Raman spectroscopy of stored red blood cell concentrate within sealed transfusion blood bags
M. Z.
Vardaki
 a,
C. G.
Atkins
a,
C. G.
Atkins
 ab,
H. G.
Schulze
a,
D. V.
Devine
cd,
K.
Serrano
ab,
H. G.
Schulze
a,
D. V.
Devine
cd,
K.
Serrano
 cd,
M. W.
Blades
*b and
R. F. B.
Turner
cd,
M. W.
Blades
*b and
R. F. B.
Turner
 *abe
*abe
aMichael Smith Laboratories, The University of British Columbia, 2185 East Mall, Vancouver, BC, Canada V6 T 1Z4. E-mail: turner@msl.ubc.ca
bDepartment of Chemistry, The University of British Columbia, 2036 Main Mall, Vancouver, BC, Canada V6 T 1Z1. E-mail: blades@chem.ubc.ca
cDepartment of Pathology and Laboratory Medicine, The University of British Columbia, 2211 Wesbrook Mall, Vancouver, BC, Canada V6 T 2B5
dCentre for Blood Research, The University of British Columbia, 2350 Health Sciences Mall, Vancouver, BC, Canada V6 T 1Z3
eDepartment of Electrical and Computer Engineering, The University of British Columbia, 2332 Main Mall, Vancouver, BC, Canada V6 T 1Z4
First published on 17th October 2018
Abstract
The standard practice in blood banks worldwide involves storage of red blood cells (RBCs) in plastic bags until they are needed for transfusion. During storage, the cells gradually degrade in functionality, a condition described as RBC storage lesion. Standard analytical techniques cannot assess the blood quality without breaching the sterility of the transfusion bag. In this study, we employed a commercially available spatially offset Raman spectroscopy (SORS) system using a custom designed protocol to non-invasively explore the biochemical changes in RBC concentrate of healthy donors over a storage period of approximately 42 days in standard transfusion bags, under standard storage conditions. The results reveal an increase in the oxygenation state of haemoglobin over the storage period for all donors, but different profiles for each donor. This study demonstrates the feasibility of acquiring consistent biochemical information relevant to the quality of stored blood, in situ through sealed blood transfusion bags using a commercially available instrument.
Introduction
Red blood cell (RBC) concentrate (or plasma-reduced blood, or packed red cells) is by far the highest demand blood product. An estimated 17.4 units of red blood cell concentrate were transfused per 1000 people worldwide in 2016.1 The collection and processing of red blood cells (RBCs) for transfusion has become routine and standardized. After being separated from the whole donated blood by centrifugation, red blood cells (RBCs) are resuspended in a defined glucose-containing storage medium and stored in plastic bags at 4 °C, where they remain until transfusion. Regulatory agencies stipulate a maximum storage period for RBC units of 42 days in North America, over which time RBCs are assumed to maintain their viability, however the cells actually degrade continuously starting from the first day of storage.2 This is a result of a multitude of chemical and morphological changes within the bag that lead to accumulation of waste products, loss of membrane integrity, and oxidative stress.3 A number of studies have suggested that older RBCs may be responsible for several post-transfusion illnesses,4–7 hence there is growing demand for an in situ technique which can provide chemical information without breaching the sterility of the blood bag.Currently, there are no techniques suitable for testing the transfusion bag contents during the storage period non-invasively. A number of studies have revealed biochemical and biomechanical cell changes through invasive sampling from blood bags.8,9 Biochemical profiles during storage have also been measured spectroscopically on sampled RBCs using NMR,10 photoacoustic imaging,11 atomic force microscopy12 and Raman spectroscopy.13,14 Multi-pathlength VIS-NIR spectroscopy has also been employed to assess the haemoglobin concentration within sealed transfusion bags.15 However, none of these techniques are readily adaptable to in situ measurements.
Raman spectroscopy (RS) is a technique that uses inelastic light scattering to reveal the chemical composition of materials based on molecular vibrations. Most RS techniques require direct exposure of the material of interest to the incident light and the Raman signal originates mainly from the surface (or a few hundred microns depth depending on the optical properties of the sample). However, a RS modality called “deep Raman spectroscopy” (spatially offset Raman and transmission Raman) allows the collection of Raman scattered photons from deeper within the sample volume by decoupling and appropriately arranging the illumination and collection optical paths.16 This modality also offers the potential to collect Raman spectra from samples through an intervening thin layer of non-sample material, such as a container wall.
RS has been employed in the past to study various aspects of the biochemistry of blood cells and under a variety of conditions, as recently reviewed by Atkins et al.17 Conventional RS on RBC concentrate sampled from transfusion bags has demonstrated a correlation between haemoglobin oxygenation and storage period over 29 different donors.18 A type of spatially offset RS (SORS) has also been employed, albeit limited to a few micrometers offset, to demonstrate the potential to non-invasively measure the changes within transfusion bags during storage time.19 Although this study revealed a correlation between haemoglobin oxygenation and storage time, the interpretation of observed trends was complicated by the low signal-to-noise ratio (SNR).
In the present study, we demonstrate that haemoglobin oxygenation of RBCs contained in sealed transfusion blood bags can be measured consistently from high-SNR spectra using an unmodified commercially available instrument. Data from six healthy donors showed variable but generally increasing haemoglobin oxygenation throughout the storage period in agreement with previous studies. For the first time, we show that it is possible to acquire these data reliably and consistently with one quick measurement and without breaching the bag sterility using a ‘turn-key’ commercially available instrument.
Experimental
Sample collection and handling
Whole blood units (450 ml ± 10%) were donated by 7 healthy male adult volunteers at the Canadian Blood Service (CBS) NetCAD facility in Vancouver, Canada. The RBCs were isolated from the whole blood with top/bottom bag system, suspended in 110 mL of SAGM additive solution (0.9% w/v saline with 1.25 mM adenine, 43.4 mM glucose and 28.8 mM mannitol), subjected to an in-line leukoreduction filtering and put into standard PVC storage/transfusion bags as previously described.20 As the paper labels were exhibiting a strong Raman signal, the contents of each transfusion bag was transferred into identical bags without adhesive backed paper labels (Macopharma, France), by connecting the bags through a sterile docking apparatus (Compodock, Fresenius HemoCare),21 transferring the blood, and then resealing the tubes using a dielectric tubing sealer (Hematron III, Baxter).In total, 7 units of RBC concentrate were used in this study which was carried out in two phases: (a) during the first phase, five freshly-collected/prepared RBC concentrate units were transferred into new label-less sterile bags to measure for a full storage period (42 days) under standard blood bank conditions at 4 °C; (b) at the end of the first phase, two more freshly-collected/prepared units were transferred into label-less bags which had been used in the first experimental phase after being thoroughly rinsed under sterile conditions using an automated cell processor (ACP 215, Haemonetics), commonly used for red blood cell glycerolization.22 The sterile rinsing protocol involved four fill/drain cycles with 234 mL saline, followed by 110 ml SAGM. A constant flow of 70 ml min−1 was maintained for all rinsing solutions. These 2 units of prepared RBC concentrate were then stored under the same conditions as the first 5 units (standard blood banking conditions at 4 °C).
Six of the seven units were used to explore biochemical changes over storage period between different donors. RBCs from donors 209, 235, 294, 455 and 471 were stored in new label-less bags. One additional unit (donor 415) was stored in one of the rinsed and refilled bags; this unit was also used to assess the repeatability of the measurements. The other unit (donor 604) that was stored in a rinsed and refilled bag was used to establish the most efficient measuring mode in terms of acquisition time and signal to noise ratio.
Ethics statement
This study was approved by the Clinical Research Ethics Board of The University of British Columbia (H11-00992, H15-00987) and the Research Ethics Board of the Canadian Blood Services (REB 2015.005, netCAD nc0003). All donors provided informed consent for use of their blood in this research. Sample handling protocols were also approved by the UBC Biosafety Committee (B15-0114).Raman spectroscopic measurements
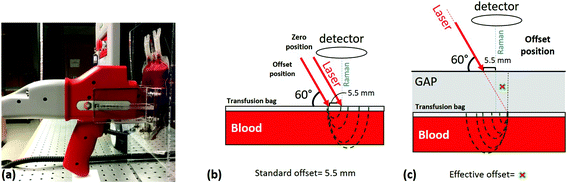
Measurements were carried out using a portable RapID™ Raman system from Cobalt Light Systems, UK (now part of Agilent Technologies, Santa Clara, CA) with 10 cm−1 spectral resolution using a 830 nm excitation laser with 400 mW maximum average laser power and a laser spot size of about 3.5 mm in diameter. Before the start of each measurement, the RBC concentrate inside the transfusion bag was mixed thoroughly and consistently by gently squeezing the bag between both hands and then inverting it seven times. Each measurement required exposing the bag to room temperature for less than 5 minutes. To achieve consistency throughout the measurements, we designed a simple apparatus for repeatedly positioning the bag in relation to the probe as shown in Fig. 1a; this apparatus also served to maintain a flat and consistently oriented transfusion bag surface such that the excitation laser and collection optical paths are consistently aligned with respect to the bag surface in order to minimize optical effects. The measurement probe itself rests with the handle on a plastic support with the nose positioned in a plastic fixture (Fig. 1a) attached to the bag cradle to eliminate any movement effects during the measurements.The RapID instrument is designed for SORS applications where highly concentrated samples (e.g. dry powders, concentrated liquids) are probed behind thin barriers of infrared-penetrable materials (e.g. polymer, glass, paper). The laser optical path impinges on the surface of the barrier at a fixed angle (60°) and the collection optical path is normal to the barrier surface as shown in Fig. 1b. The instrument makes a measurement with zero offset, primarily to characterize the barrier material, followed (automatically) by an offset measurement where the laser path is translated 5.5 mm to collect scattering from the barrier material plus contents; the instrument outputs either individual spectra or a difference spectrum. The “built-in” offset is fixed (i.e. not user adjustable) and the value is too large to be efficient for low-concentration analytes or weak Raman scatterers, such as living cells in systems such as blood bags. We therefore needed to devise a measurement protocol that enables RBC spectra to be measured with high SNR. The main parameter that needed to be optimized was the “effective offset”, given that the built-in offset is fixed. In order to accomplish this, we exploited the fixed-angle laser optical path, by introducing a defined space between the nose of the instrument and the surface of the sample bag (Fig. 1c).
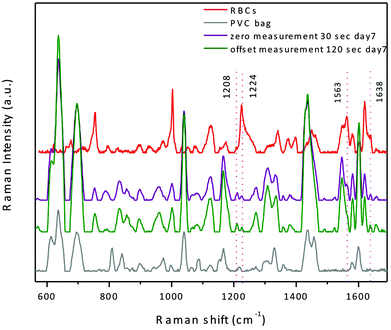
The instrument essentially operates in two modes: zero offset (as standard Raman) and non-zero offset (SORS). We characterized both of these modes using units of RBC concentrate in terms of signal to noise ratio and acquisition time. The acquisition times and laser power settings used were 30 seconds at 240 mW for the zero offset and 120 seconds at 400 mW for SORS. At the 400 mW power setting, the laser power density incident on the surface of the bag was about 4.2 W cm−2, which is well below reported damage thresholds;23 the actual power density incident on cells would be less. In order to establish the optimum effective offset, based on the optical properties of the sample, a series of measurements with decreasing offset were conducted by increasing the gap between the probe nose and the sample surface. It was determined that the optimum offset is at 6.2 mm, when the gap corresponds to 2 cm. Spectra obtained using this protocol are shown in Fig. 2 along with RBC spectra obtained from a smear of dried RBC concentrate using a conventional inVia Raman microscope at 785 nm excitation (Renishaw, Gloucestershire, UK) with no overlying barrier material. The RBC spectrum is provided for qualitative comparison only, as the spectrum obtained this way is not easily comparable to our in situ measurements using the RapID.
From Fig. 2, it is clear that spectra from both zero and optimum effective offset positions include significant contributions from both the plastic bag and the RBC contents. A custom difference spectroscopy approach could be developed to minimize the bag contribution, however we have not yet pursued this as there are several regions of interest in the spectrum where the bag and RBC contributions do not overlap, including regions that exhibit Raman markers for haemoglobin oxygenation. Thus, for measurements targeting these regions, a zero position measurement is preferable as the signal to noise ratios between the two modes are similar (>100), but the zero offset measurement requires four times less acquisition time, 40% less laser power, and does not require a spacer.
Data analysis
All data were exported through SpectraViewer version 1.3.1.1 (application software not included with the RapID, available separately from Agilent) and loaded into Matlab version R2017b (MathWorks, Natick, MA). The spectra were then baseline corrected using 17 iterations of a custom algorithm described previously.24Results and discussion
Haemoglobin oxygenation
The predominant features of blood Raman spectra are the bands associated with haemoglobin. Its vibrational spectrum in single living red blood cells has been well characterized by RS25,26 and IR absorption spectroscopy27,28 in both oxygenated (oxyHb) and deoxygenated (deoxyHb) form, where iron exists in the low-spin ferric (Fe3+) and ferrous (Fe2+) state, respectively. Specific vibrational modes of the haeme moiety have been identified as spectral markers for the Fe3+ and Fe2+ states. Appropriately normalized intensities of these bands in RBCs have been quantitatively correlated to haemoglobin oxygenation status.18,19,29–33 Significantly, this in turn has been correlated with RBC morphological changes potentially providing a link to the progression of storage lesion.18In this study, we have focused mainly on the 1208 cm−1 and 1224 cm−1 bands attributed to δ(CmH) modes of the porphyrin system;31 the ratio of intensities of these bands (1208 cm−1/1224 cm−1) yields the concentration ratio of deoxyHb to oxyHb. Our choice of these two Hb bands was simply to aid comparison with a wealth of other data involving these bands that we had compiled previously from different donors and using different instruments. This ratio, has been measured over blood storage periods ranging from day 0 through day 38–48 (depending on the donor) and the results are plotted in Fig. 3. Although the initial oxygenation status of the donors varies, which is normal, there is a general trend of increasing haemoglobin oxygenation (i.e. a consistent decrease in the deoxyHb/oxyHb ratio) that eventually reaches a saturation level. These data are generally in agreement with our previous studies,19 however we had also previously observed some anomalous trends in this ratio where the 1208 cm−1/1224 cm−1 ratio for a few donors' blood increased over the storage period. Whether or not our current technique would also reveal some anomalous (increasing) trends in an expanded pool of donors is uncertain, but further data collection is on-going. The disagreement may simply be due to the lesser quality of our previous data.
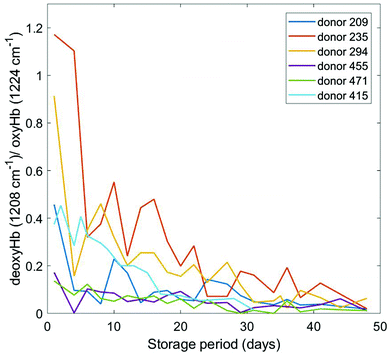 | ||
| Fig. 3 Plot of the 1208 cm−1/1224 cm−1 deoxyHb/oxyHb spectral ratio as a function of storage time for six different donors. | ||
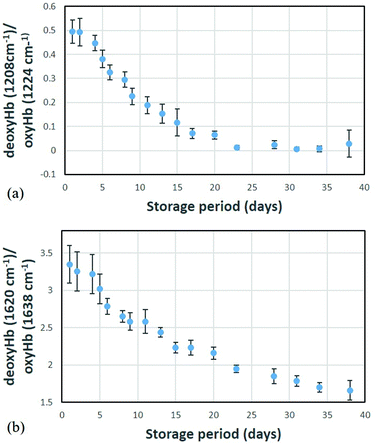
Different haemoglobin bands that have been employed by others29,34,35 for assessing haemoglobin oxygenation include the 1563 cm−1 (v(CβCβ) stretch) and 1638 cm−1 (v(CαCm) asymmetric stretch) bands. These bands are due to low spin Fe and planar porphyrin ring vibrations, respectively and have been shown to increase with storage period. We examined the behaviour of these bands in our recent data (Fig. 4 and 5) where the spectral bands were normalized to a pyrrole breathing mode (754 cm−1) which remains stable throughout the storage period. The trends for these bands are consistent with the trends for the composite deoxyHb/oxyHb ratio presented in Fig. 3, as well as the findings of others.14,36 Interestingly, there is a more distinct separation between the donors with high vs. low initial oxyHb using both of these bands (Fig. 4 and 5) compared to the 1208 cm−1/1224 cm−1 bands (Fig. 3). There also is not as pronounced a “converging” trend toward saturation, particularly in the 1563 cm−1 band.
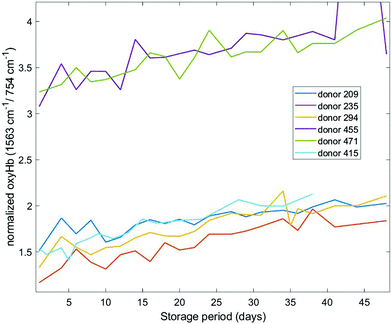 | ||
| Fig. 4 Normalized oxygenation stretch band (1563 cm−1) as a function of storage time for six different donors. | ||
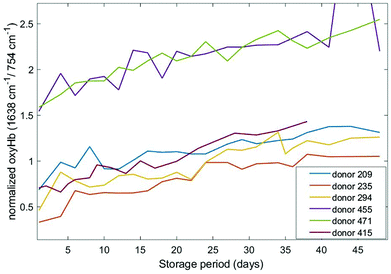 | ||
| Fig. 5 Normalized oxygenation asymmetric stretch band (1638 cm−1) as a function of storage time for six different donors. | ||
The 1563 cm−1 and 1638 cm−1 bands are up-shifted from positions that are characteristic of the oxygenated state; the corresponding peak positions in the deoxygenated states are at 1548 cm−1 and 1620 cm−1, respectively. If deoxyHb/oxyHb ratios are computed for these bands, analogous to the 1208 cm−1/1224 cm−1 ratio, the resulting profiles are shown in Fig. 6.
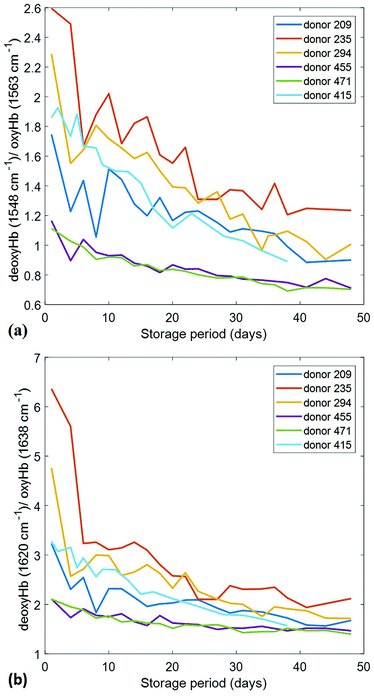 | ||
| Fig. 6 Alternate deoxyHb/oxyHb spectral ratios as a function of storage time for six different donors. (a) Plot of the 1548 cm−1/1563 cm−1 ratio; (b) plot of the 1620 cm−1/1638 cm−1 ratio. | ||
These profiles are qualitatively nearly identical to those shown in Fig. 3, exhibiting exactly the same day by day behaviours. Thus, the relative amplitudes of all of these bands precisely track one another, as would be expected if they are indeed metrics for the same analyte variations. The 1620 cm−1/1638 cm−1 ratio evidently offers the largest dynamic range and is thus likely a better (more sensitive) metric than the 1208 cm−1/1224 cm−1. It may also be the most precise metric as the component peaks are stronger in relative intensity.
All of the foregoing results are consistent with the expectation that haemoglobin oxygenation should increase during its lifetime in storage, partially due to the semi-gas-permeable nature of the PVC transfusion bag and the metabolic pathways that are disrupted.37 We should also expect to observe that RBC concentrate from different donors exhibits different initial oxygenation states. This can be attributed to a number of factors including the donor haematocrit (35–55%), donor physiology, and variations in the processing of whole blood into its components. For example, different methods of processing and equipment can effect oxygenation and contribute to early onset of storage lesion.38,39 Even if this is minimized, the elapsed time between collection of different donor's whole blood and processing to final blood products, as well as extent of manipulations during transport, can vary considerably and expose the red cells to a range of dissolved oxygen tensions for different times contributing to early differences in haemolysis. Our measurements may be particularly affected by variations in haemolysis as the haemoglobin content measured by our approach includes contributions from both extracellular and intracellular haemoglobin, as the sample is probed with a laser spot size of 3.5 mm. The extracellular fraction of haemoglobin could be more highly oxygenated,14 perhaps partly due to more facile mass-transport allowing higher accumulations near the gas-permeable bag interface.
The saturation threshold corresponds to the level where the maximum number of oxygen molecules have been bound to haemoglobin. It has been confirmed by separate studies that around 99% of the haemoglobin in RBCs is oxygen-saturated by the end of the storage period.40 However, several factors can contribute to variations in the final equilibrium fraction of oxyHb including pH changes (e.g. due to lactic acid accumulation) in the unbuffered SAGM additive solution, loss of 2,3-DPG by aging cells, and variations in the intracellular versus extracellular pools of haemoglobin.
Measurement reproducibility
In order to assess the measurement reproducibility and stability, one of the units was measured seven times in succession on each day of a 38 day storage period; the results are shown in Fig. 7 for two of the ratios considered above, 1208 cm−1/1224 cm−1 (Fig. 7a) and 1620 cm−1/1638 cm−1 (Fig. 7b). We attempted to maintain consistent mixing and handling protocols, as described in the Experimental section, however the exact probing position and sample position within the measurement fixture could not be identical for every measurement, accounting for some of the variance. A larger contribution to the variance is likely due to the fact that the volume of blood that fills the probe volume was different for each subsequent measurement. The variance exhibited in the relatively higher intensity 1620 cm−1 and 1638 cm−1 peaks supports the sample variance being the larger component. The data plotted in Fig. 3–5 were acquired from a single measurement, but if greater precision is desired, it would not be a problem to routinely make several repeat measurements and average them as each measurement takes less than a minute for the zero offset (2 minutes for offset measurements).Correlation with morphological changes
Although, the oxygenation status has not been directly linked to storage lesion, previous work in our group18 has examined the correlation between oxygenation and morphological index (MI), a standard metric used in transfusion medicine to gain qualitative insight into RBC quality and progression of storage lesion.41 The reader is referred to the original paper18 or thesis36 for the details of the methods. Briefly, a morphological “grade” (the MI) was assigned by sampling and visual assessment of a sample by a skilled technician, that MI grade was then compared to the haemoglobin oxygenation as determined by the 1208 cm−1/1224 cm−1 ratio on the same samples. The previously published,18 data are re-plotted in Fig. 8 to emphasize the cross-correlation between the MI and spectroscopically determined oxygenation state.36 The variance in both measures was substantial, but the cross-correlation appears to suggest an indirect link between oxygenation status and oxidative stress due to unbound molecular oxygen, which is one of the contributors to morphological changes in the RBCs and one of the many symptoms of storage lesion.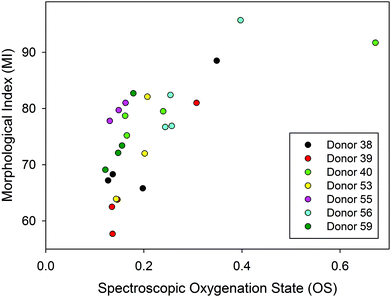 | ||
| Fig. 8 A cross-correlation plot of the morphological index (MI) and the oxygenation state (OS) on liquid RBC samples for seven donors.36 The spectroscopic oxygenation state is defined here as the ratio of intensities of 2 deoxy/oxy Hb bands (1208 cm−1/1224 cm−1). The morphological index ranges from 100 (normal, smooth discoid) to 0 (highly deformed, small, smooth spheroid). | ||
We have not conducted a separate assessment of MI for the donors evaluated in this study, but the relevance here is that the correlation hints of a potential broader utility of oxygenation measurements. The better precision and more direct (i.e. in situ vs. samples) measurements that can now be undertaken may allow an improved estimate of this correlation in future. Perhaps other symptoms of storage lesion could also be correlated with oxygenation status. If oxygenation could be correlated with actual patient outcomes, such measurements may be useful as a measure of the suitability of a unit for transfusion, especially for some patients.
Conclusions
Donated red blood cells are an extremely precious resource of our modern healthcare system. The demand is high, supplies are limited, and managing inventories by blood banks and hospitals is challenging, particularly because stored RBC concentrate is known to degrade over time, even under carefully controlled storage conditions, and blood from different donors ages differently. It would therefore be helpful to be able to gauge the condition of any particular unit prior to distribution or transfusion. However, other than visual inspection for severe haemolysis, there is currently no way to assess RBC quality in transfusion bags without breaching the sterility. In this report, we demonstrated how to employ a commercially available Raman spectroscopy instrument platform to non-invasively assess haemoglobin oxygenation status in situ within sealed transfusion bags. We presented data obtained from six units of RBC concentrate that have undergone the same processing as blood for transfusion and were stored between measurements under standard blood bank conditions. The data obtained by our procedure is qualitatively comparable to previously published data, but higher precision and more rapidly obtainable.Serious concerns have been raised in recent years over the possible effects that the storage age of a particular RBC unit may have on transfusion efficacy. Some reports suggest that many post-transfusion illnesses, especially in critically ill patients, are due to “old” blood being used instead of fresh blood.4–7,37,40 Thus, many studies have been undertaken that aimed to clarify the connection, but the results have been inconclusive or even contradictory.42,43 A problem with even properly randomized and controlled studies of large numbers of patients is that the only independent variable that can really be correlated with the patient outcomes is the number of storage days before transfusion. However, it is clear that the chemical composition of units from different donors changes at different rates, such that the age of the unit does not provide useful information about the “quality” of the unit. What is needed is an objective assessment of the composition of the unit based on a measurement that does not compromise the usability of the unit for transfusion. We have shown how one measure, haemoglobin oxygenation, could be employed today in clinical studies of transfusion efficacy. With further research, other spectral markers for the progression of storage lesion will be revealed through studies that correlate spectral measurements with biochemical tests and we are pursuing this. Such markers may include 2,3-DPG which affects haemoglobin's affinity for oxygen, or lactate, a product of RBC metabolism.
It is understood that certain regulatory approvals will first be required, but it may soon be feasible to deploy instruments such as demonstrated here to blood banks or hospital sites where measurements can be made by minimally trained personnel. This should facilitate further clinical research into the effects of storage age on transfusion efficacy. Ultimately, it could open up the prospect of blood banks making routine—perhaps even automated—measurements of units in their inventories to establish a profile of certain spectral markers for each bag that could be used to help manage those inventories in a more effective manner than the “first-in, first-out” approach used today.
Conflicts of interest
The authors report no conflict of interest.Acknowledgements
We thank the donors who provided their blood for research purposes at the Canadian Blood Service (CBS) netCAD facility in Vancouver, Canada. We also thank the technicians in the Mechanical Engineering Workshop (Chemistry, UBC) for designing the apparatus. Funding was provided by the Natural Sciences and Engineering Research Council (NSERC) of Canada.References
- Global status report on blood safety and availability 2016, World Health Organization, Geneva, 2017 Search PubMed, Licence: CC BY-NC-SA 3.0 IGO.
- M. Garcia-Roa, M. Del Carmen Vicente-Ayuso, A. M. Bobes, A. C. Pedraza, A. Gonzalez-Fernandez, M. P. Martin, I. Saez, J. Seghatchian and L. Gutierrez, Blood Transfus., 2017, 15, 222–231 Search PubMed.
- A. D'Alessandro, F. Gevi and L. Zolla, Mol. BioSyst., 2013, 9, 1196–1209 RSC.
- L. van de Watering, J. Lorinser, M. Versteegh, R. Westendord and A. Brand, Transfusion, 2006, 46, 1712–1718 CrossRef PubMed.
- A. Tinmouth, D. Fergusson, I. C. Yee, P. C. Hebert, A. Investigators and G. Canadian Critical Care Trials, Transfusion, 2006, 46, 2014–2027 CrossRef PubMed.
- A. B. Zimrin and J. R. Hess, Vox Sang., 2009, 96, 93–103 CrossRef CAS PubMed.
- G. M. Liumbruno and J. P. Aubuchon, Blood Transfus., 2010, 8, 217–219 Search PubMed.
- R. Obrador, S. Musulin and B. Hansen, J. Vet. Emerg. Crit. Care, 2015, 25, 187–199 CrossRef PubMed.
- D. J. Kor, C. M. Van Buskirk and O. Gajic, Bosnian J. Basic Med. Sci., 2009, 9(Suppl 1), 21–27 CrossRef PubMed.
- T. A. Pertinhez, E. Casali, L. Lindner, A. Spisni, R. Baricchi and P. Berni, Blood Transfus., 2014, 12, 548–556 Search PubMed.
- E. Hysi, E. M. Strohm, E. S. Berndl, M. C. Kolios and J. P. Acker, IEEE International Ultrasonics Symposium Proceedings, 2014, pp. 1662–1665 Search PubMed.
- E. Kozlova, A. Chernysh, V. Moroz, V. Sergunova, O. Gudkova and E. Manchenko, Sci. Rep., 2017, 7, 7846 CrossRef CAS PubMed.
- C. G. Atkins, K. Buckley, D. Chen, H. G. Schulze, D. V. Devine, M. W. Blades and R. F. Turner, Conference on Clinical and Biomedical Spectroscopy and Imaging IV held at the European Conferences on Biomedical Optics, 2015, vol. 9537, p. 95370X Search PubMed.
- R. Gautam, J.-Y. Oh, M. B. Marques, R. A. Dluhy and R. P. Patel, Lab. Med., 2018, 49(11), 298–310 CrossRef PubMed.
- S. Zhang, G. Li, J. Wang, D. Wang, Y. Han, H. Cao and L. Lin, Sci. Rep., 2018, 8, 2204 CrossRef PubMed.
- P. Matousek, I. Clark, E. Draper, M. Morris, A. Goodship, N. Everall, M. Towrie, W. Finney and A. Parker, Appl. Spectrosc., 2005, 59, 393–400 CrossRef CAS PubMed.
- C. G. Atkins, K. Buckley, M. W. Blades and R. F. B. Turner, Appl. Spectrosc., 2017, 71, 767–793 CrossRef CAS PubMed.
- C. G. Atkins, H. G. Schulze, D. Chen, D. V. Devine, M. W. Blades and R. F. B. Turner, Analyst, 2017, 142, 2199–2210 RSC.
- K. Buckley, C. G. Atkins, D. Chen, H. G. Schulze, D. V. Devine, M. W. Blades and R. F. Turner, Analyst, 2016, 141, 1678–1685 RSC.
- D. Devine and D. Howe, ISBT Sci. Ser., 2010, 5, 78–82 CrossRef.
- P. Van der Meer, F. Biekart, R. Pietersz, S. Rebers and H. Reesink, Transfusion, 2000, 40, 682–686 CrossRef CAS PubMed.
- A. Hansen, Q. L. Yi and J. P. Acker, Transfusion, 2013, 53, 1772–1779 CrossRef CAS PubMed.
- P. Lemler, W. R. Premasiri, A. DelMonaco and L. D. Ziegler, Anal. Bioanal. Chem., 2014, 406, 193–200 CrossRef CAS PubMed.
- H. G. Schulze, R. B. Foist, K. Okuda, A. Ivanov and R. F. Turner, Appl. Spectrosc., 2012, 66, 757–764 CrossRef CAS PubMed.
- B. R. Wood, P. Caspers, G. J. Puppels, S. Pandiancherri and D. McNaughton, Anal. Bioanal. Chem., 2007, 387, 1691–1703 CrossRef CAS PubMed.
- B. R. Wood and D. McNaughton, J. Raman Spectrosc., 2002, 33, 517–523 CrossRef CAS.
- B. L. Horecker, J. Biol. Chem., 1943, 148, 173–183 CAS.
- M. Polakovs, N. Mironova-Ulmane, N. Kurjane, E. Reinholds and M. Grube, Proceedings of the Sixth International Conference on Advanced Optical Materials and Devices (AOMD-6), International Society for Optics and Photonics, 2008, vol. 7142, p. 714214 Search PubMed.
- B. R. Wood, B. Tait and D. McNaughton, Biochim. Biophys. Acta, 2001, 1539, 58–70 CrossRef CAS.
- H. Brunner, A. Mayer and H. Sussner, J. Mol. Biol., 1972, 70, 153–156 CrossRef CAS PubMed.
- T. Yammoto and G. Palmer, J. Biol. Chem., 1973, 248, 5211–5213 CAS.
- I. P. Torres Filho, J. Terner, R. N. Pittman, E. Proffitt and K. R. Ward, J. Appl. Physiol., 2008, 104, 1809–1817 CrossRef CAS PubMed.
- K. R. Ward, R. W. Barbee, P. S. Reynolds, I. P. Filho, M. H. Tiba, L. Torres, R. N. Pittman and J. Terner, Anal. Chem., 2007, 79, 1514–1518 CrossRef CAS PubMed.
- A. Wesełucha-Birczyńska, M. Kozicki, J. Czepiel, M. Łabanowska, P. Nowak, G. Kowalczyk, M. Kurdziel, M. Birczyńska, G. Biesiada and T. Mach, J. Mol. Struct., 2014, 1069, 305–312 CrossRef.
- M. Asghari-Khiavi, A. Mechler, K. R. Bambery, D. McNaughton and B. R. Wood, J. Raman Spectrosc., 2009, 40, 1668–1674 CrossRef CAS.
- C. G. Atkins, Raman Spectroscopy of Transfusable Red Blood Cells, Doctoral Thesis, University of British Columbia, 2016 Search PubMed.
- J. D. Reynolds, D. T. Hess and J. S. Stamler, Transfusion, 2011, 51, 852–858 CrossRef CAS PubMed.
- A. L. Hansen, J. D. Kurach, T. R. Turner, C. Jenkins, M. P. Busch, P. J. Norris, J. Dugger, P. A. Tomasulo, D. V. Devine and J. P. Acker, Vox Sang., 2015, 108, 350–358 CrossRef CAS PubMed.
- S. Bakkour, J. P. Acker, D. M. Chafets, H. C. Inglis, P. J. Norris, T. H. Lee and M. P. Busch, Vox Sang., 2016, 111, 22–32 CrossRef CAS PubMed.
- E. Bennett-Guerrero, T. H. Veldman, A. Doctor, M. J. Telen, T. L. Ortel, T. S. Reid, M. A. Mulherin, H. Zhu, R. D. Buck, R. M. Califf and T. J. McMahon, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 17063–17068 CrossRef CAS PubMed.
- R. T. Usry, G. L. Moore and F. W. Manalo, Vox Sang., 1975, 28, 176–183 CrossRef CAS PubMed.
- A. C. Zubair, Am. J. Hematol., 2010, 85, 117–122 Search PubMed.
- W. A. Flegel, C. Natanson and H. G. Klein, Br. J. Haematol., 2014, 165, 3–16 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |