Bone-targeting poly(ethylene sodium phosphate)†
Yasuhiko
Iwasaki
 *ab,
Atsushi
Yokota
c,
Akihisa
Otaka
*ab,
Atsushi
Yokota
c,
Akihisa
Otaka
 b,
Naoyuki
Inoue
a,
Akane
Yamaguchi
d,
Toru
Yoshitomi
d,
Keitaro
Yoshimoto
de and
Masashi
Neo
c
b,
Naoyuki
Inoue
a,
Akane
Yamaguchi
d,
Toru
Yoshitomi
d,
Keitaro
Yoshimoto
de and
Masashi
Neo
c
aDepartment of Chemistry and Materials Engineering, Kansai University, 3-3-35, Yamate-cho, Suita-shi, Osaka 564-8680, Japan. E-mail: yasu.bmt@kansai-u.ac.jp
bORDIST, Kansai University, 3-3-35, Yamate-cho, Suita-shi, Osaka 564-8680, Japan
cDepartment of Orthopedic Surgery, Osaka Medical College, 2-7 Daigakumachi, Takatsuki, Osaka, 569-8686, Japan
dDepartment of Life Sciences, Graduate School of Arts and Sciences, The University of Tokyo, 3-8-1, Komaba, Meguro, Tokyo 153-8902, Japan
eJST, PRESTO, The University of Tokyo, 3-8-1 Komaba, Meguro, Tokyo 153-8902, Japan
First published on 21st November 2017
Abstract
Poly(ethylene sodium phosphate) (PEP·Na) showed excellent cytocompatibility and in vivo bone affinity. Moreover, PEP·Na did not interact with thrombin, which is a coagulation-related protein. Because immobilization of therapeutic agents and imaging probes on PEP·Na is easily performed, PEP·Na is a promising polymer for bone-targeted therapies.
Some phosphorus-containing polymers have recently made a large impact in biorelated fields because of their biocompatibility and structural similarity to naturally occurring molecules such as phospholipids and nucleic acids.1–3 In particular, polymers that have phosphate linkages in the backbone are of interest not only for their biodegradability but also for their unique biofunctionalities.4–6
Poly(phosphate) (polyP) is well known as a physiological polymer and plays diverse roles in nature.7,8 Recently, it has been synthesized through both biological and chemical processes and studied as biomedical materials.9,10 Müller and Wang et al. reported its bioactivity and efficacy in bone regeneration.11 polyP shows a high affinity for calcium ions, and nanocomposites of polyP and calcium ions can activate an osteoblastic function.12,13 They also proposed that polyP could be a metabolic fuel for bone mineralization.14 As it is physiologically synthesized in bone-forming osteoblast cells and blood platelets,15,16 it strongly affects the coagulation pathway and enhances fibrin formation.17 Although it is useful for bone tissue regeneration, it may not be suitable for blood circulation in injection therapy.
Polyphosphoesters are also interesting polymers that have phosphate linkages in the backbone similar to polyP. Various types of polyphosphoesters with phosphate diesters and triesters in their backbone have been synthesized.4,6,18–22 Ring-opening polymerization (ROP) is one of the most reliable synthesis processes to obtain polyphosphoesters because various cyclic monomers can be obtained by a simple condensation reaction between 2-chloro-2-oxo-1,3,2-dioxaphosphorane and alcohol.23–25 By using organocatalysts during ROP, polymerization proceeded in a living manner, and the generated polymers had a narrow molecular weight distribution.26 It has been clarified that polyphosphoesters exhibit several unique properties such as low glass transition temperature,27 thermoresponsivity,28 biodegradability,29–31 and biocompatibility.29,32 Due to these properties, numerous studies aimed at making polymeric amphiphiles to act as drug carriers have been performed.33–36 Moreover, the osteoinductivity of polyphosphoesters has also been reported, and the efficacy of polyphosphoesters for bone regeneration is anticipated.37
Recently, we synthesized poly(ethylene sodium phosphate) (PEP·Na, Fig. 1), which has phosphate diester linkage in the backbone, through the ROP of 2-methoxy-2-oxo-1,3,2-dioxaphospholane (MP) followed by its conversion into phosphate diesters from triesters, as shown in ESI Scheme S1.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 PEP·Na is a hygroscopic polymer that exists as a colorless solid. Selective inhibition of osteoclast adhesion and function was also observed following cultivation with PEP·Na. It could be considered that PEP·Na is an effective polymer for developing prodrugs for bone therapy. However, there have been no reports that show the bone affinity of PEP·Na in vivo. In the current study, we first clarified whether PEP·Na could adsorb on the bone surface in vivo. We then investigated the interaction between PEP·Na and thrombin, which is a coagulation-related protein, because the inertness of polymers toward clot formation is also important for obtaining reliable efficacy during in vivo applications.
38 PEP·Na is a hygroscopic polymer that exists as a colorless solid. Selective inhibition of osteoclast adhesion and function was also observed following cultivation with PEP·Na. It could be considered that PEP·Na is an effective polymer for developing prodrugs for bone therapy. However, there have been no reports that show the bone affinity of PEP·Na in vivo. In the current study, we first clarified whether PEP·Na could adsorb on the bone surface in vivo. We then investigated the interaction between PEP·Na and thrombin, which is a coagulation-related protein, because the inertness of polymers toward clot formation is also important for obtaining reliable efficacy during in vivo applications.
The average molecular weights and densities of the synthesized polymers are summarized in Table 1. PEP·Na was obtained via ROP; the apparent number-average molecular weight (Mn) of PEP·Na was 9.6 × 103 (DP ≈ 73) and the polydispersity index (Mw/Mn) was 1.3. During dealkylation, the degradation of the backbone of the polymer did not occur. In order to obtain fluorescent PEP·Na (Cy5-PEP·Na), a copolymer of MP and 2-(but-3-yn-1-yloxy)-2-oxo-1,3,2-dioxaphospholane (BYP)39 was synthesized, as shown in ESI Scheme S2.† The molar fraction of MP and BYP in the feed was adjusted to 0.9/0.1, and the composition of each monomer unit in the copolymer was 0.93/0.07, which was determined by 1H NMR analysis, as shown in ESI Fig. S1.† During demethylation, only methoxy groups were cleaved and BYP units were preserved. Cy5-Az was immobilized on P(EP·Na/BYP) through a copper-mediated click reaction.40 The density of Cy5-Az molecules immobilized on the polymer was determined by fluorescence spectroscopy using a linear working curve of free Cy5-Az and was approximately 2.8% (mol/mol).
| Polymer |
M
n × 10−4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
M
w/Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
Density of Cy5b (%; mol/mol) |
|---|---|---|---|
| a Determined using a GPC system equipped with a refractive index detector and size-exclusion columns, with a poly(ethylene glycol) standard in acetate buffer containing 0.1 M CH3COONa, 0.3 M NaCl, and 1.0 mM EDTA·2Na. b Determined by fluorescence spectroscopy. | |||
| PEP·Na | 0.96 | 1.30 | — |
| Cy5-PEP·Na | 1.01 | 1.38 | 2.8 |
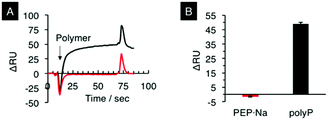
There have been several studies reporting that polyP enhances fibrin clot formation.41 Morrissey and co-workers first reported the role of polyP in thrombus formation.42 They clarified that polyP mainly acts at four points in the coagulation pathway, namely to trigger the pathway, induce factor V activation, enhance the fibrin clot structure, and act as a natural cofactor for the activation of factor XI by thrombin. They also used SPR analysis to clarify that polyP binds thrombin with a high affinity (Kd ∼ 5 nM) and that the thrombin bound to polyP mediates the activation of a coagulation factor FXI.43 We then studied the binding affinity of PEP·Na for thrombin by SPR. Fig. 2A shows SPR sensorgrams for the adsorption of PEP·Na and polyP on thrombin-immobilized sensor chips. polyP used in the current study had 60–70 repeat units and formed a sodium salt as shown in Fig. 1. When polyP was in contact with the surface, the resonance angle was dramatically changed. On the other hand, the adsorption of PEP·Na was not observed. The adsorption test was repeated several times, as shown in ESI Fig. S2,† and the result is summarized in Fig. 2B. The adsorption phenomena of the polymers were reproducible and the binding affinity of polyP for thrombin was significantly higher than that of PEP·Na.
The cytocompatibility of PEP·Na and polyP was also comparatively investigated. Fig. 3A shows the viability of osteoblastic MC3T3-E1 cells in contact with various concentrations of PEP·Na or polyP for 24 h. Although the viability decreased with an increase in the concentration of polymeric additives, it was clarified that the cell compatibility of PEP·Na was better than that of polyP. The 50% inhibition concentrations (IC50) for PEP·Na and polyP were approximately 20.0 mg mL−1 (2.09 mM) and 0.9 mg mL−1 (0.14 mM), respectively. The morphologies of MC3T3-E1 cells cultured with and without polymers are shown in Fig. 3B. Under controlled conditions without polymer additives, all adherent cells on a tissue culture dish exhibited a healthy spindle shape. The spindle shape of the adherent cells was observed even after cultivation in medium containing 10 mg mL−1 PEP·Na (75% viability in Fig. 3A). In contrast, cells became spherical in the medium containing 10 mg mL−1 of polyP (0% viability in Fig. 3A). PEP·Na shows superior biocompatibility compared with polyP, which is considered to be an interesting candidate polymer for bone tissue engineering. Therefore, PEP·Na would be useful for biomedical applications. Müller et al. discovered that polyP acts as a substrate for the principal enzyme involved in bone formation.44,45 Although further studies are needed to understand the bioactivity of PEP·Na in bone formation, the stimulatory effect of polyphosphoesters on osteoblastic functions has been mentioned in previous literature.31,37
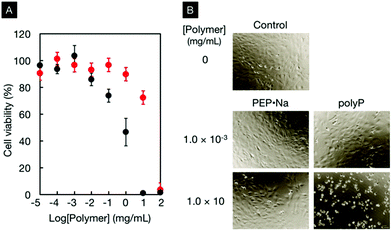 | ||
Fig. 3 Viability (A) and morphology (B) of MC3T3-E1 cells in contact with PEP·Na or polyP.  : PEP·Na; ●: polyP. : PEP·Na; ●: polyP. | ||
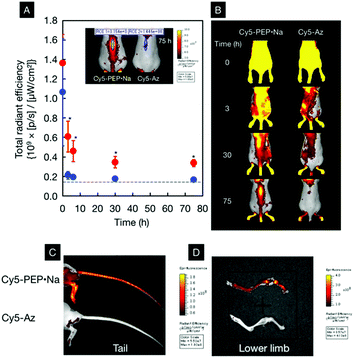
Due to the inertness of PEP·Na toward thrombin, the biodistribution of PEP·Na after intravascular injection was investigated. The animal protocol was approved by the Animal Experimentation Committee of Osaka Medical College (permit number: 29079). One hundred microliters of 100 mg mL−1 Cy5-PEP·Na or 100 μg mL−1 Cy5-Az was injected into the tail vein. The fluorescence spectra of the sample solutions are shown in ESI Fig. S3† and are nearly identical. Although the concentration of Cy5-PEP·Na was relatively high, the polymer solution was diluted immediately after mixing with blood. Fig. 4A and B show the region-of-interest (ROI) analysis of fluorescence signals from spines and the fluorescence images of ICR mice after the intravenous injection of Cy5-PEP·Na and Cy5-Az. Immediately after the intravenous injection, strong fluorescence signals were observed from the whole body of each mouse. This suggests that Cy5-PEP·Na and Cy5-Az rapidly spread throughout the mouse via the bloodstream. Although the total radiant efficiency within each ROI decreased over time, as shown in Fig. 4A, the efficiencies in mice treated with Cy5-PEP·Na were significantly higher (*p < 0.005 at each time point) than those in mice treated with Cy5-Az. After 30 h of breeding, the total radiant efficiency of the spine of Cy5-Az-treated mice became the same as that before injection, and the fluorescence signals from the spine were almost extinguished, as shown in Fig. 4B. In stark contrast, the fluorescence signals from the spine of Cy5-PEP·Na-treated mice were observed 75 h after the intravenous injection. Owing to the observation in the dorsal position, fluorescence signals from bones located near the surface were significant. The reproducibility of the biodistribution of Cy5-PEP·Na is shown in ESI Fig. S4.† We tested four mice in each sample test. The bone affinity of Cy5-PEP·Na was observed for all mice, and the long-term bone distribution of Cy5-PEP·Na can be understood from this ESI Figure.† Significant fluorescence signals from the spines of mice were observed two weeks after the injection of Cy5-PEP·Na. Even four weeks after injection, some Cy5-PEP·Na remained on the spines and limbs.
In order to make the bone affinity of Cy5-PEP·Na clearer, ex vivo tests were also performed. The mice were sacrificed and the skin was peeled from the tail. Fig. 4C and D show representative ex vivo images of murine tails and lower limbs, respectively, 75 h after the intravenous injection of Cy5-PEP·Na or Cy5-Az. Whole body images with peeled skin are also shown in ESI Fig. S5.† From these images, it can be confirmed that Cy5-PEP·Na homogeneously adsorbed on the bones of the tail, lower limbs, and spines.
In our previous study, the effect of phosphodiester composition on the mineral affinity of polyphosphoesters was studied.32 We synthesized polyphosphoester ionomers comprising phosphotriester and phosphodiester units. The amount of polyphosphoester ionomer adsorption onto hydroxyapatite (HAp) substrates increased with increasing phosphodiester units (ionized units) in the polyphosphoesters. Moreover, the polyphosphoester ionomers clearly exhibited superior physicochemical properties in relation to mineralization and biocompatibility compared with bisphosphonates. Very recently, we succeeded in synthesizing fully ionized PEP·Na, which was applied to impart mineral affinity to nanoparticles.46
Here, we propose a new candidate polymer that can be applied for bone therapy. It is well known that polymeric prodrugs are advantageous in achieving long-term circulation. Moreover, they have the capacity to immobilize functional molecules such as drugs, ligands, and imaging probes.47 The delivery of macromolecules to bones has been studied, and extravasation through vessels in or near the bone is considered to be the dominant pathway. For example, Owen and co-workers clarified that polyvinylpyrrolidone, which has an average molecular weight of 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, was able to pass through the extravascular tissue fluid.48 Several molecules, such as tetracyclines,49 bisphosphonates50,51 and acidic oligopeptides,52,53 have been adopted as ligands to target bone, and various polymeric prodrugs bearing these molecules have been proposed. In particular, bisphosphonates are promising ligands that can be used to obtain polymeric prodrugs for bone-targeting therapeutics.54–57 Miller and co-workers synthesized N-(2-hydroxypropyl)-methacrylamide (HPMA) copolymers bearing alendronate and paclitaxel, and they clarified that the copolymers demonstrated improved antitumor and antiangiogenic activities.58 Although previously proposed polymeric prodrugs have succeeded in targeting bone diseases in vivo, almost all of them are acrylic (methacrylic) polymers, and biodegradable polymers are still limited. Compared with these existing polymers, PEP·Na has the additional advantage of being biodegradable and has unique bioactivities related to bone formation.
000 g mol−1, was able to pass through the extravascular tissue fluid.48 Several molecules, such as tetracyclines,49 bisphosphonates50,51 and acidic oligopeptides,52,53 have been adopted as ligands to target bone, and various polymeric prodrugs bearing these molecules have been proposed. In particular, bisphosphonates are promising ligands that can be used to obtain polymeric prodrugs for bone-targeting therapeutics.54–57 Miller and co-workers synthesized N-(2-hydroxypropyl)-methacrylamide (HPMA) copolymers bearing alendronate and paclitaxel, and they clarified that the copolymers demonstrated improved antitumor and antiangiogenic activities.58 Although previously proposed polymeric prodrugs have succeeded in targeting bone diseases in vivo, almost all of them are acrylic (methacrylic) polymers, and biodegradable polymers are still limited. Compared with these existing polymers, PEP·Na has the additional advantage of being biodegradable and has unique bioactivities related to bone formation.
In summary, we have demonstrated that PEP·Na is inert toward thrombin and shows good affinity for bone in vivo. Due to the molecular diversity of polyphosphoesters, various types of polymeric prodrugs for bone disease treatment can be designed based on PEP·Na.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
A part of this study was supported by JSPS KAKENHI #16H03185 and MEXT Private University Research Branding Project.Notes and references
- S. Monge, B. Canniccioni, A. Graillot and J.-J. Robin, Biomacromolecules, 2011, 12, 1973 CrossRef CAS PubMed.
- B. M. Watson, F. K. Kasper and A. G. Mikos, Biomed. Mater., 2014, 9, 025014 CrossRef PubMed.
- S. Penczek, J. Pretula, P. Kubisa, K. Kaluzynski and R. Szymanski, Prog. Polym. Sci., 2015, 45, 44 CrossRef CAS.
- S. Penczek, T. Biela, P. Klosinski and G. Lapienis, Makromol. Chem., Macromol. Symp., 1986, 6, 123 CrossRef CAS.
- S. Penczek, J. Pretula and K. Kaluzynski, Biomacromolecules, 2005, 6, 547 CrossRef CAS PubMed.
- K. N. Bauer, H. T. Tee, M. M. Velencoso and F. R. Wurm, Prog. Polym. Sci., 2017, 73, 61 CrossRef CAS.
- A. Kornberg, N. N. Rao and D. Ault-Riche, Annu. Rev. Biochem., 1999, 68, 89 CrossRef CAS PubMed.
- S. A. Smith, S. H. Choi, R. Davis-Harrison, J. Huyck, J. Boettcher, C. M. Reinstra and J. H. Morrissey, Blood, 2010, 116, 4353 CrossRef CAS PubMed.
- A. Momeni and M. J. Filiaggi, J. Non-Cryst. Solids, 2013, 382, 11 CrossRef CAS.
- A. Momeni, E. M. Valliant, E. P. Brennan-Pierce, J. J. Shankar, R. Abraham, P. Colp and M. J. Filiaggi, Acta Biomater., 2016, 32, 286 CrossRef CAS PubMed.
- W. E. G. Müller, E. Tolba, H. C. Schroder and X. Wang, Macromol. Biosci., 2015, 15, 1182 CrossRef PubMed.
- W. E. G. Müller, X. Wang, B. Diehl-Seifert, K. Kropf, U. Schlossmacher, I. Lieberwirth, G. Glasser, M. Wiens and H. C. Schroder, Acta Biomater., 2011, 7, 2661 CrossRef PubMed.
- W. E. G. Müller, E. Tolba, H. C. Schröder, S. Wang, G. Glaßer, R. Muñoz-Espí, T. Link and X. Wang, Mater. Lett., 2015, 148, 163 CrossRef.
- W. E. G. Müller, E. Tolba, Q. Feng, H. C. Schröder, J. S. Markl, M. Kokkinopoulou and X. Wang, J. Cell Sci., 2015, 128, 2202 CrossRef PubMed.
- K. D. Kumble and A. Kornberg, J. Biol. Chem., 1995, 270, 5818 CrossRef CAS PubMed.
- F. Müller, N. J. Mutch, W. A. Schenk, S. A. Smith, L. Esterl, H. M. Spronk, S. Schmidbauer, W. A. Gahl, J. H. Morrissey and T. Renné, Cell, 2009, 139, 1143 CrossRef PubMed.
- J. H. Morrissey, S. H. Choi and S. A. Smith, Blood, 2012, 119, 5972 CrossRef CAS PubMed.
- K. Kaluzynski, J. Libiszowski and S. Penczek, Macromolecules, 1976, 9, 35 CrossRef.
- S. Penczek and P. Klosinski, Synthetic polyphosphates related to nucleic and teichoic acids, in Models of Biopolymers by Ring-opening Polymerization, ed. S. Penczek, CRC, Florida, USA, 1990 Search PubMed.
- Y. C. Wang, Y. Y. Yuan, J. Z. Du, X. Z. Yang and J. Wang, Macromol. Biosci., 2009, 9, 1154 CrossRef CAS PubMed.
- Polyphosphoesters: Chemistry and Application, ed. K. D. Troev, Elsevier, Waltham, USA, 2012 Search PubMed.
- T. Steinbach and F. R. Wurm, Angew. Chem., Int. Ed., 2015, 54, 6098 CrossRef CAS PubMed.
- J. Libiszowski, K. Kałużynski and S. Penczek, J. Polym. Sci., Polym. Chem. Ed., 1978, 16, 1275 CrossRef CAS.
- Y. Iwasaki and K. Akiyoshi, Macromolecules, 2004, 37, 7637 CrossRef CAS.
- S. Zhang, J. Zou, F. Zhang, M. Elsabahy, S. E. Felder, J. Zhu, D. J. Pochan and K. L. Wooley, J. Am. Chem. Soc., 2012, 134, 18467 CrossRef CAS PubMed.
- Y. Iwasaki and E. Yamaguchi, Macromolecules, 2010, 43, 2664 CrossRef CAS.
- Y. Iwasaki, K. Takemoto, S. Tanaka and I. Taniguchi, Biomacromolecules, 2016, 17, 2466 CrossRef CAS PubMed.
- Y. Iwasaki, C. Wachiralarpphaithoon and K. Akiyoshi, Macromolecules, 2007, 40, 8136 CrossRef CAS.
- S. W. Huang, J. Wang, P. C. Zhang, H. Q. Mao, R. X. Zhuo and K. W. Leong, Biomacromolecules, 2004, 5, 306 CrossRef CAS PubMed.
- D. A. Wang, C. G. Williams, F. Yang, N. Cher, H. Lee and J. H. Elisseeff, Tissue Eng., 2005, 11, 201 CrossRef CAS PubMed.
- C. Wachiralarpphaithoon, Y. Iwasaki and K. Akiyoshi, Biomaterials, 2007, 28, 984 CrossRef CAS PubMed.
- Y. Iwasaki, K. Katayama, M. Yoshida, M. Yamamoto and Y. Tabata, J. Biomater. Sci., Polym. Ed., 2013, 24, 882 CrossRef CAS PubMed.
- Y.-C. Wang, L.-Y. Tang, Y. Li and J. Wang, Biomacromolecules, 2009, 10, 66 CrossRef CAS PubMed.
- Y.-C. Wang, Y. Li, X.-Z. Yang, Y.-Y. Yuan, L.-F. Yan and J. Wang, Macromolecules, 2009, 42, 3026 CrossRef CAS.
- M. H. Xiong, Y. J. Li, Y. Bao, X. Z. Yang, B. Hu and J. Wang, Adv. Mater., 2012, 24, 6175 CrossRef CAS PubMed.
- F. Zhang, S. Zhang, S. F. Pollack, R. Li, A. M. Gonzalez, J. Fan, J. Zou, S. E. Leininger, A. Pavía-Sanders, R. Johnson, L. D. Nelson, J. E. Raymond, M. Elsabahy, D. M. P. Hughes, M. W. Lenox, T. P. Gustafson and K. L. Wooley, J. Am. Chem. Soc., 2015, 137, 2056 CrossRef CAS PubMed.
- X. Z. Yang, T. M. Sun, S. Dou, J. Wu, Y. C. Wang and J. Wang, Biomacromolecules, 2009, 10, 2213 CrossRef CAS PubMed.
- S. Kootala, M. Tokunaga, J. Hilborn and Y. Iwasaki, Macromol. Biosci., 2015, 15, 1634 CrossRef CAS PubMed.
- S. Zhang, A. Li, J. Zou, L. Y. Lin and K. L. Wooley, ACS Macro Lett., 2012, 1, 328 CrossRef CAS PubMed.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS PubMed.
- S. A. Smith and J. H. Morrissey, Curr. Opin. Hematol., 2014, 21, 388 CrossRef CAS PubMed.
- S. A. Smith, N. J. Mutch, D. Baskar, P. Rohloff, R. Docampo and J. H. Morrissey, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 903 CrossRef CAS PubMed.
- S. H. Choi, S. A. Smith and J. H. Morrissey, Blood, 2011, 118, 6963 CrossRef CAS PubMed.
- G. Leyhausen, B. Lorenz, H. Zhu, W. Geurtsen, R. Bohnensack, W. E. G. Müller and H. C. Schroder, J. Bone Miner. Res., 1998, 13, 803 CrossRef CAS PubMed.
- H. C. Schroder, L. Kurz, W. E. G. Müller and B. Lorenz, Biochemistry, 2000, 65, 296 CAS.
- Y. Hirano and Y. Iwasaki, Colloids Surf., B, 2017, 153, 104 CrossRef CAS PubMed.
- S. A. Low and J. Kopecek, Adv. Drug Delivery Rev., 2012, 64, 1189 CrossRef CAS PubMed.
- M. Owen, C. R. Howlett and J. T. Triffitt, Calcif. Tissue Res., 1977, 23, 103 CrossRef CAS PubMed.
- J. R. Neale, N. B. Richter, K. E. Merten, K. G. Taylor, S. Singh, L. C. Waite, N. K. Emery, N. B. Smith, J. Cai and W. M. Pierce Jr., Bioorg. Med. Chem. Lett., 2009, 19, 680 CrossRef CAS PubMed.
- D. Wang, S. Miller, M. Sima, P. Kopečková and J. Kopeček, Bioconjugate Chem., 2003, 14, 853 CrossRef CAS PubMed.
- Y. He, Y. Huang, Z. Huang, Y. Jiang, X. Sun, Y. Shen, W. Chu and C. Zhao, J. Controlled Release, 2017, 264, 76 CrossRef CAS PubMed.
- M. Yanagi, T. Uehara, Y. Uchida, S. Kiyota, M. Kinoshita, Y. Higaki, H. Akizawa, H. Hanaoka and Y. Arano, Bioconjugate Chem., 2013, 24, 1248 CrossRef CAS PubMed.
- Y. C. Fu, T. F. Fu, H. J. Wang, C. W. Lin, G. H. Lee, S. C. Wu and C. K. Wang, Acta Biomater., 2014, 10, 4583 CrossRef CAS PubMed.
- D. Wang, M. Sima, R. L. Mosley, J. P. Davda, N. Tietze, S. C. Miller, P. R. Gwilt, P. Kopečková and J. Kopeček, Mol. Pharm., 2006, 3, 717 CrossRef CAS PubMed.
- H. Pan, M. Sima, P. Kopečková, K. Wu, S. Gao, J. Liu, D. Wang, S. C. Miller and J. Kopeček, Mol. Pharm., 2008, 5, 548 CrossRef CAS PubMed.
- S. D'Souza, H. Murata, M. V. Jose, S. Askarova, Y. Yantsen, J. D. Andersen, C. D. Edington, W. P. Clafshenkel, R. R. Koepsel and A. J. Russell, Biomaterials, 2014, 35, 944 CrossRef PubMed.
- M. Karacivi, B. Sumer Bolu and R. Sanyal, Mol. Pharm., 2017, 14, 1373 CrossRef CAS PubMed.
- K. Miller, A. Eldar-Boock, D. Polyak, E. Segal, L. Benayoun, Y. Shaked and R. Satchi-Fainaro, Mol. Pharm., 2011, 8, 1052 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7bm00930e |
| This journal is © The Royal Society of Chemistry 2018 |