Hydrogen-bonding-induced colorimetric detection of melamine based on the peroxidase activity of gelatin-coated cerium oxide nanospheres†
Abstract
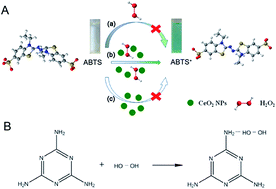
Herein, we developed a simple and rapid colorimetric assay for the detection of melamine using gelatin-coated cerium oxide (Gel-CeO2) nanospheres as peroxidase mimics. Highly monodisperse Gel-CeO2 nanospheres were synthesized through a microwave-assisted hydrothermal process. The Gel-CeO2 nanospheres showed excellent peroxidase activity, which can catalyze the oxidation of ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) by H2O2, resulting in the formation of blue oxidation products. In the presence of melamine, H2O2 will react with melamine through strong hydrogen-bonding. With the consumption of H2O2, the catalytic reaction was interrupted and the blue ABTS oxidation product solution turned pale. There was a linear relationship between the absorbance intensity of the ABTS oxidation product and the logarithm values of melamine concentrations ranging from 50 nM to 5.0 mM. Moreover, the detection limit (S/N = 3) was 5.5 nM, which is far below the regulatory level. This method was simple, rapid, sensitive and reliable, suggesting the promising practical usage of this sensing system. Finally, this method was applied to melamine detection in milk and milk powder.


 Please wait while we load your content...
Please wait while we load your content...