
Please wait while we load your content...