Emerging biosensor platforms for the assessment of water-borne pathogens
Abstract
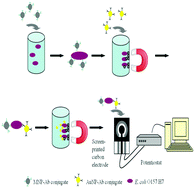
Pathogens are key contaminants in water that are responsible for the generation of various water-borne diseases, and include viruses, fungi, bacteria, and protozoan parasites. The pathogenic effects of these species in water depend on their shape, size, composition, and structure. The resulting water-borne diseases are a serious threat to the environment, including to humans and animals, and are directly responsible for environmental deterioration and pollution. The potential presence of these pathogens requires sensitive, powerful, efficient, and ideally real-time monitoring methods for their reproducible quantification. Conventional methods for pathogen detection mainly rely on time-consuming enrichment steps followed by biochemical identification strategies, which require assay times ranging from 24 h to up to a week. However, in recent years, significant efforts have been made towards the development of biosensing technologies enabling rapid and close-to-real-time detection of water-borne pathogens. This review summarizes recent developments in biosensors and sensing systems based on a variety of transducer technologies for water-quality monitoring, with specific focus on rapid pathogen detection.
- This article is part of the themed collections: Recent Review Articles and Analyst 2018 Most Downloaded Articles


 Please wait while we load your content...
Please wait while we load your content...