Left- and right-circularly polarized light-sensing based on colored and mechano-responsive chiral nematic liquid crystals†
Jun
Yoshida
 *a,
Shuhei
Tamura
a,
Hidetaka
Yuge
a and
Go
Watanabe
*a,
Shuhei
Tamura
a,
Hidetaka
Yuge
a and
Go
Watanabe
 *b
*b
aKitasato University, Department of Chemistry, School of Science, Kitasato 1-15-1, Minami-ku, Sagamihara, Kanagawa 252-0373, Japan. E-mail: yoshidaj@kitasato-u.ac.jp
bKitasato University, Department of Physics, School of Science, Kitasato 1-15-1, Minami-ku, Sagamihara, Kanagawa 252-0373, Japan. E-mail: g00325@kitasato-u.ac.jp
First published on 29th November 2017
Abstract
A liquid crystal host–guest system composed of achiral organic molecules (host) and colored chiral metal complexes (guest) was fabricated to sense both right- and left-handed circularly polarized light (r- and l-CPL), depending on the guest (dopant) concentration. The CPL-sensing can be reversibly turned on and off upon mechanical stress and heating.
There is an increasing demand for the effective production and sensing of circularly polarized light (CPL), for application in optical devices, including three-dimensional imaging/holography,1–3 optical magnetic recording,4 quantum computation,5,6 and bio-inspired materials.7 Right- or left-handed CPL is optically produced by the use of a quarter waveplate and a linear polarizer, and such a system uses multiple optical elements and tends to be large-scale. Chiral materials that preferentially absorb right- or left-handed CPL (r- and l-CPL), depending on the wavelength, are preferred for the fabrication of miniature CPL sensors and detectors. Recently, CPL detectors based on semiconducting organic molecules8 and chiral plasmonic metamaterials9 have been reported. Such chiral material-based systems show promise for the development of miniature and integrated photonic devices; however, they usually require laborious preparation of both right- and left-handed materials. In addition, the wavelength of applicable CPL often depends on the absorption band of the materials used. The utilization of both right- and left-handed CPL at any wavelength is considered the next challenge for the wider application of CPL in photonic devices.
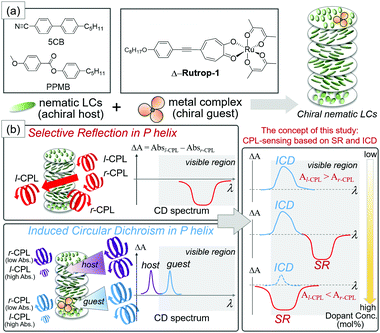
A chiral nematic liquid crystal (N*-LC) with a helicoidal structure (Fig. 1) is another useful material for producing and sensing CPL. N*-LCs with a right-handed helical structure (P helix) selectively reflect r-CPL upon the incidence of linearly polarized light (PL, Fig. 1), and vice versa for left-handed N*-LCs (M helix). The easy fabrication of N*-LCs and their thin films is promising for application in optical devices.10 Additionally, the helical pitch of N*-LCs can be tuned by applying external stimuli such as photo-irradiation, heating, and voltage.11 Thus, the wavelength of selective reflection (SR), that is the wavelength of sensing CPL, can be finely tuned. However, the sensing of both r- and l-CPLs based on N*-LCs also requires the preparation of both P and M helical structures. Although temperature-induced and photo-induced helical switching of N*-LCs has been reported,12,13 the switching is limited to special cases.
Herein, we propose the sensing of both r- and l-CPL based on a colored liquid crystal host–guest system. While N*-LCs are usually prepared by doping colorless chiral dopants (guests) with a colorless nematic liquid crystal (host) based on the requirements for optical devices, we prepared colored N*-LCs by doping colored octahedral metal complex dopants with commercially available colorless nematics. The selective reflection (SR) of CPL in N*-LCs can be monitored as a square pattern by electronic circular dichroism (CD) spectroscopy that detects the different absorptions of l-CPL and r-CPL (Fig. 1). Here, we have also focused on the induced circular dichroism (ICD) phenomenon. ICD is a signal based on the extrinsic absorption of helically arranged molecules.14–20 In our case, ICD signals based on host nematics and guest dopants can be observed in the ultraviolet and visible light regions, respectively. ICD and SR yield CD signals with opposite signs (Fig. 1) when λ0 (the reflective wavelength of the helical pitch band) is longer than λab (the wavelength of the absorption band).16 Hence, CD patterns are potentially “programmable” from the combination of ICD and SR, as schematically shown in Fig. 1(b). Saeva and co-workers reported the co-existence of SR (UV region) and ICD (visible region) bands in their pioneering work on the CD spectrum of an N*-LC sample.16 However, SR and ICD phenomena have been treated independently. One reason is that the doping of N*-LCs with dyes (usually achiral) disturbs the formation of the helicoidal structure and leads to the extension of the helical pitches.21
A red-colored ruthenium complex with Δ,Λ chirality (Δ,Λ-Rutrop-1, Fig. 1) was used in this study as a chiral dopant in combination with two nematic (N) liquid crystals, 4-cyano-4′-pentylbiphenyl (5CB) and 4-pentylphenyl-4-methoxybenzoate (PPMB). Δ,Λ–Rutrop-1 has been recently developed by us as a chiral dopant with strong absorption in the visible region (ε = ∼20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 at 530 nm in chloroform), high helical twisting powers (HTPs), and acceptable miscibility with nematics, in order to investigate dopant–host interactions by Raman spectroscopy.22 Such properties in Δ,Λ-Rutrop-1 can also be used for the induction of ICD in the visible light region.
000 at 530 nm in chloroform), high helical twisting powers (HTPs), and acceptable miscibility with nematics, in order to investigate dopant–host interactions by Raman spectroscopy.22 Such properties in Δ,Λ-Rutrop-1 can also be used for the induction of ICD in the visible light region.
Fig. 2(a) shows the CD spectra of a binary mixture of 5CB and ∼0.04 mol% enantiomeric Rutrop-1, injected in a homogeneous cell with 25 μm gap (Grandjean state). The mixture exhibits a strong signal below 350 nm, attributed to ICD based on the helical arrangements of the 5CB host molecules, while it also exhibits broad positive signals ranging from 350 to 550 nm. Considering that Δ-Rutrop-1 in chloroform solution exhibits both positive and negative CD signals in the range of 350–550 nm (Fig. 2(b)), the only positive signal in the region for a Δ-Rutrop-1/5CB mixture is attributed to ICD based on the guest Δ-Rutrop-1. A Δ-Rutrop-1/PPMB sample (∼0.04 mol%) also shows positive ICD in the region of 350–550 nm, and vice versa, for a Λ-isomer sample (Fig. S1, ESI†). The effect of linear dichroism was found to be negligible in judging the sign of ICD from the measurements with the samples rotated 90 degrees (Fig. S1 and S2, ESI†). The pitch length of Δ-Rutrop-1/5CB (0.04 mol%) was determined to be ∼25 μm based on the Cano method.11,23 Hence, SR cannot be observed in the visible region at this dopant concentration. The wavelength of selective reflection (λsr) is represented by λsr = np![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α, where n, p, and α represent the average refractive index (∼1.5 for 5CB at RT), the helical pitch, and the angle at which the helicoidal structures are viewed, respectively.
α, where n, p, and α represent the average refractive index (∼1.5 for 5CB at RT), the helical pitch, and the angle at which the helicoidal structures are viewed, respectively.
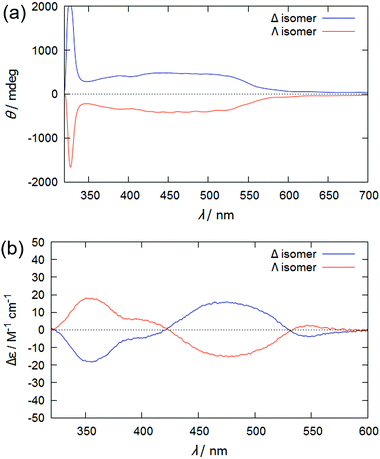 | ||
| Fig. 2 CD spectra of (a) a binary mixture of Rutrop-1 (∼0.04 mol%) and 5CB, and (b) Rutrop-1 in chloroform. In (a), the samples were injected into a homogeneous alignment cell of 25 μm thickness. | ||
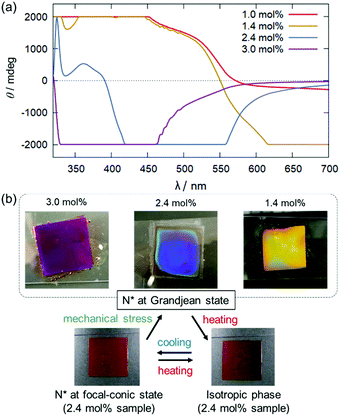
To achieve the co-existence of SR and ICD in the visible light region, binary mixtures with higher dopant concentrations (1.0, 1.4, 2.4, and 3.0 mol% for Δ-Rutrop-1/5CB; and 0.6, 1.2. 1.8, and 2.4 mol% for Δ-Rutrop-1/PPMB) were prepared. The samples were sandwiched between a glass slide and a cover glass with no surface treatment and their CD spectra were measured (Fig. 3 and Fig. S3–S8, ESI†).‡ The samples were set to be in a Grandjean state by mechanical stress as described in the following section. At 1.0 mol%, SR is still not observed in the visible light wavelength, while the SR band is observed at ∼1200 nm by transmission spectroscopy (Fig. S9, ESI†). In contrast, a huge positive band is observed in the range of 400–550 nm (red line in Fig. 3), which is attributed to ICD signals based on ruthenium complex dopants. In the 1.4 mol% sample, negative saturated CD signals attributed to SR appear from 550 to 750 nm.§ The SR of the right-handed helix corresponds to pseudo-absorption of right-handed CPL, affording negative CD signals. The SR phenomenon was also confirmed by transmission and reflection spectroscopy (Fig. S9–S11, ESI†). While the CD signals saturated at 1.4 mol% with our equipment, they were found to be significant in the wavelength region longer than 340 nm by monitoring the voltage applied to the photomultiplier tube (Fig. S4, ESI†). When the dopant concentration increases to 2.4 mol%, strong negative and small positive CD signals are observed in the region of 400–550 nm and in the region below 400 nm, respectively. The SR band is proposed to shift to a shorter wavelength because of the shortened pitch length upon the increase of dopant concentration. The pseudo-absorption of r-CPL (selective reflection) is larger than the actual absorption of l-CPL, affording a negative CD signal. In the 3.0 mol% sample,¶ only the negative band is observed at a further blue-shifted region (Fig. 3), reflecting further shortening of the pitch length. Blue phases are observed at higher dopant concentrations (3.5 and 4.0 mol%).22 Comparison of the 1.0, 1.4, 2.4, and 3.0 mol% samples clearly indicates that the inversion of CD signals occurs just by changing the dopant concentration. Similar CD signal changes are also observed in the Δ-Rutrop-1/PPMB system, upon the increase of dopant concentration (Fig. S7, ESI†). In addition, the use of the Λ isomer of Rutrop-1 certainly affords inverted ICD and SR signals (Fig. S8, ESI†). Although concentration-dependent circularly polarized luminescence has been recently reported by Ito and co-workers,24 concentration-dependent sensing of opposite-handed CPL has not yet been established. Our host–guest methodology of combining ICD and SR can also avoid the time-consuming and laborious preparation of both enantiomers.
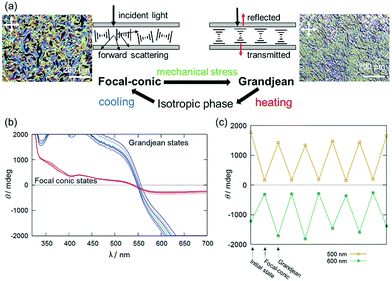
During the course of this study, we found that Δ-Rutrop-1/5CB mixtures are mechano-responsive at least for the samples containing more than 1.0 mol% dopants. The Δ-Rutrop-1/5CB (1.4 mol%) mixture sandwiched by a glass slide and a cover glass exhibited a focal conic texture with randomly distributed helicoidal domains (Fig. 3(b), 4(a) and Fig. S7, ESI†), when the sample was cooled from the isotropic phase. The focal-conic state was stable under ambient conditions, while it easily turned into a Grandjean texture with a planar alignment upon application of mechanical stress (just by pushing with a spatula, Fig. 3(b) and 4). The Grandjean texture changed back to the focal-conic texture again upon heating (Fig. S12 and a movie file in the ESI†). Responding to the reversible focal-conic/Grandjean transformation, the Rutrop-1/5CB system exhibits enhanced and diminished CD signals in the visible light region. In the focal conic state of Δ-Rutrop-1/5CB (1.4 mol%), the SR and ICD signals are not clearly observed, or are weak (red lines in Fig. 4(b)). In contrast, enhanced CD signals appear upon the application of mechanical stress (blue lines in Fig. 4(b)). The reversibility was confirmed at least five times (Fig. 4(c)). The appearance/disappearance of the ICD and SR signals could be explained by forward light scattering in the focal-conic state and Bragg reflection in the Grandjean state (Fig. 4(a)).
The response to mechanical and shear stresses is widely known in N*-LCs.25–31 However, the composition and dopant concentrations of N*-LCs that exhibit mechano-responses are known only empirically. Thus, mechano-response has not been applied to control and switching of CD signals in N*-LCs. The modulation of ICD signals by changing the sample states (focal conic and Grandjean states) is limited to the report by Saeva and co-workers,16 to the best of our knowledge. Additionally, the mechano-responsive properties were observed in the sample just sandwiched by two glasses. There is no necessity for surface alignment in the system and it is hence an accessible method for switching CD signals.
Conclusions
A liquid crystal host–guest system that can sense both right- and left-handed circularly polarized light depending on the dopant concentration was fabricated based on chiral nematic liquid crystals, focusing on selective reflection (SR) and induced CD (ICD) phenomena. Our system needs only one side of chiral dopants (Δ or Λ isomer) for sensing the opposite CPLs. The CD signal intensity is also controllable by a mechanical stress/heating process, while it remains qualitative. Although SR, ICD, and mechano-responses are each a well-known phenomenon in N*-LCs, a combination of the three properties is applied to sense CPL and control CD spectra for the first time. A key material in this approach is the guest Rutrop-1 that exhibits high helical twisting powers, good miscibility with nematic liquid crystals, and strong absorption in the visible light region. Upon the increase of the guest (dopant) concentration, the SR band is blue-shifted and the resultant CD signal undergoes inversion. Colored dopants have been considered unfavorable in terms of optical applications, whereas they are essential in our methodology. Since the absorption band of chiral octahedral metal complexes as dopants are controllable in the visible and even near-infrared regions,32 we believe that colored N*-LCs are promising materials for sensing both handed CPLs and the “programming” of CD signals (quantitative sensing) as a future study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Kitasato University Research Grant for Young Researchers, the Futaba Denshi Foundation, and a JSPS KAKENHI Grant-in-Aid for Young Scientists (B), with Grant Numbers 16K21353 and 17K14465. We thank Ms H. Watanabe (Kitasato University) for her help in the CD measurements.Notes and references
- D. Sheen, D. McMakin and T. Hall, Appl. Opt., 2010, 49, E83–E93 CrossRef PubMed.
- K.-S. Bae, U. Cha, Y.-K. Moon, J. W. Heo, Y.-J. Lee, J.-H. Kim and C.-J. Yu, Opt. Express, 2012, 20, 6927–6931 CrossRef CAS PubMed.
- L. Huang, X. Chen, H. Mühlenbernd, H. Zhang, S. Chen, B. Bai, Q. Tan, G. Jin, K.-W. Cheah, C.-W. Qiu, J. Li, T. Zentgraf and S. Zhang, Nat. Commun., 2013, 4, 2808 Search PubMed.
- C. D. Stanciu, F. Hansteen, A. V. Kimel, A. Kirilyuk, A. Tsukamoto, A. Itoh and T. Rasing, Phys. Rev. Lett., 2007, 99, 47601 CrossRef CAS PubMed.
- J. F. Sherson, H. Krauter, R. K. Olsson, B. Julsgaard, K. Hammerer, I. Cirac and E. S. Polzik, Nature, 2006, 443, 557–560 CrossRef CAS PubMed.
- E. Togan, Y. Chu, A. S. Trifonov, L. Jiang, J. Maze, L. Childress, M. V. G. Dutt, A. S. Sørensen, P. R. Hemmer, A. S. Zibrov and M. D. Lukin, Nature, 2010, 466, 730–734 CrossRef CAS PubMed.
- T.-H. Chiou, S. Kleinlogel, T. Cronin, R. Caldwell, B. Loeffler, A. Siddiqi, A. Goldizen and J. Marshall, Curr. Biol., 2008, 18, 429–434 CrossRef CAS PubMed.
- Y. Yang, R. C. da Costa, M. J. Fuchter and A. J. Campbell, Nat. Photonics, 2013, 7, 634–638 CrossRef CAS.
- W. Li, Z. J. Coppens, L. V. Besteiro, W. Wang, A. O. Govorov and J. Valentine, Nat. Commun., 2015, 6, 8379 CrossRef CAS PubMed.
- Handbook of Liquid Crystals, ed. J. W. Goodby, C. Tschierske, P. Raynes, H. Gleeson, T. Kato and P. J. Collings, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2014 Search PubMed.
- Chirality in Liquid Crystals, ed. H.-S. Kitzerow and C. Bahr, Springer, New York, 2001 Search PubMed.
- H. Hayasaka, T. Miyashita, M. Nakayama, K. Kuwada and K. Akagi, J. Am. Chem. Soc., 2012, 134, 3758–3765 CrossRef CAS PubMed.
- N. Katsonis, E. Lacaze and A. Ferrarini, J. Mater. Chem., 2012, 22, 7088–7097 RSC.
- H. de Vries, Acta Crystallogr., 1951, 4, 219–226 CrossRef CAS.
- F. D. Saeva and J. J. Wysocki, J. Am. Chem. Soc., 1971, 93, 5928–5929 CrossRef CAS.
- F. D. Saeva, P. E. Sharpe and G. R. Olin, J. Am. Chem. Soc., 1973, 95, 7656–7659 CrossRef CAS.
- W. H. Pirkle and P. L. Rinaldi, J. Org. Chem., 1980, 45, 1379–1382 CrossRef CAS.
- M. Sisido, H. Narisawa, R. Kishi and J. Watanabe, Macromolecules, 1993, 26, 1424–1428 CrossRef CAS.
- S. Allenmark, Chirality, 2003, 15, 409–422 CrossRef CAS PubMed.
- H. Qi, J. O’Neil and T. Hegmann, J. Mater. Chem., 2008, 18, 374–380 RSC.
- M. Nakata, Y. Takanishi, J. Watanabe and H. Takezoe, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2003, 68, 41710 CrossRef PubMed.
- J. Yoshida, S. Tamura, G. Watanabe, Y. Kasahara and H. Yuge, Chem. Commun., 2017, 53, 5103–5106 RSC.
- I. I. Smalyukh and O. D. Lavrentovich, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2002, 66, 51703 CrossRef CAS PubMed.
- S. Ito, K. Ikeda, S. Nakanishi, Y. Imai and M. Asami, Chem. Commun., 2017, 53, 6323–6326 RSC.
- J. M. Pochan and P. F. Erhardt, Phys. Rev. Lett., 1971, 27, 790–791 CrossRef CAS.
- D. S. Parmar, Rev. Sci. Instrum., 1991, 62, 1596–1608 CrossRef CAS.
- M. Warner, E. M. Terentjev, R. B. Meyer and Y. Mao, Phys. Rev. Lett., 2000, 85, 2320–2323 CrossRef CAS PubMed.
- C. Pujolle-Robic and L. Noirez, Nature, 2001, 409, 167–171 CrossRef CAS PubMed.
- P. V. Shibaev and C. Schlesier, Appl. Phys. Lett., 2012, 101, 193503 CrossRef.
- I. Lelidis, G. Barbero and A. L. Alexe-Ionescu, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2013, 87, 22503 CrossRef CAS PubMed.
- N. Tanaka and Y. Fukushima, J. Photopolym. Sci. Technol., 2014, 27, 719–721 CrossRef.
- J. Yoshida, K. Kuwahara, K. Suzuki and H. Yuge, Inorg. Chem., 2017, 56, 1846–1856 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7sm01975k |
| ‡ The samples were also injected into a homogeneous 5 μm cell (Fig. S6, ESI†), while more saturated signals were obtained compared to the samples just sandwiched by glass slides. |
| § The maximum scale measurable in our CD equipment (JASCO J-720) is ±2000 mdeg. |
| ¶ The color of the 3 mol% cell looks purple due to the reflection of light around 400 nm and the absorption of red-colored ruthenium dopants. |
| This journal is © The Royal Society of Chemistry 2018 |