Therapeutic applications of iron oxide based nanoparticles in cancer: basic concepts and recent advances
Abstract
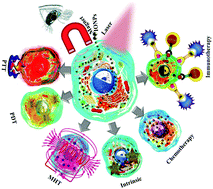
Nanotechnology has introduced new techniques and phototherapy approaches to fabricate and utilize nanoparticles for cancer therapy. These phototherapy approaches, such as photothermal therapy (PTT) and photodynamic therapy (PDT), hold great promise to overcome the limitations of traditional treatment methods. In phototherapy, magnetic iron oxide nanoparticles (IONPs) are of paramount importance due to their wide range of biomedical applications. This review discusses the basic concepts, various therapy approaches (PTT, PDT, magnetic hyperthermia therapy (MHT), chemotherapy and immunotherapy), intrinsic properties, and mechanisms of cell death of IONPs; it also provides a brief overview of recent developments in IONPs, with focus on their therapeutic applications. Much attention is devoted to elaborating the various parameters, intracellular behaviors and limitations of MHT. Bimodal therapies which act alone or in combination with other modalities are also discussed. The review highlights some limitations in the explored research areas and suggests future directions to overcome these limitations.
- This article is part of the themed collection: Nanobiointerfaces


 Please wait while we load your content...
Please wait while we load your content...