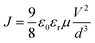Efficient polymer solar cells based on a cathode interlayer of dicyanomethylenated indacenodithiophene derivative with large π-conjugation and electron-deficient properties†
Yang
Miao
,
Hanbo
Yu
,
Yuewei
Zhang
,
Xianju
Yan
,
Jingying
Zhang
* and
Yue
Wang
 *
*
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: yuewang@jlu.edu.cn
First published on 27th November 2017
Abstract
Two indacenodithiophene (IDT) based water/alcohol-soluble small molecular cathode interlayers (CILs), namely TBIDTD and TBIDTCN, were designed, synthesized and applied in typical PTB7:PC71BM-based conventional structural single-junction polymer solar cells (PSCs). This is the first report where the IDT core was used as a large π-conjugated backbone for CILs. These two molecules adopt the same polar end-groups. However, compared to TBIDTD, TBIDTCN exhibits a lower LUMO (lowest unoccupied molecular orbital) level due to the introduction of dicyanomethylene groups, which is beneficial for electron transport and extraction at the interface. Systematic optimizations were carried out to confirm the interfacial modification ability of the CILs. The results indicate that both CILs can significantly improve the performance of PSCs, and the TBIDTCN-modified devices presented better performance because of the further improved electron mobility and further reduced Rs (series resistance). A PCE (power conversion efficiency) of 9.19% was achieved by the TBIDTCN-modified devices, which was nearly 1.66 times that of the bare cathode device, due to the simultaneously enhanced Voc (circuit voltage), Jsc (short-circuit current) and FF (fill factor). Our work demonstrates that the IDT-based CILs are effective in improving the performance of PSCs and presents an attractive design strategy for CILs with large π-conjugation and electron-deficient properties.
Introduction
In recent years, polymer solar cells (PSCs) with a bulk heterojunction structure have been extensively studied because of their unique advantages, including the large area flexibility, light weight and potentially low cost of fabrication.1 To date, power conversion efficiencies (PCEs) have exceeded 13% for single-junction solar cells.2 Rapid advances in PCE have been realized due to the development of new photoactive materials such as polymer donors3 and non-fullerene acceptors,4 optimization of active layer morphology5 and interfacial engineering.6 One of the most effective interfacial strategies is the incorporation of appropriate interface-modified materials to alleviate the interfacial energy barriers and optimize the extraction of electrons from the photoactive layer to the electrodes.7 Among the large family of interfacial materials, water/alcohol-soluble organic polymeric/small molecular cathode interlayer (CIL) materials have attracted significant attention. CILs can significantly improve the performance of devices; in particular, CILs can simplify the device fabrication procedures for roll-to-roll processes due to their orthogonal solubility, affording better device stability.Generally, these CILs comprise conjugated backbones and pendant polar functional groups to ensure good electron mobility and alcohol/water solubility. A bulk of such bipolar CILs have been developed, including polymers and small molecules, and amine derivatives are chosen as the pendant functional groups in most cases. One typical example is poly[(9,9-dioctyl-2,7-fluorene)-alt-(9,9-bis(30-(N,N-dimethylamino)propyl)-2,7-fluorene)] (PFN), which has been widely used in optoelectronic devices.8 In these works, the amino-functionalized fluorene moiety not only allows the solution-processed deposition of water/alcohol solutions, but can also form interfacial dipoles between the CIL and the cathode, which plays very important roles in interfacial modification. As a result, CIL-modified PSCs exhibit a remarkably improved performance due to the simultaneously enhanced circuit voltage (Voc), short-circuit current (Jsc) and fill factor (FF). In addition, compared to polymers, CILs based on small molecules are more attractive as they possess several advantages, such as well-defined structures, simple synthesis and purification processes, and good batch to batch reproducibility.9 Therefore, many small molecular bipolar CILs with extensive molecular structures have been explored to fabricate high-performance PSCs, such as ammonium salts or amine-terminated triphenylamine-fluorene,10 perylene diimide11 and fullerene derivatives.12 In this context, we focused on developing novel CILs based on electron-deficient backbones. This design is favorable for electron transfer at the interface due to the narrowed energy barriers. It is also meaningful to design a new kind of large π-conjugated skeleton with tunable LUMO (lowest unoccupied molecular orbital) levels to achieve CILs with electron-deficient properties.
Recently, indacenodithiophene (IDT) based small molecular acceptors have attracted a lot of attention, since IDT-based IEIC was firstly used in PSCs as an acceptor by Zhan and co-workers.13 As the first non-fullerene small molecular acceptor that exceeds 10% efficiency, IDT-based ITIC has been widely studied and has achieved many great successes.14 However, although ITIC derivatives could work well as acceptors for multiple donors, the IDT skeleton is rarely applied as the large π-conjugated backbone for n-type CILs like the perylene diimide and fullerene, due to its electron-rich nature. Thus, it is necessary to introduce electron-withdrawing groups to the electron-rich IDT skeleton to endow it with electron-deficient character.
In this context, we introduced strong electron-withdrawing dicyanomethylene groups to the IDT skeleton and combined the resulting electron-deficient dicyanomethylenated indacenodithiophene (IDTCN) with a flexible pendant polar functional group affording a unique small molecular CIL named as TBIDTCN (Fig. 1). It was noted that several groups had demonstrated that IDTCN-based materials can achieve high electron mobility.15 In addition, a reference electrolyte without dicyanomethylene groups, namely TBIDTD, was also synthesized. Both molecules utilize quaternary ammonium ion-terminated 1,2,3-trihexyloxybenzene as the same end-groups to ensure the orthogonal solubility to the photoactive layer. Furthermore, TBIDTCN and TBIDTD were employed as CILs in the PTB7:PC71BM (PTB7:poly[[4,8-bis[(2-ethylhexyl)oxy]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl][3-fluoro-2-[(2-ethylhexy)carbonyl]thieno[3,4-b]thiophenediyl]]; PC71BM:[6,6]-phenyl C71-butyric acid methyl ester) based PSCs (Fig. 1b and c). The effect of the CILs on the PSC performance will be presented below. It is noted that our work not only achieved a new kind of large π-conjugated and electron-deficient CILs with tunable LUMO levels, but also revealed an attractive molecular design strategy for the future development of efficient CILs.
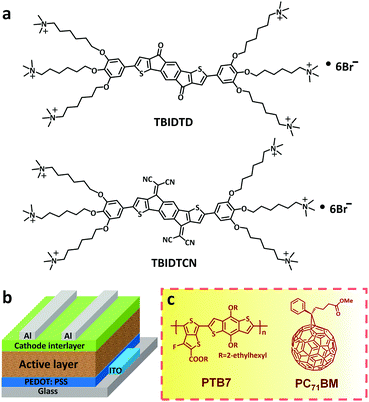 | ||
| Fig. 1 (a) Molecular structures of TBIDTD and TBIDTCN; (b) device architecture of conventional PSCs; (c) chemical structure of PTB7 and PC71BM. | ||
Results and discussion
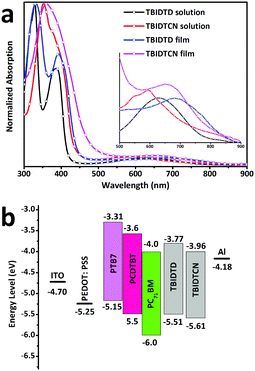
THBBr-IDTD was synthesized by Suzuki coupling of 2,7-dibromo-s-indacenodithiophene-4,9-dionethe (Br-IDTD) and 3,4,5-tris((6-bromohexyl)oxy)phenylboronic ester (PBE-THBBr), and the intermediate THBBr-IDTCN was synthesized by Knoevenagel condensation of THBBr-IDT and malononitrile. Ionization of THBBr-IDTD and THBBr-IDTCN with trimethylamine was then performed to afford TBIDTD and TBIDTCN. The detailed synthetic route of the new n-type small molecular CIL (TBIDTCN) is shown in Schemes S1 and S2 (see ESI†). The molecular structures of the intermediates and the target CILs were well confirmed by 1H NMR, elemental analysis and mass spectrometry. The detailed synthetic procedures and characterization data are summarized in the Experimental section. The target TBIDTD and TBIDTCN were easy to dissolve in methanol, but were insoluble in chloroform, chlorobenzene, tetrahydrofuran, etc., which indicated that they could be used as a cathode interlayer for the solution-processed fabrication of multilayer PSCs without interface erosion. Optical absorption spectra of TBIDTD and TBIDTCN in methanol solution and a solid thin film are shown in Fig. 2a. The optical band gap (Eoptg) was determined from the thin film absorption onset to be 1.46 eV for TBIDTD and 1.40 eV for TBIDTCN, respectively. It is worth noting that for TBIDTD and TBIDTCN, the donor–acceptor charge transfer absorption intensities in the range of 500–800 nm are very weak. This is an important advantage of indacenodithiophene derivatives, as the cathode interlayers in other low-bandgap organic semiconductors have strong absorption in this range. Therefore, the introduction of TBIDTD or TBIDTCN does not result in absorption loss in PSCs. Cyclic voltammetry (CV) measurements were carried out to determine the frontier molecular orbitals of TBIDTD and TBIDTCN (Fig. S2a, ESI†). Based on the reduction potential onset, the lowest unoccupied molecular orbital (LUMO) energy levels were estimated to be −3.77 eV for TBIDTD and −3.96 eV for TBIDTCN. In addition, the highest occupied molecular orbital (HOMO) energy levels were estimated to be −5.51 eV for TBIDTD and −5.61 eV for TBIDTCN, based on the oxidation onset. The Eclg calculated from the electrochemical data was in the same range as Eoptg. The data are summarized in Table 1. Compared to TBIDTD, the LUMO level of TBIDTCN is 0.2 eV lower due to the introduction of electron-withdrawing dicyanomethylene groups, which means lower energy barriers at the interface for TBIDTCN-modified devices. Fig. 2b presents the energy level diagrams and TBIDTCN exhibits almost the same LUMO levels as PC71BM, indicating that the electron transfer from PC71BM to TBIDTCN should jump over a little energy barrier. It is worth noting that the deep LUMOs could also lead to intramolecular electron transfer in TBIDTCN. EPR (electron paramagnetic resonance) measurements revealed that dicyanomethylenated TBIDTCN showed a single intense resonance signal (Fig. S2b, ESI†), but TBIDTD displays no EPR signal. So far, the precise mechanism for the formation of single-electron radicals remains unclear. The intramolecular electron transfer between electron-rich donors and electron-deficient acceptors may generate single-electron radicals. It is commonly accepted that lower LUMO levels could provide a stronger electron-accepting ability, which may explain the EPR signal of dicyanomethylenated TBIDTCN.16 It has been reported that such intrinsic electron transfer could benefit the electron extraction and mobility of CILs.11b,17 The EPR property of TBIDTCN implies that the self-doped behavior may be used to explain the properties of these CIL materials.18| CIL | Abs. λonset [nm] | E optg [eV] | E onsetre [ev] | E LUMO [eV] | E HOMO [eV] | E clg [eV] |
|---|---|---|---|---|---|---|
| TBIDTD | 849 | 1.46 | −1.03 | −3.77 | −5.51 | 1.74 |
| TBIDTCN | 886 | 1.40 | −0.84 | −3.96 | −5.61 | 1.65 |
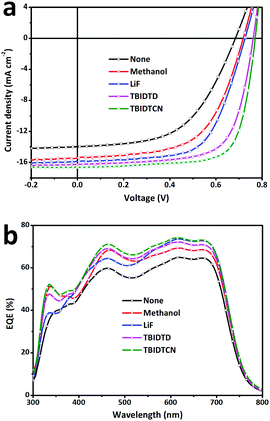
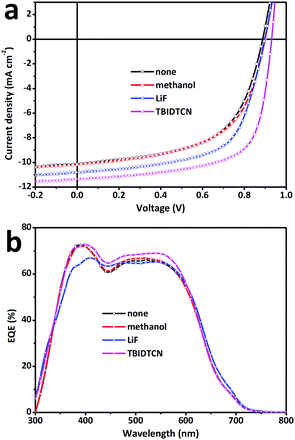
To study the effect of CILs with different LUMOs on the PSC performance, conventional structural PTB7:PC71BM-based PSCs were fabricated with the two CILs deposited onto the active layer by spin-coating from their methanol solutions. Different concentrations of both CILs in methanol solution were tested and the optimized one was found to be 0.5 mg mL−1 (3000 rpm spin speed). The optimized thickness of both CILs was around 3 nm. For comparison, three control devices were also fabricated, those being the Al-only device without the CILs, the methanol-treated device via spin-coating methanol on the active layer and the device modified with 0.7 nm LiF. The current density–voltage (J–V) characteristics of the devices under 100 mW cm−2 AM 1.5G irradiation are shown in Fig. 3a and the performance parameters of Jsc, Voc, FF and PCE are summarized in Table 2. As a control device, the PSCs without any CIL film or any post-treatments (Al-only device) exhibited a PCE of 5.55% with a Jsc of 13.98 mA cm−2, a Voc of 0.682 V and a FF of 58.3%. Both of the new CILs significantly enhanced the performance of the devices. In particular, the TBIDTCN-modified devices could achieve a PCE of 9.19%, which was increased by nearly 66% compared to the Al-only device, with an obviously enhanced FF of 72.2%. Even when compared to the PCE of LiF-modified devices (PCE: 7.56%), the PCE value was apparently improved by 22%. However, the methanol/Al-only device exhibited a slightly improved PCE of 6.62%. This suggests the enhanced PCEs of the devices with the solution-processed CILs are mainly attributed to the CILs themselves rather than the solvent (methanol). In order to ensure the validity and repeatability of the data, we measured at least 30 pixels (size = 2 × 2 mm2) for all device configurations. The average PCE values of the Al-only, methanol-treated, LiF/Al, TBIDTD/Al and TBIDTCN/Al devices are also listed in Table 2. Furthermore, the series resistance (Rs) was demonstrated to be important in indicating the FF of the PSCs, as a higher FF is generally accompanied by a lower Rs value.19 Therefore, we measured the Rs values of all devices (Table 2). As shown in Table 2, compared to the Al-only device, the Rs values of the methanol-treated device and LiF-modified device were slightly decreased. However, the incorporation of CILs could obviously depress the Rs value to be 4.7 Ω cm2 for TBIDTD and 3.7 Ω cm2 for TBIDTCN, indicating that better ohmic contact for charge transfer and extraction was achieved at the cathode interface when the CILs were inserted. This result is consistent with the higher FF that was achieved for these devices. To check the discrepancy of Jsc, which easily leads to an overvalued PCE, external quantum efficiency (EQE) spectra for the corresponding devices in the range of 300–800 nm were collected and are shown in Fig. 3b. The EQE value indicates the ratio of the number of incident photons to the number of change-carrying electrons, and thus is a measure of the conversion efficiency. JEQEsc values integrated from the EQE spectra, as listed in Table 2, are basically in agreement with the Jsc values obtained from the J–V characteristics under illumination within 5% error. This indicates that the Jsc data presented in Fig. 3a are reliable and the PCE values presented in Table 2 are not overvalued. These results indicate that both IDT-based CILs are efficient in improving the device performance and a highest PCE can be achieved via the intercalation of dicyanomethylenated TBIDTCN.
| CILs | J sc (JEQEsc) [mA cm−2] | V oc [V] | FF [%] | PCEmax(ave) [%] | R s [Ω cm2] |
|---|---|---|---|---|---|
| None | 13.98/13.56 | 0.682 | 58.3 | 5.55/5.36 | 13.5 |
| Solvent | 15.38/14.82 | 0.718 | 59.8 | 6.60/6.49 | 9.6 |
| LiF | 15.85/15.11 | 0.732 | 65.2 | 7.56/7.42 | 9.4 |
| TBIDTD | 16.23/15.43 | 0.766 | 69.3 | 8.62/8.48 | 4.7 |
| TBIDTCN | 16.57/15.82 | 0.768 | 72.2 | 9.19/9.10 | 3.7 |
The J–V characteristics of all devices were also measured in the dark to further demonstrate the higher FFs achieved by the IDT-based CIL-modified devices. As shown in the Fig. 4a, in the range from −1 to 0 V, the reverse current of the CIL-modified devices were clearly suppressed compared to the bare Al or methanol-treated devices. It can be seen that the reverse current of the TBIDTCN-modified device was suppressed the most, which indicated that TBIDTCN could work more effectively in improving the charge selectivity and blocking holes at the interface. At a forward bias, a higher turn-on voltage was also observed in the IDT-based CIL-modified devices, indicating a bigger built-in potential (Vbi) across the devices. In fact, Vbi is an important indicator for Voc, as an increased Vbi is essential for a higher Voc.
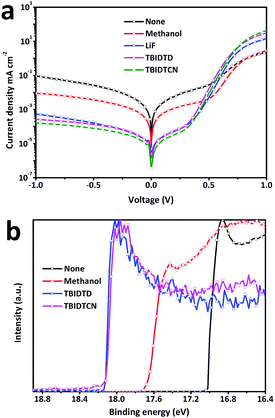 | ||
| Fig. 4 (a) J–V curves of the PTB7:PC71BM-based devices with or without CILs in the dark; (b) UPS spectra of bare Al, methanol-treated Al, TBIDTD-modified Al and TBIDTCN-modified Al. | ||
The Voc change could also be explained by the change in the work function (WF) of the CIL-modified Al, which was confirmed by the ultraviolet photoelectron spectroscopy (UPS) measurements with monochromatized HeI radiation at 21.22 eV. Previous studies have shown that the introduction of CILs can effectively depress the work function (WF) of the cathode through the surface dipole interaction between the pedant polar functional groups and the cathode.20 As shown in Fig. 4b, the WF values, which were defined by the secondary electron cut-off in the UPS spectra, were 4.18 eV for bare Al and 3.54 eV for the methanol-treated Al. The values are in accordance with other reported results. Furthermore, the CIL-covered Al nearly had the same WF values, 3.11 eV for TBIDTD and 3.10 eV for TBIDTCN. Obviously, both CILs could effectively depress the WF of the cathode, which was in accordance with the sequence of Voc values.
In addition, the interfacial dipole could be further studied via water contact angle (θ) measurements. A reduced water contact angle of a CIL-covered active layer indicates more ionic components accumulated at the top of the organic active layer surface, which means a stronger interfacial dipole.21 As shown in Fig. 5, the surfaces of the pristine PTB7:PC71BM film and the methanol-treated PTB7:PC71BM film were largely hydrophobic with water contact angles (θ) of 98.6° and 97.8°, respectively. However, the coverage of CILs obviously changed the surface nature, with water contact angles (θ) of 69.4° for TBIDTD and 61.2° for TBIDTCN. Obviously, TBIDTCN with a lower LUMO energy level could form a stronger interfacial dipole than TBIDTD and favors better charge injection and collection in the PSCs, which could explain the higher Jsc and FF for the TBIDTCN-modified devices.
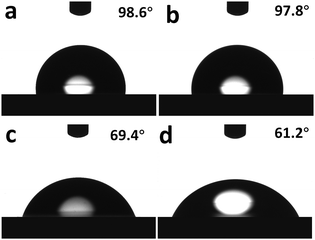 | ||
| Fig. 5 Water contact angle images of (a) PTB7:PC71BM BHJ film; (b) methanol-treated PTB7:PC71BM BHJ film; (c) TBIDTD on PTB7:PC71BM BHJ film; (d) TBIDTCN on PTB7:PC71BM BHJ film. | ||
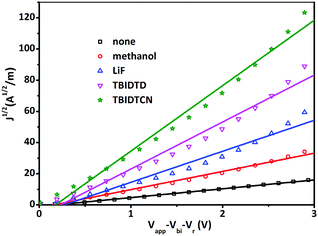
Meanwhile, the electron mobility in the PSCs was investigated via the space charge limited current (SCLC) technique with a device architecture of [ITO/Al/PTB7:PC71BM/with or without CILs/Al]. The J1/2–V curves of the electron-only devices are presented in Fig. 6. The electron mobilities were estimated according to the following equation:
To explore the surface morphologies of the CIL-modified films, which is important for deeply understanding the enhanced Jsc and FF, atomic force microscopy (AFM) was subsequently carried out at ambient temperature. Generally, a good film-formation ability of CILs at the top of the hydrophobic active layer could be helpful for better ohmic contact at the interface. Fig. 7 presents the images of the pristine PTB7:PC71BM film, the methanol-treated PTB7:PC71BM film and the two CIL-modified films. The surface of pristine PTB7:PC71BM appeared to be a relatively smooth surface with a root mean square (RMS) roughness of 1.48 nm. The methanol treatment caused a slightly smoother surface with RMS roughness of 1.26 nm. The surface of the TBIDTCN-modified film exhibited a RMS roughness of 2.44 nm, which was smoother than the surface of the TBIDTD-modified film (2.99 nm), due to the introduction of dicyanomethylene groups. It was noted that both CILs display a leopard print-like pattern on the PTB7:PC71BM surface, which may be beneficial to ohmic contact at the interface.
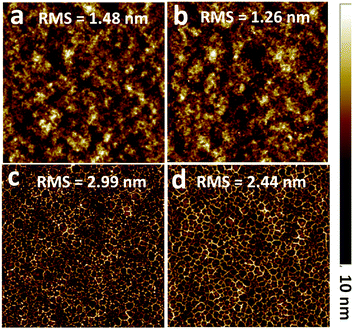 | ||
| Fig. 7 AFM images (5 × 5 μm) of (a) PTB7:PC71BM BHJ film; (b) methanol-treated PTB7:PC71BM BHJ film; (c) TBIDTD on PTB7:PC71BM BHJ film; (d) TBIDTCN on PTB7:PC71BM BHJ film. | ||
PCDTBT:PC71BM-based PSCs were also fabricated to testify the universality of TBIDTCN as a CIL. The J–V characteristics of the PCDTBT:PC71BM-based devices under 100 mW cm−2 AM 1.5G irradiation are presented in Fig. 8a, and the corresponding EQE curves of the solar cells are shown in Fig. 8b with the device parameters summarized in Table 3. As we expected, the solar cells that employed TBIDTCN as the CIL with the optimized thickness of 3 nm showed a maximum PCE value of 7.11% with a Jsc of 11.35 mA cm−2, Voc of 0.928 V and FF of 67.5%, respectively. The PCE of the TBIDTCN-modified device was increased by nearly 36% compared to the value of the Al-only device and 17% compared to the value of the LiF-modified device, showing the universality of TBIDTCN, and confirming its great potential as a CIL in PSC applications.
| CIL | J sc (JEQEsc) [mA cm−2] | V oc [V] | FF [%] | PCEmax(ave) [%] | R s [Ω cm2] |
|---|---|---|---|---|---|
| None | 10.24/10.02 | 0.878 | 58.3 | 5.24/5.19 | 13.3 |
| Solvent | 10.31/10.08 | 0.903 | 58.7 | 5.46/5.32 | 12.8 |
| LiF | 10.82/10.47 | 0.892 | 63.2 | 6.10/6.02 | 12.5 |
| TBIDTCN | 11.35/11.09 | 0.928 | 67.5 | 7.11/7.05 | 5.9 |
Conclusions
In conclusion, two new indacenodithiophene (IDT) based small molecular water/alcohol-soluble CILs with the same polar end groups (quaternary ammonium ion-terminated trihydroxybenzene) were successfully synthesized and applied in conventional PTB7:PC71BM-based PSCs. Both CILs could significantly enhance the device performance. Dicyanomethylenated TBIDTCN displayed a better performance and could improve the PCE to a value of 9.19%, which is 1.66 times that of the Al-only devices. It was demonstrated that the CILs could effectively reduce the series resistance (Rs), increase the built-in potential (Vbi) and enhance the electron mobility of PSCs via systematic measurements. This is the first time that an IDT core was used as conjugated backbone for CILs. Compared to TBIDTD, dicyanomethylenated TBIDTCN has lower LUMO levels, which can narrow the energy barriers and facilitate electron transportation. The introduction of dicyanomethylene groups could also lower the RMS roughness of the CIL surface, which is beneficial to the ohmic contact at the interface. The further application of TBIDTCN in the PCDTBT:PC71BM-based PSCs indicates that TBIDTCN is a relatively universal CIL for different active layers. These results demonstrate that IDT-based bipolar molecules could be used to develop CILs for the fabrication of high performance PSCs.Experimental section
Materials and characterization
All reagents were purchased from Sigma-Aldrich Chemical Co., Alfa Aesar Co. and Aladdin Co., and were directly used without further purification. All reactions were performed under N2 atmosphere, unless otherwise stated. 2,7-Dibromo-s-indacenodithiophene-4,9-dionethe (Br-IDTD) and 5-iodo-1,2,3-trihydroxybenzene were prepared according to the previously reported procedures.22 The synthetic route of PBE-THBBr is shown in the ESI.†1H NMR spectra were recorded on a Bruker Avance-500 spectrometer or a Varian Mercury 300 MHz spectrometer (Palo Alto, CA) with tetramethylsilane as the internal standard. Mass spectra were recorded on a Kratos AXIMA-CFR Kompact MALDI mass spectrometer with anthracene-1,8,9-triol as the matrix or a GC-MS spectrometer (ITQ1100, Thermo Fisher). Elemental analyses were performed on a flash EA 1112 spectrometer (Germany). Optical spectra were obtained on a Shimadzu UV-2550 spectrophotometer. TGA (thermogravimetric analyses) were performed on a TAQ500 thermogravimeter at a heating rate of 10 °C min−1 under nitrogen. Details of the synthesis and characterization procedures are presented below.Synthesis
Materials and device fabrication
PTB7 and PCDTBT were purchased from 1-material Chemscitech Inc. (Canada), PC71BM was purchased from American Dye Source, Inc. LiF, 1,2-dichlorobenzene (DCB), chlorobenzene (CB) and methanol were purchased from Sigma-Aldrich and used as delivered.All PSC devices were fabricated with a simple conventional structure. The ITO-coated glass was carefully precleaned with detergent, deionized water, acetone and isopropyl alcohol in an ultrasonic bath, and treated with plasma for 7 min. After filtration through a 0.45 μm filter, the PEDOT:PSS (Baytron PVP Al 4083) layer was spin coated (3000 rpm, 60 s) and baked at 120 °C for 10 min to form a thin film with a thickness of 35 nm on the cleaned ITO substrate. The device was then transferred to a nitrogen glove box. Then an active layer was prepared on top of the PEDOT:PSS layer. For the PTB7:PC71BM devices, a blended chlorobenzene solution of PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 w/w, total concentration of 25 mg mL−1) with 3 vol% 1,8-diiodoctane that had been stirred for a week at room temperature was spin-coated on the substrate at 1000 rpm for 40 s and then the solvent was removed under vacuum. The film thickness was nearly 120 nm. For the PCDTBT:PC71BM devices, a blend of 7 mg PCDTBT and 28 mg PC71BM was dissolved in 1 mL of the mixed solvent (chlorobenzene
1.5 w/w, total concentration of 25 mg mL−1) with 3 vol% 1,8-diiodoctane that had been stirred for a week at room temperature was spin-coated on the substrate at 1000 rpm for 40 s and then the solvent was removed under vacuum. The film thickness was nearly 120 nm. For the PCDTBT:PC71BM devices, a blend of 7 mg PCDTBT and 28 mg PC71BM was dissolved in 1 mL of the mixed solvent (chlorobenzene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) o-dichlorobenzene = 1
o-dichlorobenzene = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) and stirred for two days. Then the solution was spin-coated on the substrate at 2000 rpm for 40 s and annealed at 75 °C for 10 min. The film thickness was nearly 70 nm. The CILs were deposited onto the active layer from the methanol solutions with a concentration of 0.5 mg mL−1 at 3000 rpm for 40 s, subsequently. The thickness of the TBIDTCN layer was estimated to be 3 nm after spin-coating a 0.5 mg mL−1 TBIDTCN methanol solution on the ITO substrate (3000 rpm, 40 s), by XPS experiments using a VG Scienta R3000 spectrometer in an ultrahigh vacuum at a base pressure of 2 × 10−10 mbar. Finally, 100 nm of Al was thermally-deposited under a pressure of 2 × 10−6 mbar. The measurement chamber was equipped with a monochromatic Al (Kα) X-ray source providing photons of 1486.6 eV. The device area was 0.04 cm2.
3, v/v) and stirred for two days. Then the solution was spin-coated on the substrate at 2000 rpm for 40 s and annealed at 75 °C for 10 min. The film thickness was nearly 70 nm. The CILs were deposited onto the active layer from the methanol solutions with a concentration of 0.5 mg mL−1 at 3000 rpm for 40 s, subsequently. The thickness of the TBIDTCN layer was estimated to be 3 nm after spin-coating a 0.5 mg mL−1 TBIDTCN methanol solution on the ITO substrate (3000 rpm, 40 s), by XPS experiments using a VG Scienta R3000 spectrometer in an ultrahigh vacuum at a base pressure of 2 × 10−10 mbar. Finally, 100 nm of Al was thermally-deposited under a pressure of 2 × 10−6 mbar. The measurement chamber was equipped with a monochromatic Al (Kα) X-ray source providing photons of 1486.6 eV. The device area was 0.04 cm2.
Measurements and characterization
The photovoltaic performance of the device was tested under 1 Sun, AM 1.5 full-spectrum solar simulator (Photo Emission Tech., model #SS50AAA-GB) with an irradiation intensity of 100 mW cm−2, which was monitored by a standard silicon solar cell traced to the National Institute of Metrology, China. The current–voltage (J–V) characteristics were measured via a Keithley 2400 source meter. A Q Test station (Crowntech Inc., Macungie, PA) was used to achieve the EQE (external quantum efficiency) spectra under ambient conditions. Ultraviolet photoemission spectroscopy (UPS) measurements were carried out with monochromatized HeI radiation at 21.2 eV using a VG Scienta R3000 analyzer under ultrahigh vacuum with a base pressure of 1 × 10−10 mbar. The contact angles were measured on a commercial contact angle system (DatePhysics, OCA 20) at ambient temperature with a 4 μL water droplet as the indicator. AFM measurements were directly performed on the surface of the fabricated devices with an S II Nanonavi probe station 300 Hz (Seiko, Japan) in the DFM mode under ambient conditions. The AFM images and RMS values were presented and calculated by the software Nanonavi Analysis. In addition, the electron mobilities of the devices were measured by the SCLC (space charge limited current) method with a device structure of [ITO/Al/PTB7:PC71BM/with or without CIL/Al] and were tested using a Keithley 2400 source meter.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the National Basic Research Program of China (2014CB643506), Natural Science Foundation of China (51173065), program for JLU Science and Technology Innovative Research Team and project of SXGJSF2017-3.Notes and references
- (a) J. Peet, J. Y. Kim, N. E. Coates, W. L. Ma, D. Moses, A. J. Heeger and G. C. Bazan, Nat. Mater., 2007, 6, 497 CrossRef CAS PubMed; (b) G. Li, R. Zhu and Y. Yang, Nat. Photonics, 2012, 6, 153 CrossRef CAS; (c) W. Zhao, S. Li, S. Zhang, X. Liu and J. Hou, Adv. Mater., 2017, 29, 1604059 CrossRef PubMed.
- W. Zhao, S. Li, H. Yao, S. Zhang, Y. Zhang, B. Yang and J. Hou, J. Am. Chem. Soc., 2017, 139, 7148 CrossRef CAS PubMed.
- (a) Y. Li, Acc. Chem. Res., 2012, 45, 723 CrossRef CAS PubMed; (b) L. Ye, S. Zhang, L. Huo, M. Zhang and J. Hou, Acc. Chem. Res., 2014, 47, 1595 CrossRef CAS PubMed; (c) Y. Lin, Y. Li and X. Zhan, Chem. Soc. Rev., 2012, 41, 4245 RSC; (d) J. E. Coughlin, Z. B. Henson, G. C. Welch and G. C. Bazan, Acc. Chem. Res., 2013, 47, 257 CrossRef PubMed; (e) T. S. van der Poll, J. A. Love, T.-Q. Nguyen and G. C. Bazan, Adv. Mater., 2012, 24, 3646 CrossRef CAS PubMed; (f) M. Wang, Z. Wang, W. Ma, S. C. Chen and Q. Zheng, Adv. Energy Mater., 2016, 2, 1600340 Search PubMed; (g) T. Liu, X. Pan, X. Meng, Y. Liu, D. Wei, W. Ma, L. Huo, X. Sun, T. H. Lee, M. Huang, H. Choi, J. Y. Kim, W. C. H. Choy and Y. Sun, Adv. Mater., 2017, 29, 1604251 CrossRef PubMed; (h) Q. Tu, D. Cai, L. Wang, J. Wei, Q. Shang, S.-C. Chen, Y. Ma, Z. Yin, C. Tanga and Q. Zheng, J. Mater. Chem. C, 2016, 4, 6160 RSC; (i) Y. Abe, H. Li, J. Yin, C. Soci, A. C. Grimsdalea and Y. M. Lam, J. Mater. Chem. C, 2016, 4, 9656 RSC; (j) P. Zhou, Y. Yang, X. Chen, Z.-G. Zhang and Y. Li, J. Mater. Chem. C, 2017, 5, 2951 RSC.
- (a) Y. Fu, B. Wang, J. Qu, Y. Wu, W. Ma, Y. Geng, Y. Han and Z. Xie, Adv. Funct. Mater., 2016, 26, 5922 CrossRef CAS; (b) W. Ni, M. Li, B. Kan, F. Liu, X. Wan, Q. Zhang, H. Zhang, T. P. Russellcd and Y. Chen, Chem. Commun., 2016, 52, 465 RSC; (c) J. Lee, R. Singh, D. Sin, H. Kim, K. Song and K. Cho, Adv. Mater., 2016, 28, 69 CrossRef CAS PubMed; (d) W. Zhao, D. Qian, S. Zhang, S. Li, O. Inganas, F. Gao and J. Hou, Adv. Mater., 2016, 28, 4734 CrossRef CAS PubMed; (e) Y. Lin, F. Zhao, Y. Wu, K. Chen, Y. Xia, G. Li, S. K. K. Prasad, J. Zhu, L. Huo, H. Bin, Z.-G. Zhang, X. Guo, M. Zhang, Y. Sun, F. Gao, Z. Wei, W. Ma, C. Wang, J. Hodgkiss, Z. Bo, O. Inganäs, Y. Li and X. Zhan, Adv. Mater., 2017, 29, 1604155 CrossRef PubMed; (f) S. Chen, G. Zhang, J. Liu, H. Yao, J. Zhang, T. Ma, Z. Li and H. Yan, Adv. Mater., 2017, 29, 1604231 CrossRef PubMed; (g) N. Qiu, H. Zhang, X. Wan, C. Li, X. Ke, H. Feng, B. Kan, H. Zhang, Q. Zhang, Y. Lu and Y. Chen, Adv. Mater., 2017, 29, 1604964 CrossRef PubMed; (h) P. Cheng, M. Zhang, T.-K. Lau, Y. Wu, B. Jia, J. Wang, C. Yan, M. Qin, X. Lu and X. Zhan, Adv. Mater., 2017, 29, 1605216 CrossRef PubMed; (i) Z. Liu, Y. Wu, Q. Zhang and X. Gao, J. Mater. Chem. A, 2016, 4, 17604 RSC; (j) S. Li, W. Liu, C.-Z. Li, F. Liu, Y. Zhang, M. Shi, H. Chen and T. P. Russell, J. Mater. Chem. A, 2016, 4, 10659 RSC.
- (a) J. S. Moon, J. Jo and A. J. Heefer, Adv. Energy Mater., 2012, 2, 304 CrossRef CAS; (b) M. T. Dang, L. Hirsch, G. Wantz and J. D. Wuest, Chem. Rev., 2013, 113, 3734 CrossRef CAS PubMed; (c) J. H. Lee, K. M. Kim, W. Jang, S. Ahn, Y. Y. Kim, O. O. Park and D. H. Wang, J. Mater. Chem. C, 2017, 5, 1106 RSC.
- (a) C. C. Chueh, C. Z. Li and A. K. Y. Jen, Energy Environ. Sci., 2015, 8, 1160 RSC; (b) Z. Hu, K. Zhang, F. Huang and Y. Cao, Chem. Commun., 2015, 51, 5572 RSC; (c) Y. Chen, S. Wang, L. Xue, Z. Zhang, H. Li, L. Wu, Y. Wang, F. Li, F. Zhang and Y. Li, J. Mater. Chem. A, 2016, 4, 19189 RSC; (d) J. G. Manion, D. Gao, P. M. Brodersen and D. S. Seferos, J. Mater. Chem. C, 2017, 5, 3315 RSC.
- (a) Z. A. Page, Y. Liu, V. V. Duzhko, T. P. Russell and T. Emrick, Science, 2014, 346, 441 CrossRef CAS PubMed; (b) C. C. Chueh, C. Z. Li and A. K. Y. Jen, Energy Environ. Sci., 2015, 8, 1160 RSC; (c) W. Jiao, D. Ma, M. Lv, W. Chen, H. Wang, J. Zhu, M. Lei and X. Chen, J. Mater. Chem. A, 2014, 2, 14720 RSC; (d) Z. Liu, X. Ouyang, R. Peng, Y. Bai, D. Mi, W. Jiang, A. Facchettic and Z. Ge, J. Mater. Chem. A, 2016, 4, 2530 RSC; (e) B. Zhang, S. Yi, G. Chen, Z. He, H.-B. Wu, W. Yang, F. Niu, J. Qu, P. Zeng and Y. Cao, J. Mater. Chem. C, 2017, 5, 4858 RSC; (f) X. Liu, R. Xu, C. Duan, F. Huang and Y. Cao, J. Mater. Chem. C, 2016, 4, 4288 RSC.
- (a) Z. He, C. Zhong, X. Huang, W.-Y. Wong, H. Wu, L. Chen, S. Su and Y. Cao, Adv. Mater., 2011, 23, 4636 CrossRef CAS PubMed; (b) Z. He, B. Xiao, F. Liu, H. Wu, Y. Yang, S. Xiao, C. Wang, T. P. Russell and Y. Cao, Nat. Photonics, 2015, 9, 174 CrossRef CAS; (c) J. D. Chen, C. Cui, Y. Q. Li, L. Zhou, Q. D. Ou, C. Li, Y. Li and J. X. Tang, Adv. Mater., 2015, 27, 1035 CrossRef CAS PubMed; (d) L. Zuo, C.-Y. Chang, C.-C. Chueh, S. Zhang, H. Li, A. K.-Y. Jen and H. Chen, Energy Environ. Sci., 2015, 8, 1712 RSC; (e) Z. Zheng, S. Zhang, M. Zhang, K. Zhao, L. Ye, Y. Chen, B. Yang and J. Hou, Adv. Mater., 2015, 27, 1189 CrossRef CAS PubMed.
- W. Chen, J. Lv, J. Han, Y. Chen, T. Jia, F. Li and Y. Wang, J. Mater. Chem. A, 2016, 4, 2169 RSC.
- (a) D. Chen, H. Zhou, M. Liu, W.-M. Zhao, S.-J. Su and Y. Cao, Macromol. Rapid Commun., 2013, 34, 595 CrossRef CAS PubMed; (b) Z. Wang, Z. Li, X. Xu, Y. Li, K. Li and Q. Peng, Adv. Funct. Mater., 2016, 26, 4643 CrossRef CAS.
- (a) Z.-G. Zhang, B. Qi, Z. Jin, D. Chi, Z. Qi, Y. Li and J. Wang, Energy Environ. Sci., 2014, 7, 1966 RSC; (b) Z. Wang, N. Zheng, W. Zhang, H. Yan, Z. Xie, Y. Ma, F. Huang and Y. Cao, Adv. Energy Mater., 2017, 1700232 CrossRef; (c) B. Russ, M. J. Robb, F. G. Brunetti, P. L. Miller, E. E. Perry, S. N. Patel, V. Ho, W. B. Chang, J. J. Urban, M. L. Chabinyc, C. J. Hawker and R. A. Segalman, Adv. Mater., 2014, 26, 3473 CrossRef CAS PubMed; (d) Z. Hu, R. Xu, S. Dong, K. Lin, J. Liu, F. Huang and Y. Cao, Mater. Horiz., 2017, 4, 88 RSC.
- (a) C.-Z. Li, C.-Y. Chang, Y. Zang, H.-X. Ju, C.-C. Chueh, P.-W. Liang, N. Cho, D. S. Ginger and A. K.-Y. Jen, Adv. Mater., 2014, 26, 626 Search PubMed; (b) N. Cho, C.-Z. Li, H.-L. Yipa and A. K.-Y. Jen, Energy Environ. Sci., 2014, 7, 638 RSC; (c) Z. G. Zhang, H. Li, Z. Qi, Z. Jin, G. Liu, J. Hou, Y. Li and J. Wang, Appl. Phys. Lett., 2013, 102, 143902 CrossRef.
- Y. Lin, Z. Zhang, H. Bai, J. Wang, Y. Yao, Y. Li, D. Zhu and X. Zhan, Energy Environ. Sci., 2015, 8, 610 Search PubMed.
- (a) Y. Lin, J. Wang, Z. G. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170 CrossRef CAS PubMed; (b) H. Bin, Z. G. Zhang, L. Gao, S. Chen, L. Zhong, L. Xue, C. Yang and Y. Li, J. Am. Chem. Soc., 2016, 138, 4657 CrossRef CAS PubMed; (c) G. Han, Y. Guo, X. Song, Y. Wang and Y. Yi, J. Mater. Chem. C, 2017, 5, 4852 RSC.
- (a) H. Tian, Y. Deng, F. Pan, L. Huang, D. Yan, Y. Geng and F. Wang, J. Mater. Chem., 2010, 20, 7998 RSC; (b) J.-D. Peltier, B. Heinrich, B. Donnio, J. R. Berthelot, E. Jacques and C. Poriel, ACS Appl. Mater. Interfaces, 2017, 9, 8219 CrossRef CAS PubMed.
- (a) C. H. Park and H. E. Simmons, J. Am. Chem. Soc., 1968, 90, 2431 CrossRef CAS; (b) Y. Matsunaga, K. Goto, K. Kubono, K. Sako and T. Shinmyozu, Chem. – Eur. J., 2014, 20, 7309 CrossRef CAS PubMed; (c) B. Russ, M. J. Robb, B. C. Popere, E. E. Perry, C.-K. Mai, S. L. Fronk, S. N. Patel, T. E. Mates, G. C. Bazan, J. J. Urban, M. L. Chabinyc, C. J. Hawker and R. A. Segalman, Chem. Sci., 2016, 7, 1914 RSC; (d) S. Guha, F. S. Goodson, L. J. Corson and S. Saha, J. Am. Chem. Soc., 2012, 134, 13679 CrossRef CAS PubMed.
- (a) T. H. Reilly, A. W. Hains, H.-Y. Chen and B. A. Gregg, Adv. Energy Mater., 2012, 2, 455 CrossRef CAS; (b) G. Xu, L. Gao, H. Xu, L. Huang, Y. Xie, X. Cheng, Y. Li, L. Chen and Y. Chen, J. Mater. Chem. A, 2017, 5, 13807 RSC.
- (a) L. Chan, Y. Tan, X. Liu and Y. Chen, Nano Energy, 2016, 27, 492 CrossRef; (b) Z. Wu, C. Sun, S. Dong, X. F. Jiang, S. Wu, H. Wu, H. L. Yip, F. Huang and Y. Cao, J. Am. Chem. Soc., 2016, 138, 2004 CrossRef CAS PubMed; (c) Z. Hu, Z. Chen, K. Zhang, N. Zheng, R. Xie, X. Liu, X. Yang, F. Huang and Y. Cao, Sol. RRL, 2017, 1, 1700055 CrossRef.
- S. Liu, G. Zhang, J. Lu, J. Jia, W. Li, F. Huang and Y. Cao, J. Mater. Chem. C, 2015, 3, 4372 RSC.
- C. K. Mai, R. A. Schlitz, G. M. Su, D. Spitzer, X. Wang, S. L. Fronk, D. G. Cahill, M. L. Chabinyc and G. C. Bazan, J. Am. Chem. Soc., 2014, 136, 13478 CrossRef CAS PubMed.
- X. Cheng, S. Sun, Y. Chen, Y. Gao, L. Ai, T. Jia, F. Li and Y. Wang, J. Mater. Chem. A, 2014, 2, 12484 RSC.
- (a) L. Cai, T. Moehl, S.-J. Moon, J.-D. Decoppet, R. Humphry-Baker, Z. Xue, L. Bin, S. M. Zakeeruddin and M. Grätzel, Org. Lett., 2014, 16, 106 CrossRef CAS PubMed; (b) L. Han, Y. Zhang, W. Chen, X. Cheng, K. Ye, J. Zhang and Y. Wang, Chem. Commun., 2015, 51, 4477 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7tc05055k |
| This journal is © The Royal Society of Chemistry 2018 |