Biocompatibility of polymer-based biomaterials and medical devices – regulations, in vitro screening and risk-management
Abstract
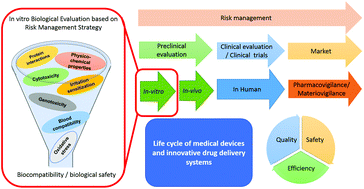
Biomaterials play an increasing role in modern health care systems. Biocompatibility poses a significant challenge for manufacturers of medical devices and contemporary intelligent drug delivery technologies from materials development to market approval. Despite a highly regulated environment, biocompatibility evaluation of biomaterials for medical devices is a complex task related to various factors that include mainly chemical nature and physical properties of the material, the contact tissue and duration of contact. Although international standards, such as ISO 10993-1, are generally employed to prove regulatory compliance needed for market clearance or for initiating clinical investigations, they may not offer sufficient guidance, or risk-management perspective when it comes to choosing materials or appropriate in vitro biocompatibility screening methods when developing medical devices. The global normative approach towards the biocompatibility evaluation of medical devices is presented in this review, with a focus on in vitro studies. Indeed, a risk-management approach towards the judicial choice of in vitro tests throughout the development and production of medical devices and drug delivery systems will facilitate rapid regulatory approval, avoid unnecessary animal studies, and ultimately reduce risks for patients. A detailed overview towards the construction of a comprehensive biological evaluation plan is described herein, with a focus on polymer-based materials used in medical applications. Polymeric materials offer a broad spectrum of applications in the manufacturing of medical devices. They are extensively employed within both conventional and innovative drug delivery systems with superior attributes supporting robust, extended use capacity, capable of meeting specific requirement such as adhesion, drug release, and more. Various methods of biocompatibility assessment are detailed within, with an emphasis on scientific analysis. This review may be of interest to those involved in the design, manufacturing and in vitro testing of medical devices and innovative drug delivery technologies, specifically with respect to a risk-management approach towards the biocompatibility assessment of polymer-based devices.


 Please wait while we load your content...
Please wait while we load your content...