Analysis of natural and synthetic folates in pharmaceuticals and foods: a review
Shuo
Yin
 a,
Yi
Yang
a,
Yi
Yang
 a,
Yongxin
Li
a,
Yongxin
Li
 ab and
Chengjun
Sun
ab and
Chengjun
Sun
 *ab
*ab
aWest China School of Public Health, Sichuan University, Chengdu 610041, China. E-mail: sunchj@scu.edu.cn; Tel: +86-28-85501301
bProvincial Key Laboratory for Food Safety Monitoring and Risk Assessment of Sichuan, Chengdu 610041, China
First published on 28th November 2017
Abstract
Folate (vitamin B9) plays an important role in cell division and tissue growth by participating in the amino acid metabolism and nucleotide metabolism. Folate deficiency among pregnant women and children is common around the world, particularly in developing countries. Accurate folate analysis is critical for quality control in pharmaceutical and food products. This review briefly introduces the physiochemical properties, folate deficiency and food fortification of synthetic and natural folates, and the emphases are focused on the progress of sample pretreatment and analysis methods of these compounds in pharmaceuticals and foods in the last decade.
1 Introduction
Folate is a generic term for compounds sharing a common chemical backbone and the same vitamin activity. It acts as a coenzyme in several single-carbon transfer reactions, and thus plays an important part in numerous metabolic processes like the synthesis of DNA and RNA.1 A few authors have summarized research progress on natural and synthetic folates recently,2–5 mainly focusing on low folate status and related diseases,2 folate bioavailability and biofortification,3 electrochemical determination of folic acid,4 and unsubstituted food folates.5In this review, we will systematically summarize the recently reported sample treatment and analysis methods for natural and synthetic folates in pharmaceuticals and foods. The classification, physiochemical properties of folate and its deficiency and food fortification will briefly be discussed.
1.1 Classification
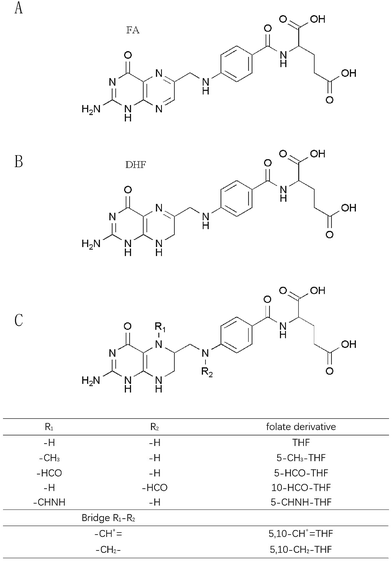
Folates include naturally occurring folates and synthetic folic acid in supplements and fortified foods.6 Natural folates exist in different forms that vary in both their oxidation state and the carbon group linked to the N5 and N10 positions of the pteridine ring.7 Based on the literature, common natural folates are grouped into 5-methyl-tetrahydrofolate (5-CH3-THF), formyl folates and unsubstituted folates. According to the oxidation states of the pteridine moiety, unsubstituted folates mainly consist of three types: fully oxidized folic acid (FA), reduced 7,8-dihydrofolate (DHF) and 5,6,7,8-tetrahydrofolate (THF).5 Formyl folates include 5-formyl-tetrahydrofolate (5-HCO-THF) and 10-formyl-tetrahydrofolate (10-HCO-THF) as well as their interconversion products such as 5,10-methenyl-tetrahydrofolate (5,10-CH+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) THF), 5,10-methylene-tetrahydrofolate (5,10-CH2-THF), and 5-formimino-tetrahydrofolate (5-CHNH-THF).8 Natural folates occur widely in green leafy vegetables, citrus fruit, egg yolk, liver, certain berries, nuts, beans, cereals, wheat flour and yeast.
THF), 5,10-methylene-tetrahydrofolate (5,10-CH2-THF), and 5-formimino-tetrahydrofolate (5-CHNH-THF).8 Natural folates occur widely in green leafy vegetables, citrus fruit, egg yolk, liver, certain berries, nuts, beans, cereals, wheat flour and yeast.
In 1931, Wills found a biological factor from yeast. This substance cured a megaloblastic anemia. In 1940, Snell and Peterson extracted one biological factor from spinach leaf and they named it “folic acid”. This name has been used ever since, but today folic acid (FA) only refers to the synthetic form of folate. FA was synthesized for the first time in 1945 by Shane and Carpenter.9 FA is industrially produced through chemical synthesis. China is the main producer of synthetic folic acid with a capacity of ca. 3000 metric tons per year. Nowadays, FA is widely used in various pharmaceuticals and fortified foods, such as flour, bread, fruit juices and infant food.2
1.2 Chemical structures and physiochemical properties
Various folates have a similar chemical structure. FA is composed of a pteridine ring, p-aminobenzoic acid (p-ABA) and L-glutamic acid. The chemical structures of folates are displayed in Fig. 1. Changes of chemical structures cause some differentiation of FA from natural folates. The pteridine moiety of FA exists in a fully oxidized state so that FA is much more stable than natural folates. Furthermore, natural folates often contain additional glutamate residues, which reduce their bioavailability.3The water solubility of FA is 1.6 mg L−1 at 25 °C and it is slightly soluble in methanol, less in ethanol and butanol, and insoluble in acetone, chloroform, ether and benzene. FA and natural folates are unstable in acidic solution and relatively stable in a neutral to basic medium. They are sensitive to ultraviolet light, air and heat. Suitable antioxidants, low temperature and a nitrogen atmosphere can slow down their degradation process.
1.3 Folate deficiency
Folate is an essential nutrient for humans. For folate deficiency, the most classical disease is megaloblastic anemia. Insufficient intake of folate in early pregnancy may increase the risks of neural tube defects (NTDs), low birth weight, cleft palate and other birth defects. The symptoms for folate deficiency also include canker sores, sore tongue, headaches, heart palpitations, irritability, and behavioral disorders. Furthermore, folate deficiency could increase plasma homocysteine concentrations, the frequency of chromosomal breaks and causes disruption of DNA synthesis, repair and methylation.10 Hence, it may increase the incidences of cardiovascular disease, stroke and some cancers.11 However, the evidence is insufficient, and further research is also needed.1.4 Recommended dietary allowances (RDA)
Folate can't be self-synthesized by the human body and must be supplied from the diet. Humans can obtain folate from natural foods, FA fortified foods and some pharmaceuticals such as FA tablets and multivitamin tablets. One dietary folate equivalent (DFE) is defined as 1 μg food folate. The bioavailability of natural folate is lower than that of FA. Thus, one DFE is equivalent to 0.6 μg FA from fortified foods or dietary supplements consumed with foods. The U.S. National Institutes of Health (NIH) suggested that folate RDA for adults was 400 μg DFE.12 Pregnant women need more folate because of the higher metabolic rate, greater DNA synthesis and rapid cell division.13 Hence, pregnant women should ingest 600–1000 μg DFE per day through folic acid tablets and foods to reduce the risk of birth defects.In 2015, the World Health Organization (WHO) published a guideline for folate. It says that high folic acid intake has not been reliably shown to be associated with negative health effects.14 However, a recent study reported that excessive FA intake may be associated with vitamin B12 deficiency, lower natural killer cell activity, higher insulin resistance and higher risk of unilateral retinoblastoma, breast cancer and other cancers.15 As yet, little research has been done in this area.
1.5 Food fortification
Poor folate intake leading to folate deficiency is common around the world, especially in developing countries. Since the 1990s, the United States and Canada firstly introduced mandatory fortification of cereal-grain products with FA in a concentration of 1.4 and 1.5 parts per million, respectively.16 Nowadays, the prevalence of folate deficiency is decreased in more than 70 countries by supplying FA fortified foods. Epidemiological studies have led to the identification of FA as a primary prevention strategy for NTDs.17 Red cell folate levels can reflect folate status perfectly. According to the statistics of the United Nations International Children's Emergency Fund (UNICEF), the serum folate level is on the rise. Today, there is virtually no folate deficiency anaemia in North America. In Canada, the incidence of neuroblastoma has fallen by more than 60% since the country began using FA fortified flour.18 Ocampo et al.19 analyzed the data from MotherToBaby cohort studies in the United States and Canada, and found that the use of folic acid-containing supplements might reduce the risk of gestational hypertension (GH) and preeclampsia (PE) for pregnant women. What's more, along with the increase of the duration of FA use came a lower risk for GH and PE. Statistical data are rather sparse about folate status in developing countries.Along with extensive use of fortified foods, the safety of synthetic FA in fortified foods has become an increasing concern recently. It's reported that FA may have adverse effects including vitamin B12 deficiency, changing DHF reductase enzyme activity and inducing cancer under certain conditions.20 A good alternative would be to increase the natural folate level in food crops. Vitamin bio-fortification strategies using food grade microorganisms have been reported in recent years.21 A study by Laiño and colleagues20 demonstrated, for the first time, that milk fermented with selected strains of lactic acid bacteria can improve folate status and prevent folate deficiency. Among lactic acid bacteria, S. thermophilus is regarded as an important industrial dairy starter to synthesize folates. Meucci et al.22 studied 50 strains of S. thermophilus, and they found that only 14 strains were able to grow and accumulate different amounts of FA in a culture medium without folates.
2 Sample pretreatment
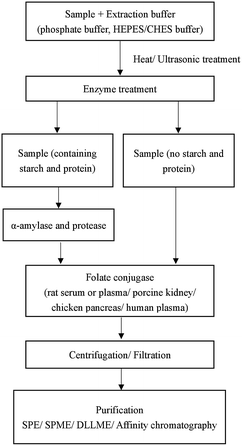
The precise analysis of folates is complicated because of their low level in samples, multiplicity of folate forms, their instability to chemical and physical factors and complex matrix interference of food.23 Different methods used in pretreatment can influence the quantitative accuracy. The choice of the sample pretreatment method depends on sample matrixes and the following detection methods. There is no standard pretreatment method for folate analysis. Generally, sample pretreatment involves several steps: extraction of folates from the matrix; incubation with α-amylase, protease, and folate conjugase; purification and concentration6 (Fig. 2).2.1 Sample extraction
Several types of buffers such as phosphate,24 ammonium acetate25 and 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid/2-(cyclohexylamino)ethanesulfonic acid (HEPES/CHES)26 at pH 4.5–7.5 have been used in folate extraction. The parameters such as the sample amount, the volume, the concentration and pH of the buffer, heating temperature and time could affect the extraction efficiency. In some food samples, part of folate is often bound to a protein. Heat treatment is usually used to liberate the bound form of folate from the protein.27 However, high temperature may increase folate degradation. As an alternative to heat treatment, high hydrostatic pressure (HHP) is an emerging and non-thermal technique used in the food industry for high-quality and safe food products.28 HHP is also used in sample pretreatment to improve extraction efficiency. As shown by Nassim's group,29 HHP can dissolve non-soluble granules in egg yolk and improve the recovery of 5-CH3-THF from egg yolk. The parameters of HHP were set to 400 MPa for 5 min. Under these conditions, the folate degradation was negligible.2.2 Enzyme treatment
The choice of enzymes depends on the sample matrices and the subsequent detection methods. When the sample contains protein and starch, α-amylase and protease are used to release folates that are trapped in carbohydrates and proteins. FA interacts with α-amylase and protease by multi non-covalent interactions including hydrophobic interactions, hydrogen bonding or electrostatic interactions.30 Natural folates are often in polyglutamyl forms, and folate conjugase can break down natural polyglutamyl folates into mono- or di-glutamate forms. Rat serum or plasma, porcine kidney, chicken pancreas and human plasma are the major sources of folate conjugase.27 Nevertheless, when the sample is fortified food, the folate conjugase is not always required.Tri-enzyme treatment using chicken pancreas conjugase, α-amylase, and protease was first advocated for microbiological assay of total folate in foods in 1990.31 This procedure is particularly effective for cereal-based and milk-based foods. Motta et al.32 used a tri-enzymatic extraction technique for extraction of five folates in quinoa, amaranth and buckwheat. LC-MS/MS was applied to the determination of the target folates with satisfactory results. As they said, the detection method is also a key factor that influences the selection of enzymes. Czarnowska-Kujawska et al.23 used HPLC for folate analysis in vegetables and fruit samples, and compared the effectiveness of two commonly used folate conjugases from porcine kidney and rat plasma. Rat plasma was chosen as the source of folate conjugase in this research. Chandra-Hioe and coworkers27 compared enzymatic treatments to release FA and endogenous 5-CH3-THF from infant milk with enzyme-free heat extraction. The results indicated that enzyme-free heat extraction of folate was more compatible with UPLC-MS/MS than enzymatic treatment.
2.3 Purification and concentration
The sample matrix interference should be eliminated before determination because the folates are at a very low level in the extraction solution. Many techniques including solid phase extraction (SPE), solid phase microextraction (SPME), dispersive liquid–liquid microextraction (DLLME) and affinity chromatography have been reported for purification and concentration of folates in sample solutions.Folate and FA and its derivatives are prone to be oxidized during the sample pretreatment. A dark place, lower temperature and neutral to alkaline environment can slow the oxidative process. What's more, antioxidants are also important in resisting oxidation. Ascorbic acid,44 sodium ascorbate,45 β-mercaptoethanol,46 2,3-dimercapto-1-propanol24 and 1,4-dithiothreitol34 are commonly used antioxidants. Sometimes, two or more antioxidants are used together to have a better antioxidant effect. Nitrogen can also prevent folates from oxidation.
3 Analysis methods for folates
A variety of methods have been developed for the separation and quantification of FA and its analogues in different matrices, including microbiological assay (MA), bio-specific procedures, high-performance liquid chromatography (HPLC), liquid chromatography-tandem mass spectrometry (LC-MS/MS) and capillary electrophoresis (CE). Other techniques such as various sensors, spectrophotometry47 and a flow injection chemiluminescence method48 have been reported too.3.1 Microbiological assay (MA)
Some microorganisms require folate as a nutritional factor for their growth and multiplication, which they are unable to synthesize by themselves. These microorganisms are used for quantitative folate analysis. Microbiological assay was firstly used for the determination of folate using Lactobacillus casei in 1968.14 MA had been a standard method of folate analysis over the past few decades. To date, most of the available data about folate content in foods have been obtained by MA. MA is sensitive to various forms of folate with bioactivity. Along with continuously going deeper into research, some new MA methods were established during recent years. Fajardo et al.49 reported a 96-well microtiter plate MA method for the determination of the total folate in 21 fresh-cut vegetables and fruits using chloramphenicol-resistant Lactobacillus casei subspecies rhamnosus (NCIMB 10463). The detection range of FA was 0–30 pg/100 μL and the recoveries were 85–110%. The sensitivity and reproducibility were improved, compared with traditional MA methods. However, MA can't distinguish the different forms of folate, so it can only analyze the total folate in the samples.3.2 Bio-specific assay
Bio-specific assay can be classified into two types, i.e. the assays based on the use of naturally occurring vitamin binding proteins with either radiolabels or enzyme labels; and those based on the specific interaction of an antibody with its antigen, e.g. enzyme linked immunosorbent assay (ELISA).50 As for the present, much of the bio-specific assays are used in clinical diagnosis rather than food analysis. The national health and nutrition examination survey (NHANES) of U.S. monitored the folate status of U.S. populations from 1998–2010. The Bio-Rad radioassay (BR) was used to measure serum and RBC concentrations from 1988–2006, and MA was used from 2007–2010. The latter is considered to be more accurate.51 Most bio-specific assays are tedious, time-consuming and unstable. Immunoassay has been also applied in some biosensors recently.3.3 High performance liquid chromatography (HPLC)
The limitation of MA in analysis of folate derivatives has promoted the use of liquid chromatographic techniques.50 High performance liquid chromatography (HPLC) has become a popular method for analysis of folates in the past decade. The HPLC methods for analysis of folates in pharmaceuticals and foods are shown in Table 1. Various detectors including ultraviolet detectors (UVDs),35,42 diode array detectors (DADs),52,53 electrochemical detectors (ECDs)54 and fluorescence detectors (FLDs)23,26,29 have been used. An UVD responds to all folates, but the UVD lacks the sensitivity to detect folates at the trace levels required. As a result, a FLD is a better choice for analyzing folates in complex matrices. Czarnowska-Kujawska et al.23 reported an HPLC method with fluorometric detection for analysis of 5-CH3-THF and THF in fresh fruits and vegetables with the LOQs of 0.4–0.6 ng mL−1. A SPE cartridge using strong anion exchange was used for sample purification and the recoveries were between 92% and 105%. THF and 5-CH3-THF are natively fluorescent. In contrast, FA is a weakly fluorescent molecule and it needs fluorescence derivatization. Tornero et al.26 developed a HPLC-FLD method for simultaneous determination of FA, THF and 5-CH3-THF in cereals, spinach and tomatoes. A post-column on-line photoderivatization was used for FA analysis. The LODs of FA, THF and 5-CH3-THF were 7.47 ng mL−1, 15.3 ng mL−1, 22.9 ng mL−1, respectively. Wang et al.55 developed ultra-performance liquid chromatography (UPLC) coupled with ion chromatography (IC) to simultaneously determine folic acid and inorganic anions in folic acid tablets. As for folic acid, the LOD was 0.0061 mg L−1, and the RSDs for peak area and retention time were 0.49% and 0.14%, respectively.| Analytes | Sample matrix and preparation | Analytic conditions | Method performances | Ref. |
|---|---|---|---|---|
| FA, 5-CH3-THF, 5-HCO-THF, THF, 10-HCO-THF, 10-HCO-DHF | Two fruits and six vegetables; extracted, enzyme-treated (α-amylase, rat plasma), and SPE (SAX) | Column: phenomenex synergi C18 (250 × 4.6 mm, 4 μm); mobile phase: acetonitrile (ACN)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 mmol L−1 phosphoric acid buffer (pH 2.3); detector: UVD (290 nm), FLD: ex 290 nm and em 360 nm 30 mmol L−1 phosphoric acid buffer (pH 2.3); detector: UVD (290 nm), FLD: ex 290 nm and em 360 nm |
LOQ = 0.4 (5-CH3-THF); 0.6 (THF) ng mL−1; range = 0.3–66.3 (5-CH3-THF), 0.4–55.7 (THF) ng mL−1; recovery = 109% ± 9 (5-CH3-THF); 93% ± 8 (THF) | 23 |
| FA, THF, 5-CH3-THF, 10-HCO-THF, 5-HCO-THF and pteroic acid | New Zealand spinach; extracted, heated, centrifuged, and filtered | Column: C18 (100 × 2.0 mm, 5 μm); mobile phase: 100 mmol L−1 potassium phosphate buffer (pH 2.0)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (85 methanol (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, v/v); detector: DAD 15, v/v); detector: DAD |
LOD = 1.33–36.2 mg/100 g | 25 |
| FA, THF, 5-CH3-THF | Cereals, spinach, tomatoes; extracted, enzymatically deconjugated (rat plasma), and SPE (SAX) | Column: C8 (50 × 4.6 mm, 2.7 μm); mobile phase: 2 mmol L−1 formic acid (pH 4)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN (6 ACN (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 94, v/v); detector: fast scanning fluorimetric detector (FSFD) (ex 280 nm and em 360 nm for THF and 5-CH3-THF and 440 nm for FA) 94, v/v); detector: fast scanning fluorimetric detector (FSFD) (ex 280 nm and em 360 nm for THF and 5-CH3-THF and 440 nm for FA) |
LOD = 7.47; 15.3; 22.9 ng mL−1; range = 50–1000; 100–1000; 100–1000 ng mL−1; RSD (intra-day) = 5.28%; 1.28%; 1.36% (n = 5); recovery = 92.73–105.0% | 26 |
| FA | Spinach, orange; extracted, filtered, and SPE (10 mg MMIPs) | Column: C18 (250 × 4.6 mm); mobile phase: ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (90 water (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v); detector: UVD (280 nm) 10, v/v); detector: UVD (280 nm) |
Recoveries = 95–104% (orange), 99.5–102.5% (spinach); RSD (for recoveries) < 0.5% | 35 |
| 5-HCO-THF, 10-HCO-THF, 5-CH3-THF, 5,10-CH2-THF, FA | Wheat-flour, egg yolk, and orange juice; extracted, filtered, and IP-DLLME (extraction solvent: 1-octanol, disperser solvent: ethanol) | Column: ODS (250 × 4 mm, 5 μm); mobile phase: phosphate buffer (pH 3.5)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN (90 ACN (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v); detector: UVD (280 nm) 10, v/v); detector: UVD (280 nm) |
LOD = 2–4.1 ng g−1; range = 1–200 ng g−1; RSD = 5.2–7.4%; recoveries = 98–102% | 42 |
| FA, VC and five other water-soluble vitamins B | Vitamins with mineral tablets (VMT); diluted, ultrasound, and filtered | Column: C18 (250 × 4.6 mm, 5 μm); mobile phase: 50 mmol L−1 ammonium dihydrogen phosphate buffer (pH 3.0)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN (85 ACN (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, v/v for FA); detector: DAD (280 nm for FA) 15, v/v for FA); detector: DAD (280 nm for FA) |
LOD = 0.010 mg L−1 (FA); range = 2.952–8.856 mg L−1 (FA); RSD = 1.37% (intra-day) and 1.75% (inter-day) (FA); recoveries = 97.95–99.51% (FA) | 52 |
| FA, VC and four other water-soluble vitamins B | Pharmaceutical preparations; centrifuged | Column: C18 (150 × 3.0 mm, 5 μm); mobile phase: 0.1% formic acid (pH 2.6)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN; detector: DAD (285 nm) ACN; detector: DAD (285 nm) |
Range = 0.6–12 μg mL−1 (FA); RSD < 2.06% (FA); recoveries = 99.13–100.48% (FA) | 53 |
| FA and five inorganic anions | Folic acid tablets; dissolved, ultrasound, diluted, and filtered | Column: C18 (150 × 4.6 mm, 2.6 μm); mobile phase: methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) deionized water (3 deionized water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97, v/v); detector: UVD (280 nm) 97, v/v); detector: UVD (280 nm) |
LOD = 0.0061 mg L−1 (FA); range = 0.1–10.0 mg L−1 (FA); RSD = 0.14% (retention time) and 0.49% (peak area) (FA); recovery = 96.4–105.8% (FA) | 55 |
| FA | Fortified cereals and juices; extracted, centrifuged, filtered | Column: Diamond Hydride™ (75 × 4.6 mm, 4.2 μm); mobile phase: (A) H2O + 10 mmol L−1 ammonium formate, (B) 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 acetonitrile 10 acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O + 10 mmol L−1 ammonium formate; detector: VWUVD (284 nm) H2O + 10 mmol L−1 ammonium formate; detector: VWUVD (284 nm) |
Range = 0.1–2.0 mg L−1; RSD = 0.02–0.14% (samples), 0.02–0.08% (standard solutions); recoveries = 90% | 82 |
| 5-CH3-THF | Nutritional products; centrifuged | Column: C18 analytical column (250 × 4.6 mm, 5 μm); mobile phase: 0.05 mol L−1 KH2PO4 (pH 2.9): ACN; detector: FLD | LOD = 10 μg kg−1; RSD = 1.1%; recovery = 92.8% | 83 |
As for HPLC and UPLC, some detectors by themselves are not confirmatory such as UVDs. Some auxiliary methods are used for confirmatory analysis. For example, a DAD detector can provide a 3-D scanning spectrum which can be used for qualitative analysis. However, the most efficient method for confirmatory analysis is MS/MS detection.
3.4 LC-MS/MS analysis
Although HPLC holds a high position for analysis of folates in foods and pharmaceuticals, it has some limitations. A strong matrix effect in complex samples influences the precision of analysis. Furthermore, conventional LC detectors are unable to detect the very low level of folates and FA in the samples. LC (UPLC) coupled with MS/MS has very high separation efficiency as well as strong identification ability. Therefore, the application of UPLC-MS/MS in the analysis of FA and folates in foods and pharmaceuticals increased sharply in recent years. In AOAC Method 2011.06 (ref. 37) and AOAC Method 2013.13 (ref. 34), UPLC coupled to a triple quadrupole mass spectrometer is applied to the determination of total folates in infant formulas and adult nutrients. Shohag et al.24 established an UPLC-tandem quadrupole mass spectrometry method for the determination of seven folates in plant leaves within five minutes of running time for the first time. A SPE (SAX) technique was used for sample purification after the tri-enzyme treatment. The range was 0.1–60 μg/100 g. The recoveries were 71.27–99.01%, and the total folate in four plant leaves were 117.45–223.74 μg/100 g fresh weight (FW). Stable isotopes such as 13C-labeled (13C5) isotopes27,34 and deuterium-labeled (2H4) isotopes56 of folates are usually used as internal standards (ISs) to improve the accuracy in MS analysis. Methotrexate has also been used as an IS for quantification of five folates.57Table 2 shows the LC-MS/MS methods for the determination of folate in pharmaceuticals and foods.| Analytes | Sample matrix and preparation | Analytic conditions | Method performances | Ref. |
|---|---|---|---|---|
THF, 5-CH3-THF, 5-HCO-THF, 5,10-CH+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) THF, FA, FA-glu3 10-HCO-THF THF, FA, FA-glu3 10-HCO-THF |
Plant leaf; extracted (tri-enzyme), centrifuged, filtered, and SPE (SAX) | Column: ACQUITY UPLC BEH C18 column (50 mm × 2.1 mm, 1.7 μm); mobile phase: 0.1% formic acid in water and 0.1% formic acid in acetonitrile; detector: tandem quadrupole mass spectrometer | LOD = 0.003–0.021 μg/100 g FW; range = 0.1–60 μg/100 g; RSD = 1.7–7.8%; recoveries = 71.27–99.01% | 24 |
| FA, 5-CH3-THF | Infant milk formulae and adult nutritionals; heated, centrifuged, filtered, and SPE (styrene divinylbenzene) | Column: waters Acquity HSS T3 (100 × 1.0 mm, 1.8 μm); mobile phase: 0.1% (v/v) formic acid and ACN; detector: TSQ quantum access tandem quadrupole mass spectrometer (Thermo-Scientific, San Jose, CA) (FA: m/z 442 → 295; 5-CH3-THF: m/z 460 → 313); internal standard: [13C5]-FA and [13C5]-5-CH3-THF | LOD = 1.4 (FA) and 7.5 (5-CH3-THF) ng mL−1; range = 3.1–1400 (FA) and 16.2–1400 (5-CH3-THF) ng mL−1; RSD = 6% (intra-day), 8% (inter-day); recoveries = 85% (FA) and 95% (5-CH3-THF) | 27 |
| FA, 5-CH3-THF, THF, 5-HCO-THF, 10-HCO-THF | Quinoa, amaranth, and buckwheat; tri-enzyme treatment (α-amylase, protease and rat serum), centrifuged, and filtered | Column: HSS T3 (150 × 2.1 mm, 1.8 μm); mobile phase: 0.1% formic acid solution in water and 0.1% formic acid solution in ACN; detector: triple quadrupole mass spectrometer (Waters ACQUITY® TQD, Waters Co., Milford, USA); internal standard: (13C5) FA and (13C5) 5-CH3-THF | LOD = 0.56–1.02 μg/100 g; range = 7–100 ng mL−1; RSD < 10% | 32 |
| FA, 5-CH3-THF | Standard reference materials (SRMs): infant/adult nutritional formula, fortified breakfast cereal, whole milk powder, and whole egg powder; tri-enzyme treatment (α-amylase, protease and rat serum), and SPE (C18) | Column: Agilent Zorbax SB-C18 (150 × 2.1 mm, 3.5 μm); mobile phase: 0.1% formic acid in water and 0.1% formic acid in ACN; detector: triple quadrupole mass spectrometer | RSD < 8% | 33 |
| FA, 5-CH3-THF | Infant formula and adult/pediatric nutritional formula; enzyme-treatment (α-amylase, protease), and SPE (SAX) | Column: HSS T3 (150 × 2.1 mm, 1.8 μm); mobile phase: 0.5% (v/v) acetic acid in water and ACN; detector: triple quadrupole mass spectrometer (Agilent 6460) | LOD = 0.10 (FA) and 0.05 (5-CH3-THF) μg/100 g; range = 0.33–300 μg/100 g; RSD < 5.4%; recoveries = 89–114% | 34 |
| THF, 5-CH3-THF, 10-FFA, 5-HCO-THF, FA, FA-glu3, 10-MFA | Infant formula and adult nutritionals; tri-enzyme treatment (α-amylase, protease and rat plasma), and SPE (WAX) | Column: HSS T3 (150 × 2.1 mm, 1.8 μm); mobile phase: 10 mmol L−1 ammonium formate buffer (pH 2.8); detector: triple quadrupole mass spectrometer (Waters Quattro Premier) | Range = 10–19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 μg/100 g; RSD = 4.69–13.89%; recoveries = 94.10–101.34% 100 μg/100 g; RSD = 4.69–13.89%; recoveries = 94.10–101.34% |
37 |
FA, 5-CH3-THF, THF, 5-HCO-THF, 5,10-CH+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) THF THF |
Tomatoes; homogenized, tri-enzyme treatment (α-amylase, protease and rat serum), centrifuged, and filtered | Column: Luna C18 (250 × 4.60 mm, 5 μm); mobile phase: 0.1% (v/v) formic acid in water and ACN; detector: Exactive™ Plus Orbitrap mass spectrometer (Thermo Fisher Scientific, USA) | LOD = 0.15–0.37 ng mL−1; range = 1–30 (FA, 5-CH3-THF, 5-HCO-THF), 0.5–30 (5,10-CH+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) THF), 2–30 (THF) ng mL−1; recoveries = 48–131% THF), 2–30 (THF) ng mL−1; recoveries = 48–131% |
57 |
3.5 Capillary electrophoresis (CE)
In the past decade, CE has been applied to analysis of folic acid and its analogues in pharmaceuticals and foods. Various detectors can be coupled to CE, including UVDs,58 DADs,59 laser-induced fluorescence (LIF) detectors,60 chemiluminescence detectors (CLDs)61 and MS,62 which makes CE a powerful analytical tool. Silva and colleagues58 developed a CE-UVD method for FA and nine other water-soluble vitamins in food supplements with a detection limit of 0.28 mg L−1. The sensitivity of CE analysis is unsatisfactory because of its short light path and small sample volume. To overcome this limitation, a laser-induced fluorescence detector has been used for analyzing trace-level folic acid. The sensitivity of the LIF detector is about 1000 times higher than that of the UVD. Zhao et al.60 reported a CE-LIF method for the simultaneous determination of three essential amino acids and three B vitamins (FA, niacinamide and riboflavin). The assay had a linear range of 0.005 to 0.80 μmol L−1 for FA, with a detection limit of 5.0 nmol L−1. Chemiluminescence detectors (CLDs) are also regarded as one of the most sensitive detectors and furthermore, it does not require a bulky light source.63 Zhao et al.61 established a CE-CLD method for the detection of FA in pharmaceutical tablets, apple juice, and human urine. Its LOD was 2.0 × 10−8 mol L−1, and the recoveries were 94.3–105.8%. In general, CE has the advantages of high resolution, high speed, and low cost. However, its main drawbacks are bad reproducibility and unsatisfactory sensitivity. A variety of CE methods for folate analysis are summarized in Table 3.| Analytes | Sample matrix and preparation | Analytic conditions | Method performances | Ref. |
|---|---|---|---|---|
| FA and nine other water-soluble vitamins | Food supplements (drinks, tablets, powders, and injection solutions); diluted, and filtered | Column: a fused silica capillary (60 cm × 50 μm) and 49.85 cm of effective length; mobile phase: hydroxide solution and water; detector: UVD (214 nm) | LOD = 0.28 mg L−1 (FA); range = 6–60 mg L−1; RSD = 1.9% (intra-day) and 6.7% (inter-day); recoveries = 93.57–100.05% (FA) | 58 |
| FA | Green, red and mignon lentils; extracted, centrifuged, and filtered | Column: agilent uncoated fused silica capillary (effective lengths 55 cm, 75 μm I.D.); mobile phase: 10 mM sodium tetraborate in 10% (v/v) methanol at pH 9; detector: DAD (200 nm); internal standard: methylparaben | LOD = 6.12 × 10–7 mol L−1; range = (1.2–4.8) × 10–5 mol L−1; RSD = 0.65% (inter-day); 0.48–0.79% (intra-day); recoveries = 112% | 59 |
| FA, two other water-soluble vitamin B and three amino acids | Four health drink samples; diluted and derivatized | Column: uncoated fused-silica capillaries (60 cm × 50 μm), with an effective length of 50 cm); mobile phase: running buffer (Na2B4O7), NaOH, water solution; detector: LIF (488 nm excitation, 520 nm emission) | LOD = 5.0 nmol L−1; range: 0.005–0.80 μmol L−1 (FA); RSD = 1.09% (migration time) and 3.57% (peak area) (FA); recoveries = 93.32% ± 2.58 (FA) | 60 |
| FA | FA tablets, apple juice, and human urine; diluted and heated | Column: a 50 cm × 75 μm I.D. uncoated fused silica capillaries; electrophoresis electrolyte: 35 mmol L−1 sodium borate (pH 9.4) containing 0.8 mM luminol; detector: chemiluminescence detector (CLD) | LOD = 2.0 × 10−8 mol L−1; range = 5.0 × 10−8 to 1.0 × 10−5 mol L−1; RSD = 1.5% (peak area) and 1.1% (migration time); recoveries = 94.3–105.8% (FA tablet) | 61 |
| FA and eight other water-soluble vitamin B | Pharmaceuticals (one injection solution and two tablets); diluted and filtered | Column: Agilent CZE column (50 μm I.D. uncoated fused silica capillary tube of an 820 mm total length; mobile phase: 50 mmol L−1 formic acid (pH 2.05); detector: MS/MS | LOD = 0.1715 μg mL−1 (FA); range = 3.125–50 μg mL−1; RSD = 4.44% (FA); recoveries = 93.08% (FA) | 62 |
| FA | Fortified instant Asian noodles; extracted, enzyme-treated (α-amylase), centrifuged, and filtered | Column: uncoated fused-silica capillaries (75 μm I.D., 50 cm effective length); mobile phase: 8 mM phosphate-12 mmol L−1 borate run buffer with 5% MeOH at pH 9.5; detector: DAD (214 nm); internal standard: nicotinic acid | LOD = 5.3 mg L−1; recoveries = 96–103% | 84 |
3.6 Sensors
Sensors have been playing an increasingly important role in analysis of FA and its analogues due to its high sensitivity, small size and negligible environmental impact. In recent years, there have been numerous sensors reported, such as luminescent sensors,64–67 electrochemical sensors,68–71 and biosensors,46,72 for the quantification of folates. Luminescent sensors can be classed into photoluminescent sensors, chemiluminescent sensors and electrochemiluminescent sensors. Many materials are used as fluorescent probes in the photoluminescent sensors such as 8-aminonaphthalene-1,3,6-trisulfonate anchored to Zn–Al–CO3-layered double hydroxide (ANTS-anchored Zn–Al–CO3-LDH) particles,64 polyethylenimine-capped silver nanoclusters (PEI-AgNCs)66 and quantum dots (QDs).67 Liu et al.64 designed a fluorescent sensor based on ANTS-anchored Zn–Al–CO3-LDH particles. This method was rapid, inexpensive and label-free, and it had excellent sensitivity (LOD = 0.1 μmol L−1) and repeatability (RSD ≤ 3.5%). However, it was only applicable to the analysis of FA in the simulated phosphate buffer solution (pH = 7.4) instead of real samples. Zhang et al.66 detected FA in FA tablets, wheat flour, liquid and powdered milk using PEI-AgNCs as a fluorescence probe in the luminescent sensors (LOD = 0.032 nmol L−1) (Fig. 3A). Electrochemiluminescent sensors have also been applied to the folate analysis. Li et al.73 established a rapid and sensitive electrochemiluminescence sensor based on a 3D graphene/CdSeTe/Ru(bpy)32+-doped silica nanocomposite modified electrode (Fig. 3B). The linear range of FA detection in FA tablets was 1.0 × 10−11 mol L−1 to 1.0 × 10−6 mol L−1, and the LOD was 3.6 × 10−12 mol L−1.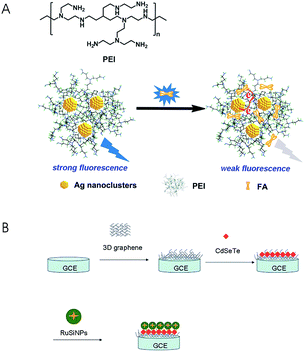 | ||
| Fig. 3 (A) Chemical structure of PEI and schematic representation of FA detection (reproduced from ref. 66); (B) the preparation procedures for a 3D graphene/CdSeTe/RuSiNP modified electrode (reproduced from ref. 73). | ||
Electrochemical techniques are much more sensitive and easy to use, and offer low detection limits compared to conventional methods.4 Electrochemical impedance spectroscopy (EIS) has been used for folate analysis and electrochemical characterization of electrodes such as square wave voltammetry (SWV),68 differential pulse voltammetry (DPV),74 cyclic voltammetry (CV),75 and linear sweep voltammetry (LSV).76 In order to improve selectivity and sensitivity for folate analysis, many chemically modified electrodes (CMEs) have been used in electrochemical sensors. Numerous nanomaterials such as nanowires,71 nanoparticles,77 nanotubes76 and nanocomposites78 are used as modifiers for electrodes. Recently, room temperature ionic liquids (RTILs)79,80 have been applied in chemically modified electrodes because of their higher ionic conductivity, wider electrochemical windows and environmental friendliness. Jamali et al.79 developed a sensitive nanosensor based nanoalloy (Pt:Co) room temperature ionic liquid (RTIL) modified carbon paste electrode for analysis of FA in fortified juice, FA tablets and mint vegetable. The LOD was 4.0 × 10−8 mol L−1, and the RSD was 0.94%.
Biosensors also provide a new choice in folate analysis. The biosensor incorporating surface plasmon resonance (SPR) offers an excellent platform for the detection of folate in pharmaceuticals and foods. Indyk81 developed an optical biosensor assay utilizing folate-binding proteins. In his study, the cross-reactivity of folate-binding proteins for both FA and 5-CH3-THF facilitated a more reliable estimate of the total folate in samples. It may provide a precise and accurate alternative to conventional methods for folate routine analysis in milk-based products. A pencil graphite electrode modified with salmon sperm ds-DNA was used in an electrochemical biosensor for FA analysis by Mirmoghtadaie and co-workers.72 Biosensors have a higher selectivity and specificity for bio-active substances than other sensors in complex matrices. Nevertheless, sensors have some drawbacks such as poor reproducibility, difficulty in simultaneous determination and complex packaging techniques, which limit their wide application. Table 4 shows the various types of sensors for analysis of folates in pharmaceuticals and foods.
| Analytes | Sample matrix and preparation | Detection techniques and employed materials | Method performances | Ref. |
|---|---|---|---|---|
| FA | Infant formula and adult/pediatric nutritional formula; enzymatic and heat treatments | SPR optical biosensor assay; exchangeable carboxymethyldextranfunctionalized chip using folate binding protein (FBP) | LOD = 6.5 μg/100 g; RSD = 3.48% (intra-day) and 4.63% (inter-day) | 46 |
| FA | Aqueous media | Fluorescence detection (ex 355 nm and em 506 nm); ANTS-anchored Zn–Al–CO3-LDH particles | LOD = 0.1 μmol L−1; range = 1–200 μmol L−1; RSD = 0.7–3.4%; recoveries = 97.4–104.0% | 64 |
| FA | Pharmaceutical tablets | Fluorescence detection; BSA-stabilized gold nanoclusters (AuNCs) | Range = 120.0 ng mL−1 to 33.12 μg mL−1; LOD = 18.3 ng mL−1; RSD < 4%; recoveries = 93.5–95.7% | 65 |
| FA | FA tablets, wheat flour, liquid milk and milk powder, urine; centrifuged | Fluorescence detection (ex 375 nm and em 452 nm) PEI-AgNCs | LOD = 0.032 nmol L−1; range = 0.1 nmol L−1 to 2.75 μmol L−1; RSD < 3.5%; recoveries = 96.0–105.0% | 66 |
| FA | Aqueous media | Fluorescence detection; copper- or manganese-doped ZnS QDs | LOD = 11 μmol L−1 | 67 |
| FA and vanillin | Chocolate, biscuit and coffee milk; sonicated, centrifuged, and diluted | Electrochemical detection (SWV); cadmium oxide nanoparticle decorated with single wall carbon nanotubes (CdO/SWCNTs) and 1,3-dipropylimidazolium bromide (DPIB) as a binder (CPE/CdO/SWCNTs/DPIB) | LOD = 0.06 ± 0.01 μmol L−1 (FA); range = 0.1–1200 μmol L−1 (FA) | 68 |
| FA and 6-thioguanine | Folic acid tablets, 6-thioguanine tablets and urine samples | Electrochemical detection (SWV); 2-chlorobenzoylferrocene/ZnO–CuO nanoplate modified carbon paste electrode (2CBFZCCPE) | Range = 15–2000 μmol L−1; RSD = 1.8–3.4% (FA); recoveries = 97.4–103.3% (FA) | 69 |
| FA, ascorbic acid and uric acid | B complex tablets, FA and human urine; centrifuged and filtered | Electrochemical detection; Mn–SnO2 nanoparticle modified glassy carbon electrode | Range = 1–500 μmol L−1 (FA); LOD = 25 nmol L−1 (FA); RSD = 3.51% (FA); recoveries = 99.4–102.3% | 70 |
| FA | FA tablets; dissolved and filtered | Electrochemical detection (SWV); bismuth nanowires (BiNWs) on glassy carbon (GC) substrates | Range = 10–150 nmol L−1; LOD = 9.53 nmol L−1; RSD = 2.5% recovery = 92.33% | 71 |
| FA | FA tablets, fortified wheat flour, and spinach; centrifuged and filtered | Electrochemical biosensor (DPV); a pencil graphite electrode modified with salmon sperm ds-DNA | Range = 0.1–10.0 μmol L−1; LOD = 1.06 × 10−8 μmol L−1; RSD < 4.6%; recoveries = 98.4–100.8% | 72 |
| FA | FA tablets | Electrochemical detection (DPV); nanosized gold and graphene modified carbon ionic liquid electrode | Range = 0.01–50.0 μmol L−1; LOD = 2.7 nmol L−1; RSD = 2.1%; recoveries = 97.2–98.4% | 74 |
| FA | Vitamin supplement formulations and human serum; vitamin supplement: filtered and diluted | Electrochemical detection (SWV); polypyrrole-α-polyoxometalate-AuNPs (PPy-α-POM-AuNP modified gold (Au) electrode) | Range = 1.0–44.0 nmol L−1; LOD = 0.12 nmol L−1; RSD = 5.3% (intra-day), 5.47% (inter-day) | 75 |
| FA | FA tablets, oats; centrifuged | Photoelectrochemical detection; carbon nanohorn supported interwoven titanate nanotubes | Range = 1 × 10−10 mol L−1 to 5 × 10−5 mol L−1; LOD = (2.5 ± 0.005) × 10−11 mol L−1; recoveries = 96.1–101.5% | 76 |
| FA, VC | Five pharmaceutical tablets, blood serum, and lemon juice; filtered and diluted | Electrochemical detection (DPV); magnetite (Fe3O4) nanoparticle modified carbon paste electrode (Fe3O4-CME) | Range = 6.50 × 10−8–9.80 × 10−5 mol L−1 (FA); LOD = 2.01 nmol L−1 (FA); RSD = 2.23% (intra-day), 1.49% (inter-day) (FA); recoveries = 98.9–99.5% (FA) | 77 |
| FA, glutathione, nicotinamide adenine dinucleotide; | Pharmaceutical tablets, human blood, and urine; tablets: filtered and diluted | Electrochemical detection (SWV); carbon paste electrode (CPE) modified ZnO/CNT nanocomposite | Range = 3.0–700 μmol L−1 (FA); LOD = 1.0 μmol L−1 (FA); RSD = 2.9% | 78 |
| FA | Fortified juice, FA tablets and mint; centrifuged and filtered | Electrochemical detection (SWV); a nanoalloy (Pt:Co) room temperature ionic liquid (RTIL) modified carbon paste electrode | Range = 1.0 × 10−7–5.0 × 10−4 mol L−1; LOD = 4.0 × 10−8 mol L−1; RSD = 0.94% | 79 |
| FA | Tablets, apple juice, and urine; centrifuged and filtered | Electrochemical detection (SWV); ZnO/nanoparticle (NP) modified carbon ionic liquid paste electrode (ZnO/NP/CILPE) | Range = 0.05–1.5 μmol L−1 (slope = 1.776 μA μM−1) and 1.5–550 μmol L−1 (slope = 0.033 μA μM−1); LOD = 0.01 μmol L−1 | 80 |
| FA | Pharmaceutical products (foligen and folvite); dissolved, sonicated, filtered, and diluted | Electrochemical detection (CV, LSV and chronoamperometry); hydroxyapatite (HA) nanoparticle (NP) modified glassy carbon electrode (GCE) | Range = 1.0 × 10−7–3.5 × 10−4 mol L−1; LOD = 75 nmol L−1; RSD = 5.6%; recoveries = 98.48–103.25% | 85 |
| FA | Water | Surface enhanced Raman scattering (SERS); gold nanorod (AuNR) grafting of molecularly imprinted polymers (MIPs) | Range = 1.0 × 10−8–1.0 × 10−4 mol L−1; LOD = 0.1 μmol L−1 | 86 |
| FA | Electrochemical detection; on/off-switchable molecularly imprinted polymer (MIP) modified electrode | Range = 1.0–200 μmol L−1; LOD = 0.9 μmol L−1 | 87 | |
| FA | Aqueous, serum and pharmaceutical samples | Electrochemical detection (differential pulse and cathodic stripping voltammetric); molecularly imprinted polymer (MIP)-carbon consolidated composite fiber | LOD = 0.191–0.197 ng mL−1; RSD = 0.9–1.4%; recoveries = 98.5–100.7% | 88 |
4 Conclusion and perspectives
Folate deficiency is one of the major global public health issues. Low folate intake and poor folate status are generally present in pregnant women and children, especially in developing countries. More data are needed on various natural folate bioavailabilities and the folate content in foods typically consumed should be established for the different regions of the world. For different forms of folates in pharmaceuticals and foods, it is necessary to develop more easy methods with high sensitivity, specificity and good reproducibility. Compared with traditional MA methods, HPLC and CE can distinguish different folate forms. Various detectors are used in HPLC and CE. A MS/MS detector has higher sensitivity and specificity and it can accurately quantify various forms of folates at trace levels. In recent years, sensors have become a better alternative for folate analysis, especially electrochemical sensors and biosensors. The application of various novel materials as modifiers for electrodes increased the selectivity and decreased the detection limit of the sensors. In a word, the determination of natural and synthetic folates should be developed in a rapid, easy, sensitive, green way. The development of the techniques for rapid pretreatment and simultaneous determination of FA and its derivatives will be still the key research point in the future.Conflicts of interest
There are no conflicts to declare.References
- F. Martin, E. C. Giménez and E. Konings, J. AOAC Int., 2016, 99, 19–25 CrossRef CAS PubMed.
- R. Obeid, K. Oexle, A. Rißmann, K. Pietrzik and B. Koletzko, J. Perinat. Med., 2016, 44, 261–268 CAS.
- R. K. Saini, S. H. Nile and Y. Keum, Food Res. Int., 2016, 89, 1–13 CrossRef CAS PubMed.
- S. Akbar, A. Anwar and Q. Kanwal, Anal. Biochem., 2016, 510, 98–105 CrossRef CAS PubMed.
- H. S. Strandler, J. Patring, M. Jägerstad and J. Jastrebova, J. Agric. Food Chem., 2015, 63, 2367–2377 CrossRef CAS PubMed.
- R. Iyer and S. K. Tomar, J. Food Sci., 2009, 74, R114–R122 CrossRef CAS PubMed.
- C. Serrano-Amatriain, R. Ledesma-Amaro, R. López-Nicolás, G. Ros, A. Jiménez and J. L. Revuelta, Metab. Eng., 2016, 38, 473–482 CrossRef CAS PubMed.
- M. Jägerstad and J. Jastrebova, J. Agric. Food Chem., 2013, 61, 9758–9768 CrossRef PubMed.
- W. G. P. B. Brown, Adequate food for all: culture, science, and technology of food in the 21st century, CRC press, Boca Raton, 2009 Search PubMed.
- B. C. Blount, M. M. Mack, C. M. Wehr, J. T. MacGregor, R. A. Hiatt, G. Wang, S. N. Wickramasinghe, R. B. Everson and B. N. Ames, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 3290–3295 CrossRef CAS.
- J. Y. Park, S. E. Vollset, A. Melse-Boonstra, V. Chajès, P. M. Ueland and N. Slimani, Mol. Nutr. Food Res., 2013, 57, 562–581 CAS.
- Folates, https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/#h2, accessed October 2017.
- C. McStay, S. Prescott, C. Bower and D. Palmer, Nutrients, 2017, 9, 123 CrossRef PubMed.
- WHO (World Health Organization), Guideline: Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects, WHO, 2015 Search PubMed.
- J. Selhub and I. H. Rosenberg, Biochimie, 2016, 126, 71–78 CrossRef CAS PubMed.
- C. M. Witthöft, Encyclopedia of Dairy Sciences, ed. J. W. Fuquay, P. F. Fox and P. L. H. McSweeney, Elsevier/Academic Press, Amsterdam; Boston, 2nd edn, 2011, Vitamins | Folates, pp. 678–686 Search PubMed.
- J. B. Wallingford, L. A. Niswander, G. M. Shaw and R. H. Finnell, Science, 2013, 339, 1222002 CrossRef PubMed.
- Vitamin & Mineral Deficiency: a Global Progress Report. Part 8 the case of folate, http://https://www.unicef.org/media/files/vmd.pdf, accessed October 2017 Search PubMed.
- M. P. G. De Ocampo, A. Mrg, M. Ca, A. Je and M. Tr, Women Birth, 2017 DOI:10.1016/j.wombi.2017.08.128.
- J. E. Laiño, H. Zelaya, M. Juárez Del Valle, G. Savoy De Giori and J. G. LeBlanc, J. Funct. Foods, 2015, 17, 22–32 CrossRef.
- V. Capozzi, P. Russo, M. T. Dueñas, P. López and G. Spano, Appl. Microbiol. Biotechnol., 2012, 96, 1383–1394 CrossRef CAS PubMed.
- A. Meucci, L. Rossetti, M. Zago, L. Monti, G. Giraffa, D. Carminati and F. Tidona, Food Microbiol., 2018, 69, 116–122 CrossRef CAS PubMed.
- M. Czarnowska-Kujawska, E. Gujska and J. Michalak, J. Food Compos. Anal., 2017, 57, 64–72 CrossRef CAS.
- M. J. I. Shohag, Q. Yang, Y. Wei, J. Zhang, F. Z. Khan, M. Rychlik, Z. He and X. Yang, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2017, 1040, 169–179 CrossRef CAS PubMed.
- E. P. D. P. Azevedo, C. A. de Azevedo Filho, B. S. Santos, L. T. Da Silva, A. E. C. Fai and J. A. Da Paix O, Food Anal. Methods, 2016, 9, 1321–1332 CrossRef.
- E. Martín Tornero, A. Espinosa-Mansilla and I. Durán Merás, Microchem. J., 2017, 133, 333–345 CrossRef.
- M. V. Chandra-Hioe, M. P. Bucknall and J. Arcot, Food Chem., 2017, 234, 365–371 CrossRef CAS PubMed.
- C. Y. Wang, H. W. Huang, C. P. Hsu and B. B. Yang, Crit. Rev. Food Sci. Nutr., 2016, 56, 527–540 CrossRef PubMed.
- N. Naderi, Y. Pouliot, J. D. House and A. Doyen, Innovative Food Sci. Emerging Technol., 2017, 43, 191–200 CrossRef CAS.
- W. Shi, Y. Wang, H. Zhang, Z. Liu and Z. Fei, Food Chem., 2017, 226, 128–134 CrossRef CAS PubMed.
- J. I. Martin, W. O. Landen Jr, A. G. Soliman and R. R. Eitenmiller, J. - Assoc. Off. Anal. Chem., 1990, 73, 805–808 CAS.
- C. Motta, I. Delgado, A. S. Matos, G. B. Gonzales, D. Torres, M. Santos, M. V. Chandra-Hioe, J. Arcot and I. Castanheira, J. Food Compos. Anal., 2017, 64, 181–187 CrossRef CAS.
- J. E. Camara, M. S. Lowenthal and K. W. Phinney, Anal. Bioanal. Chem., 2013, 405, 4561–4568 CrossRef CAS PubMed.
- K. Meisser-Redeuil, S. Bénet, C. Gimenez, E. Campos-Giménez and M. Nelson, J. AOAC Int., 2014, 97, 1121–1126 CrossRef CAS PubMed.
- S. Hussain, S. Khan, S. Gul, M. I. Pividori and M. Del Pilar Taboada Sotomayor, React. Funct. Polym., 2016, 106, 51–56 CrossRef CAS.
- M. V. Chandra-Hioe, M. P. Bucknall and J. Arcot, Anal. Bioanal. Chem., 2011, 401, 1035–1042 CrossRef CAS PubMed.
- J. Szpylka, J. DeVries, A. Cheney and S. House, J. AOAC Int., 2012, 95, 1547–1554 CrossRef CAS PubMed.
- S. Ansari and M. Karimi, Talanta, 2017, 164, 612–625 CrossRef CAS PubMed.
- B. B. Prasad, M. P. Tiwari, R. Madhuri and P. S. Sharma, Anal. Chim. Acta, 2010, 662, 14–22 CrossRef CAS PubMed.
- M. Rezaee, Y. Assadi, M. M. Hosseini, E. Aghaee and F. Ahmadi, J. Chromatogr. A, 2006, 1161, 1–9 CrossRef PubMed.
- H. Yan and H. Wang, J. Chromatogr. A, 2013, 1295, 1–15 CrossRef CAS PubMed.
- Y. Nojavan, M. Kamankesh, F. Shahraz, M. Hashemi and A. Mohammadi, Talanta, 2015, 137, 31–37 CrossRef CAS PubMed.
- R. K. Saini, P. Manoj, N. P. Shetty, K. Srinivasan and P. Giridhar, J. Food Sci. Technol., 2016, 53, 511–520 CrossRef CAS PubMed.
- P. Upadhyaya, K. Tyagi, S. Sarma, V. Tamboli, Y. Sreelakshmi and R. Sharma, Food Chem., 2017, 217, 610–619 CrossRef CAS PubMed.
- S. Vishnumohan, J. Arcot and R. Pickford, Food Chem., 2011, 125, 736–742 CrossRef CAS.
- H. Indyk and D. Dowell, J. AOAC Int., 2012, 95, 298–300 CrossRef CAS PubMed.
- R. Matias, P. R. S. Ribeiro, M. C. Sarraguça and J. A. Lopes, Anal. Methods, 2014, 6, 3065–3071 RSC.
- S. M. Wabaidur, S. M. Alam, S. H. Lee, Z. A. Alothman and G. E. Eldesoky, Spectrochim. Acta, Part A, 2013, 105, 412–417 CrossRef CAS PubMed.
- V. Fajardo, E. Alonso-Aperte and G. Varela-Moreiras, Food Chem., 2015, 169, 283–288 Search PubMed.
- J. Arcot and A. Shrestha, Trends Food Sci. Technol., 2005, 16, 253–266 CrossRef CAS.
- C. M. Pfeiffer, J. P. Hughes, D. A. Lacher, R. L. Bailey, R. J. Berry, M. Zhang, E. A. Yetley, J. I. Rader, C. T. Sempos and C. L. Johnson, J. Nutr., 2012, 142, 886–893 CrossRef CAS PubMed.
- P. Jin, L. Xia, Z. Li, N. Che, D. Zou and X. Hu, J. Pharm. Biomed. Anal., 2012, 70, 151–157 CrossRef CAS PubMed.
- E. Deconinck, S. Crevits, P. Baten, P. Courselle and J. De Beer, J. Pharm. Biomed. Anal., 2011, 54, 995–1000 CrossRef CAS PubMed.
- P. A. Ramos-Parra, R. Urrea-López and R. I. Díaz De La Garza, Food Res. Int., 2013, 54, 177–185 CrossRef CAS.
- F. Wang, M. Cao, N. Wang, N. Muhammad, S. Wu and Y. Zhu, Food Chem., 2018, 239, 62–67 CrossRef CAS PubMed.
- A. Freisleben, P. Schieberle and M. Rychlik, Anal. Bioanal. Chem., 2003, 376, 149–156 CrossRef CAS PubMed.
- K. Tyagi, P. Upadhyaya, S. Sarma, V. Tamboli, Y. Sreelakshmi and R. Sharma, Food Chem., 2015, 179, 76–84 CrossRef CAS PubMed.
- D. Silva, J. V. Visentainer, N. Souza and C. C. Oliveira, Food Anal. Methods, 2013, 6, 1592–1606 CrossRef.
- U. D. Uysal, E. M. Oncu-Kaya and M. T. El, Chromatographia, 2010, 71, 653–658 CAS.
- D. Zhao, M. Lu and Z. Cai, Electrophoresis, 2012, 33, 2424–2432 CrossRef CAS PubMed.
- S. Zhao, H. Yuan, C. Xie and D. Xiao, J. Chromatogr. A, 2006, 1107, 290–293 CrossRef CAS PubMed.
- K. Marakova, J. Piestansky, E. Havranek and P. Mikus, Pharmazie, 2014, 69, 663–668 CAS.
- X. Wang, K. Li, L. Yao, C. Wang and A. Van Schepdael, J. Pharm. Biomed. Anal., 2018, 147, 278–287 CrossRef CAS PubMed.
- P. Liu, D. Liu, Y. Liu and L. Li, J. Solid State Chem., 2016, 241, 164–172 CrossRef CAS.
- B. Hemmateenejad, F. Shakerizadeh-shirazi and F. Samari, Sens. Actuators, B, 2014, 199, 42–46 CrossRef CAS.
- J. R. Zhang, Z. L. Wang, F. Qu, H. Q. Luo and N. B. Li, J. Agric. Food Chem., 2014, 62, 6592–6599 CrossRef CAS PubMed.
- M. Geszke-Moritz, G. Clavier, J. Lulek and R. Schneider, J. Lumin., 2012, 132, 987–991 CrossRef CAS.
- S. Cheraghi, M. A. Taher and H. Karimi-Maleh, J. Food Compos. Anal., 2017, 62, 254–259 CrossRef CAS.
- H. Beitollahi, S. G. Ivari and M. Torkzadeh-Mahani, Mater. Sci. Eng., C, 2016, 69, 128–133 CrossRef CAS PubMed.
- N. Lavanya, E. Fazio, F. Neri, A. Bonavita, S. G. Leonardi, G. Neri and C. Sekar, J. Electroanal. Chem., 2016, 770, 23–32 CrossRef CAS.
- A. Ananthi, S. S. Kumar and K. L. Phani, Electrochim. Acta, 2015, 151, 584–590 CrossRef CAS.
- L. Mirmoghtadaie, A. A. Ensafi, M. Kadivar and P. Norouzi, Mater. Sci. Eng., C, 2013, 33, 1753–1758 CrossRef CAS PubMed.
- X. Li, X. Tan, J. Yan, Q. Hu, J. Wu, H. Zhang and X. Chen, Electrochim. Acta, 2016, 187, 433–441 CrossRef CAS.
- X. Wang, Z. You, Y. Cheng, H. Sha, G. Li, H. Zhu and W. Sun, J. Mol. Liq., 2015, 204, 112–117 CrossRef CAS.
- A. Babakhanian, S. Kaki, M. Ahmadi, H. Ehzari and A. Pashabadi, Biosens. Bioelectron., 2014, 60, 185–190 CrossRef CAS PubMed.
- H. Dai, Y. Li, S. Zhang, L. Gong, X. Li and Y. Lin, Sens. Actuators, B, 2016, 222, 120–126 CrossRef CAS.
- M. P. Kingsley, P. B. Desai and A. K. Srivastava, J. Electroanal. Chem., 2015, 741, 71–79 CrossRef CAS.
- J. B. Raoof, N. Teymoori, M. A. Khalilzadeh and R. Ojani, Mater. Sci. Eng., C, 2015, 47, 77–84 CrossRef CAS PubMed.
- T. Jamali, H. Karimi-Maleh and M. A. Khalilzadeh, LWT--Food Sci. Technol., 2014, 57, 679–685 CrossRef CAS.
- A. Taherkhani, T. Jamali, H. Hadadzadeh, H. Karimi-Maleh, H. Beitollahi, M. Taghavi and F. Karimi, Ionics, 2014, 20, 421–429 CrossRef CAS.
- H. E. Indyk, Int. Dairy J., 2011, 21, 783–789 CrossRef CAS.
- J. E. Young, M. T. Matyska and J. J. Pesek, J. Chromatogr. A, 2011, 1218, 2121–2126 CrossRef CAS PubMed.
- P. W. Johns, J. H. Baxter and M. C. Terp, Food Anal. Methods, 2017, 10, 3255–3263 CrossRef.
- R. Hau Fung Cheung, J. G. Hughes, P. J. Marriott and D. M. Small, Food Chem., 2009, 112, 507–514 CrossRef.
- P. Kanchana and C. Sekar, Spectrochim. Acta, Part A, 2015, 137, 58–65 CrossRef CAS PubMed.
- R. Ahmad, N. Félidj, L. Boubekeurlecaque, S. Lautruong and S. Gamderouich, Chem. Commun., 2015, 51, 9678–9681 RSC.
- N. Karimian, M. H. A. Zavar, M. Chamsaz, A. P. F. Turner and A. Tiwari, Electrochem. Commun., 2013, 36, 92–95 CrossRef CAS.
- B. B. Prasad, R. Madhuri, M. P. Tiwari and P. S. Sharma, Biosens. Bioelectron., 2010, 25, 2140–2148 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |