Involvement of histone hypoacetylation in INH-induced rat liver injury
Ling-yan
Zhu
,
Qi
Ren
,
Yu-hong
Li
,
Yi-yang
Zhang
,
Jin-feng
Li
,
Ying-shu
Li
,
Zhe
Shi
and
Fu-min
Feng
 *
*
Hebei Province Key Laboratory of Occupational Health and Safety for Coal Industry, School of Public Health, North China University of Science and Technology, Tangshan 063210, China. E-mail: fm_feng@sina.com; Fax: +86 8805562; Tel: +86 8805562
First published on 4th October 2017
Abstract
This study explores the mechanism of histone acetylation under the effect of oxidative stress in rat liver injury induced by isoniazid (INH). Fifty-six adult SD rats were selected and divided randomly into INH groups (48) and control (8). Rats in INH groups were intragastrically injected with 55 mg kg−1 day−1 for 3, 7, 10, 14, 21, and 28 days, and control rats were given an equal volume of distilled water. Pathological changes in liver tissues were observed by HE staining. Western blot analysis was conducted to measure the expression levels of H3k14ac and H4k8ac. The activities of HAT, HDAC and IL-1β, and TNF-α were detected by ELISA in liver tissues. Real-time RT-PCR analysis was performed to determine the protein expression levels of HAT, HDAC, and IL-1β and the mRNA expression of TNF-α. The levels of superoxide dismutase (SOD) and malondialdehyde (MDA) were assayed by biochemical methods in liver tissues. At different time points, the SOD activity decreased, whereas the MDA content significantly increased after 14 days (FSOD = 11.15, FMDA = 7.42, P < 0.01). During this period, the expression of histone acetylated H3K14 and H4K8 acetylation decreased compared with the control group (FH3K14 = 4.18, FH4K8 = 3.87, P < 0.05); by contrast, HDAC1 and HDAC2 showed a high expression level compared with those in the control group (FHDAC1 = 29.13, FHDAC2 = 58.34, P < 0.01). Moreover, the expression of CBP/P300 was lower than that in the control group (FCBP/P300 = 12.18, P = 0.001), and the protein contents of IL-1β and TNF-α in rat liver tissues were up-regulated (FIL-1β = 44.88, FTNF-α = 41.56, P < 0.01). These results suggest that histone acetylation is involved in INH-induced rat liver injury. Furthermore, the hypoacetylation of histones H3K14 and H4K8 is negatively correlated with oxidative stress-mediated rat liver injury.
1 Introduction
Isoniazid (INH) is a widely used and effective first-line agent for treatment of tuberculosis; the most important clinical adverse reaction of INH is anti-tuberculosis drug-induced liver injury (ADLI).1 In the process of liver injury, INH metabolizes acetyl hydrazine and hydrazine, which are directly involved in oxidative stress in liver cells and may be an important factor in ADLI.2,3 However, oxidative stress caused by excessive reactive oxygen species can aggravate the secretion of inflammatory factors and enhance liver injury.4 Endogenous and exogenous oxidants in the body could activate or inhibit the post-translational modification of proteins in the cell, leading to chromatin remodeling of histones and abnormal expression of some inflammatory and antioxidant genes.5Histone, one of the main components of chromatin, contains an amino terminal that can be covalently modified and can change the chromatin configuration, leading to transcriptional activation or gene silencing.6 Acetylation is an important modification of histone tail chromatin remodeling. The lysine residues of four core histones (H2A, H2B, H3, and H4) can be acetylated,7 and histones H3 and H4 are more easily modified. Histone acetylation is a reversible dynamic process regulated by histone acetyltransferase (HAT) and histone deacetylase (HDAC). HAT and HDAC can regulate chromatin remodeling by enhancing or inhibiting histone acetylation, thereby controlling the expression of genes to influence cell activity.8 Both enzymes also regulate cell growth, oxidative stress, and inflammatory gene expression.9 Therefore, the effect of oxidative stress on INH-induced hepatic injury is important in research on acetylation of aberrantly expressed genes.
This study aims to explore the relationship between histone acetylation and INH-induced liver injury in rats. INH-induced oxidative stress during liver injury in rats is discussed in relation to the histone H3 and H4 acetylation status and the interaction of oxidative stress factors, inflammatory factors, and HAT/HDAC.
2 Materials and methods
2.1 Animal grouping and treatment
Nine-week-old Sprague-Dawley rats weighing 258–365 g (28 males and 28 females) were purchased from Beijing Hua Fukang Biotechnology Co. Ltd (Animal Permit no. SCXK (jing) 2009-0004). The rats were fed in a barrier laboratory from the Experimental Animal Center of North China University of Science and Technology with the rearing environment license number of SYXK (ji) 2010-0038. The rats were maintained in a controlled environment at 20 °C to 25 °C and 50% ± 5% relative humidity under a 12 h dark/light cycle and acclimatized for 1 week before use. All experimental procedures involving animals were approved by the Animal Care and Use Committee of North China University of Science and Technology (approval no. 2014-006).After 1 week, the rats were divided into experimental (n = 48) and control (n = 8) groups by using simple randomization. Rats in the experimental group were treated with INH (55 mg kg−1 day−1 body weight, dissolved in distilled water) by intragastric administration to induce acute hepatic injury. The rats were sacrificed at different time points (3, 7, 10, 14, 21, and 28 days) after INH administration (n = 8 for each time point).
2.2 Reagents
INH was obtained from Shenyang Hongqi Pharmaceutical Co. Ltd (Production batch number: 1404022). Rabbit monoclonal anti-histone H3 (acetyl K14) antibody and total histone H3, H4 antibody were purchased from Abcam (U.S.). Goat anti-rabbit immunoglobulinG (IgG) horseradish peroxidase and enhanced chemiluminescence were acquired from Xi'an Jingcai Biotechnology Co. Ltd. An enzyme-linked immunosorbent assay (ELISA) kit for detecting rat CBP/P300, HDAC1, HDAC2, IL-1β, and TNF-α was purchased from Beijing Dong Ge Bo Ye Biotechnology Co. Ltd. SOD and MPO detection kits were purchased from Nanjing Jiancheng Bioengineering Institute. A TRI pure Reagent Total RNA extraction kit was obtained from Beijing Baitaike Biotechnology Co. Ltd. A platinum SYBR Green quantitative polymerase chain reaction (qPCR) kit and a M-MLV kit were supplied by Invitrogen (USA). The specific primers for CREBBP, P300, HDAC1, HDAC2, IL-1β, and TNF-α were acquired from Invitrogen (USA).2.3 Histopathology
After the rats were sacrificed, rat liver tissues were collected, that is, the cross-section of liver tissues over the hepatic portal. The tissues were fixed in 4% paraformaldehyde and subsequently embedded in paraffin. The sections were stained with hematoxylin and eosin by using a standard protocol and analyzed under light microscopy.2.4 Measurement of SOD activity and the MDA content in liver
Liver tissues were prepared to make 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 homogenates, and SOD activity and the MDA content in hepatic homogenates were measured using assay kits according to their instructions.
10 homogenates, and SOD activity and the MDA content in hepatic homogenates were measured using assay kits according to their instructions.
2.5 Determination of global histone H3K14 and H4K8 acetylation in the liver
Specific proteins were analyzed by immunoblotting with the appropriate rabbit antibodies recognizing histone H3k14ac and histone H4k8ac. Proteins were separated along with specific protein standards and molecular weight markers in 15% polyacrylamide gels and transferred onto 0.22 μm polyvinylidine fluoride membranes. After blocking with 5% non-fat dried milk for 1.5–2 h, the membrane was incubated with the primary antibody diluted at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 for anti-H3k14ac, 1
2000 for anti-H3k14ac, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for anti-H4k8ac, 1
000 for anti-H4k8ac, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000 for total H3 antibody, or 1
3000 for total H3 antibody, or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 for total H4 antibody at 4 °C overnight. The membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody diluted at 1
1000 for total H4 antibody at 4 °C overnight. The membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody diluted at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 at room temperature for 2 h. Antibody binding was visualized using an ECL chemiluminescence system. Changes in H3k14ac and H4k8ac histone levels were analyzed compared with total histone H3 and histone H4 levels.
5000 at room temperature for 2 h. Antibody binding was visualized using an ECL chemiluminescence system. Changes in H3k14ac and H4k8ac histone levels were analyzed compared with total histone H3 and histone H4 levels.
2.6 CBP/P300, HDAC1, HDAC2, IL-1β, and TNF-α protein assay and mRNA expression in the liver
Liver tissues were prepared to make 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 homogenates. The contents of CBP/P300, HDAC1, HDAC2, IL-1β, and TNF-α in the hepatic homogenate were measured using assay kits according to the instructions (supplied by Beijing Dong Ge Bo Ye Biotechnology Co. Ltd).
5 homogenates. The contents of CBP/P300, HDAC1, HDAC2, IL-1β, and TNF-α in the hepatic homogenate were measured using assay kits according to the instructions (supplied by Beijing Dong Ge Bo Ye Biotechnology Co. Ltd).
RNA was isolated from rat liver tissues by using the RNeasy mini-kit following the manufacturer's instructions. RNA integrity was electrophoretically verified by ethidium bromide staining and by an OD260/OD280 nm absorption ratio at 1.8–2.0. Briefly, 2 μg of RNA of each sample was reverse-transcribed to cDNA in 20 μL reactions by using a M-MLV first-strand synthesis kit.
Quantitative real-time PCR was performed in a 20 μL final volume. cDNA was used for PCR amplification under the following conditions: preheating at 94 °C for 5 min, denaturation at 94 °C for 30 s, annealing at 60 °C (CREBBP, P300, HDAC1, HDAC2, IL-1β, and TNF-α) for 30 s, and extension at 72 °C for 30 s. The reaction was repeated for 40 cycles, followed by incubation at 72 °C for 5 min. β-Actin, a reference gene, was used to normalize each sample and gene. Relative mRNA expression in each sample was calculated as the ratio of target gene concentration to β-actin concentration. Primers used are listed in Table 1.
| Gene | Primer | Sequences(5′–3′) |
|---|---|---|
| CREBBP | Forward | CCTTGCACAGAGAGTGAGGG |
| Reverse | CAGGAACCACCTCAAGTCCC | |
| P300 | Forward | GAGGTCACTGTTCGGGTTGTTC |
| Reverse | TGGTTCGATATGGAAAAGATTCTG | |
| HDAC1 | Forward | TCTGACAAACGCATTGCCTG |
| Reverse | AGGGACTTGGAGAGAAGATGGA | |
| HDAC2 | Forward | GCTGCTTCAACCTAACTG |
| Reverse | CTCATACGTCCAACATCG | |
| IL-1β | Forward | AGGCTTCCTTGTGCAAGTGT |
| Reverse | TGTCGAGATGCTGCTGTGAG | |
| TNF-α | Forward | GATCGGTCCCAACAAGGAGG |
| Reverse | TCCCTCAGGGGTGTCCTTAG |
2.7 Statistical analysis
Data were obtained from eight animals in each group and expressed as mean ± SD. P values were determined by one-way ANOVA. P-Values less than 0.05 were considered significant.3 Results
3.1 Histological findings of ADLI
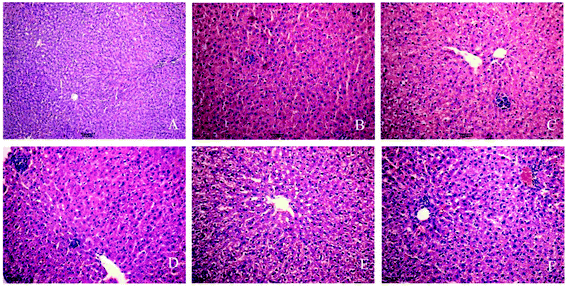
Experimental rats were stained with HE by using a standard protocol and analyzed under light microscopy. In the control group (Fig. 1A), the liver cells showed normal morphology and complete arrangement. No evident pathological changes were found in the 3-day group. The 7-day and 10-day groups showed liver cell swelling and a slight accumulation of inflammatory cells (Fig. 1B and C). In the 14-, 21-, and 28-day, liver cell cytoplasm appeared with osteoporosis, and most of the cells showed piecemeal necrosis, focal necrosis, and portal area presented considerable inflammatory cell infiltration, and hepatic cords disappeared (Fig. 1D–F). The phenomena can show the success of INH-induced liver injury in a rat model and reflect the occurrence of liver injury.3.2 Oxidative stress and antioxidant enzyme activities in INH-challenged rats
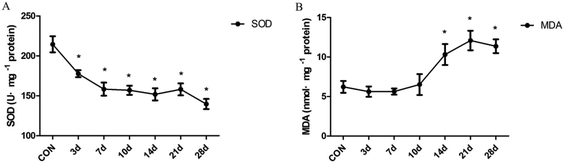
The degree of antioxidant and oxidative liver injury was determined by measuring the levels of SOD and MDA in liver homogenates. The resultant data indicate that SOD activity decreased significantly in INH-treated rats from the 3 day to the 28 day(F = 11.15, P < 0.01) (Fig. 2A). In addition, we found that treatment with INH further increased the hepatic level of MDA, an end product of lipid peroxidation; moreover, the content of MDA is in a high level after 14 days (F = 7.42, P < 0.01) (Fig. 2B). The results suggest that the lipid peroxidation products (MDA) accumulated in the rat liver tissues, which inhibited the expression of the antioxidant substances (SOD) and then aggravated the oxidative damage of liver cells.3.3 Effect of INH treatment on the histones H3K14ac and H4K8ac at different times in the rat liver
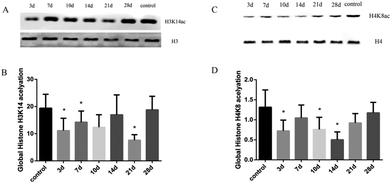
The related stimuli can change the state of histone H3 and H4 acetylation, which affect the activation and transcription of specific genes; thus, histones H3 and H4 are involved in the development of the disease. Liver tissues in experimental rats of histone H3K14 and H4K8 acetylation were significantly lower than the control group, with statistically significant significance (FH3K14 = 4.18, FH4K8 = 3.87, P < 0.05). With a high MDA level at the 21 day, the expression levels of histone H3K14 and H4K8 acetylation decreased compared with the control group, whereas H3K14 was at the lowest level at 21 days (Fig. 3). The results suggest that the hypoacetylation of histones H3K14 and H4K8 was negatively correlated with oxidative stress-mediated rat liver injury induced by INH.3.4 Expression levels of HAT and HDAC in rat liver
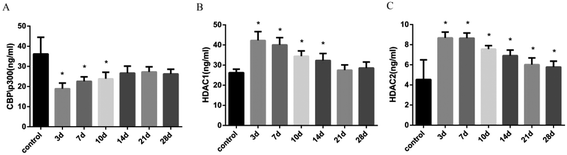
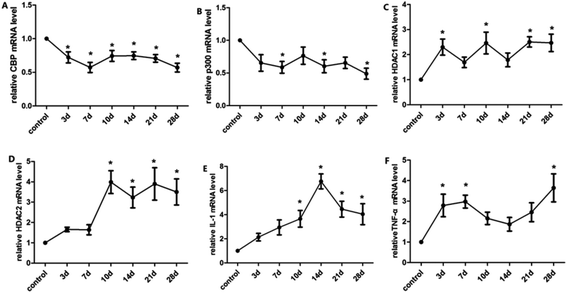
The balance of histone acetylation is regulated by HAT and HDAC. The expression levels of HAT and HDAC after INH exposure in rats were determined by ELISA analysis, and we found that the expression level of CBP/P300 was lower than that in the control group at 3 days to 10 days, whereas the expression levels of HDAC1 and HDAC2 in liver were time-dependently reduced after INH challenge but were higher than the control group (Fig. 4). Considering that the high level of HDAC1 and HDAC2 would lead to reduce histone acetylation, the results shown in Fig. 3 were consistent with the hypo-acetylation of histones H3K14 and H4K8, and the mRNA expression levels of the genes and its corresponding protein expression were approximately the same (Fig. 5).3.5 Expression levels of proinflammatory cytokines in rat liver
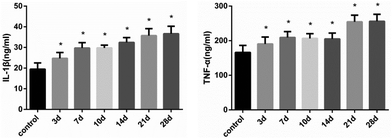
After continuous administration, the expression levels of IL-1β and TNF-α in each time group were up-regulated. As shown in Fig. 5, the concentrations of IL-1β and TNF-α in liver were significantly elevated after 21 days (FIL-1β = 44.88, FTNF-α = 41.56, P < 0.01) (Fig. 5). The mRNA expression was consistent with the level of protein (Fig. 6). The results suggest that histones H3K14 and H4K8 promote the release of inflammatory cytokines and aggravate the injury of hepatocytes.4 Discussion
Chromatin remodeling induced by acetylation is related to the regulation of eukaryotic gene expression, which is an important mechanism of gene transcription. Oxidative stress is not only one of the multiple factors affecting chromatin remodeling10 but an important factor in the pathogenesis of drug-induced liver injury. Several reports showed that high levels of oxidative stress globally inhibit histone acetylation.11 According to the results of this experiment, under the stimulus of INH, different time points of SOD activity decreased in SD rat liver tissues, whereas the content of MDA was significantly increased after 14 days. This finding shows that the metabolism of INH acting on rat liver cells caused by oxidative damage. When MDA is at a high level at 21 days, the expression levels of histone H3K14 and H4K8 acetylation decreased compared with the control group, whereas histone H3K14 was at the lowest level at 21 days and histone H3K14 was the lowest at 14 days. During the SOD rising stage from 14 days to 21 days and MDA in 21 days to 28 days, histone H3K14 and H4K8 acetylation exhibited a recovery trend. Excessive reactive oxygen species (ROS) is suggested to induce the response of the antioxidant system and decrease the inhibitory effect of high level oxidative stress on histone H3 and H4 acetylation.The reversible process of histone acetylation is controlled by HAT and HDAC. HAT and HDAC could induce abnormal gene expression by the acetylation of a series of transcription factors.12 Studies showed that excess ROS acts on homocysteine, and oxidative stress could influence the transcriptional function of the conserved region of CBP/P300, thereby reducing the cell histone acetylation degree.13,14 HDAC1 and HDAC2 are the prominent members of the class I HDAC family. They could regulate the expression of genes and signaling pathways involved in the process of diseases and participate in the regulation of oxidative stress and inflammatory response through direct or indirect combination with the NF-κB and MKP-1 signal pathway.15,16 According to the results of this experiment, we found that the expression of CBP/P300 was lower than that in the control group and increased after 14 days. Given the changes in SOD and MDA, we consider the expression correlated with the dynamic changes of oxidative stress induced by drug metabolism; furthermore, the mRNA expression of CBP/P300 was similar to the proteins. CBP/P300 acetyl transfer function is inhibited under oxidative stress, and the acetylation degree of histones H3 and H4 decreased. Simultaneously, HDAC1 and HDAC2 show high-level expression compared with the control group, although a small downward trend was observed after 14 days, but the above indicators are basically the same expression but still with the above indicators approximate expression. Similarly, the mRNA expression of HDAC1 and HDAC2 was similar to that of the proteins. In addition, HDAC2 could modulate the stability of nuclear factor erythroid 2-related factor 2 (Nrf2), the Nrf2 stability was decreased and Nrf2 acetylation increased in the presence of an HDAC inhibitor, trichostatin A. Therefore, HDAC2 may also be involved in the defensive response against oxidative stress.17 One explanation is that with the changes in SOD and MDA in the experiment after 14 days, HDAC1 and HDAC2 decrease, while histone H3, H4 acetylation increases.
In addition, inflammatory factors play an important role in liver diseases. Liver inflammation results in the fact the INH metabolite acetyl hydrazine and hydrazine can stimulate the overproduction of endogenous hydrogen peroxide.18 The acetylation of histone H4 is mainly related to the inflammatory reaction,19 and HDAC can also affect the expression. In a study involving chronic obstructive pulmonary disease (COPD), the expression of IL-8 and the induced inflammatory reaction could be promoted when the acetylation of histone H4 increased in rat with COPD. However, the significant decrease of HDAC2 in lung tissues may activate some inflammatory and apoptotic genes.20–22 as the inflammatory mediators of liver toxicity, IL-1β and TNF-α can promote the occurrence of the inflammatory reaction in liver injury. According to this experiment, results show an inflammatory reaction occurring in the liver tissue after continuous INH administration, in which the protein contents of IL-1β and TNF-α in rat liver tissue were upregulated, consistent with the pathological changes. In the period of increasing mRNA expression levels of IL-1β and TNF-α, the mRNA expression of HDAC1 and HDAC2 was gradually expressed at a high level; by contrast, the expression levels of CBP/P300 mRNA decreased. After 14 days with the INH stimulus, histone H4K5 and H4K8 acetylation levels rebounded, and IL-1β and TNF-α increased significantly. During the phenomenon, the content of MDA decreased in 21 to 28 days. Oxidative stress induced by INH metabolites is suggested to promote the protection of the antioxidant system response, and functional inhibition of ROS inhibits histone H4 acetylation; moreover, the balance of HAT and HDAC is broken, and the secretion of IL-1β and TNF-α can promote the formation of liver injury.
In conclusion, histone acetylation is involved in INH-induced rat liver injury. This reaction can influence the levels of histone H3 and H4 acetylation and induce the overproduction of inflammatory factors that could accelerate liver cell damage when the balance of HAT and HDAC was broken under oxidative stress induced by INH in rat liver. Although we concluded that oxidative stress affects histone acetylation and eventually lead to high expression of inflammatory factors, how to influence the function and the expression of inflammatory factor in histone acetylation needs to be clarified. Finally, we combined in vivo and clinical studies to clarify the relationship among histone modification, oxidative stress, and inflammatory factor expression in drug-induced liver injury.
Conflicts of interest
There are no conflicts of interest to declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work of the manuscript entitled “Involvement of Histone Hypoacetylation in INH-induced Rat Liver Injury”Acknowledgements
This study was supported by the Natural Science Foundation of Hebei Province, China (No. H2016209300).References
- A. Jaswal, N. Sinha and M. Bhadauria, et al., Therapeutic potential of thymoquinone against anti-tuberculosis drugs induced liver damage, Environ. Toxicol. Pharmacol., 2013, 36(3), 779–786 CrossRef CAS PubMed.
- U. A. Boelsterli and K. K. Lee, Mechanisms of isoniazid-induced idiosyncratic liver injury: Emerging role of mitochondrial stress, J. Gastroenterol. Hepatol., 2014, 29(4), 678–687 CrossRef CAS PubMed.
- C. Enriquez-Cortina, M. Almonte-Becerril and D. Clavijo-Cornejo, et al., Hepatocyte growth factor protects against isoniazid/rifampicin-induced oxidative liver damage, Toxicol. Sci., 2013, 135(1), 26–36 CrossRef CAS PubMed.
- S. Tafazoli, M. Mashregi and P. J. O'Brien, Role of hydrazine in isoniazid-induced hepatotoxicity in a hepatocyte inflammation model, Toxicol. Appl. Pharmacol., 2008, 229(1), 94–101 CrossRef CAS PubMed.
- I. K. Sundar, Y. Hongwei and R. Irfan, Oxidative stress and chromatin remodeling in chronic obstructive pulmonary disease and smoking-related diseases, Antioxid. Redox Signaling, 2013, 18(15), 1956–1971 CrossRef CAS PubMed.
- J. Gräff and L. H. Tsai, Histone acetylation: molecular mnemonics on the chromatin, Nat. Rev. Neurosci., 2013, 14(2), 97–111 CrossRef PubMed.
- G. M. Shahbazian, Functions of site-specific histone acetylation and deacetylation, Annu. Rev. Biochem., 2007, 76(76), 75–100 CrossRef PubMed.
- G. Legube and D. Trouche, Regulating histone acetyltransferases and deacetylases, EMBO Rep., 2003, 4(10), 944–947 CrossRef CAS PubMed.
- J. Kang, C. Jie and Y. Shi, et al., Curcumin-induced histone hypoacetylation: the role of reactive oxygen species, Biochem. Pharmacol., 2005, 69(2), 1205–1213 CrossRef CAS PubMed.
- M. Berthiaume, N. Boufaied and A. Moisan, et al., High Levels of Oxidative Stress Globally Inhibit Gene Transcription and Histone Acetylation, DNA Cell Biol., 2006, 25(2), 124–134 CrossRef CAS PubMed.
- I. Moraes, Z. F. Yuan and S. Liu, et al., Analysis of Histones H3 and H4 Reveals Novel and Conserved Post-Translational Modifications in Sugarcane, PLoS One, 2015, 10(7), e0134586 Search PubMed.
- S. G. Swygert, Chromatin dynamics: Interplay between remodeling enzymes and histone modifications, Biochim. Biophys. Acta, 2014, 1839(8), 728–736 CrossRef CAS PubMed.
- B. David, The role of cysteine residues as redox-sensitive regulatory switches, Curr. Opin. Struct. Biol., 2004, 14(6), 679–686 CrossRef PubMed.
- C. Lin, J. Kang and R. Zheng, Oxidative stress is involved in inhibition of copper on histone acetylation in cells, Chem.-Biol. Interact., 2005, 151(3), 167–176 CrossRef CAS PubMed.
- F. J. Dekker, T. van den Bosch and N. I. Martin, Small molecule inhibitors of histone acetyltransferases and deacetylases are potential drugs for inflammatory diseases, Drug Discovery Today, 2014, 19(5), 654–660 CrossRef CAS PubMed.
- O. H. Krämer, HDAC2: a critical factor in health and disease, Trends Pharmacol. Sci., 2009, 30(12), 647–655 CrossRef PubMed.
- N. Mercado, R. Thimmulappa and C. M. R. Thomas, et al., Decreased histone deacetylase 2 impairs Nrf2 activation by oxidative stress, Biochem. Biophys. Res. Commun., 2011, 406(2), 292–298 CrossRef CAS PubMed.
- I. G. Metushi and J. Uetrecht, Lack of liver injury in Wistar rats treated with the combination of isoniazid and rifampicin, Mol. Cell. Biochem., 2013, 387(1–2), 9–17 Search PubMed.
- Q. Zeng, J. Shu and Q. Hu, et al., Apoptosis in human myelodysplastic syndrome CD34+ cells is modulated by the upregulation of TLRs and histone H4 acetylation via a β-arrestin 1 dependent mechanism, Exp. Cell Res., 2015, 12(8), 1–10 Search PubMed.
- Y. Chen, H. Ping and A. Wen, et al., Histone deacetylase activity is decreased in peripheral blood monocytes in patients with COPD, J. Inflammation, 2012, 9(6), 10 CrossRef CAS PubMed.
- T. Masako, T. Dai and A. Kenichi, et al., Sputum Plasminogen Activator Inhibitor-1 Elevation by Oxidative Stress-Dependent Nuclear Factor-κB Activation in COPD, Chest, 2013, 144(2), 15–521 Search PubMed.
- A. R. Winkler, K. N. Nocka and C. M. M. Williams, Smoke exposure of human macrophages reduces HDAC3 activity, resulting in enhanced inflammatory cytokine production, Pulm. Pharmacol. Ther., 2012, 25(4), 286–292 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |