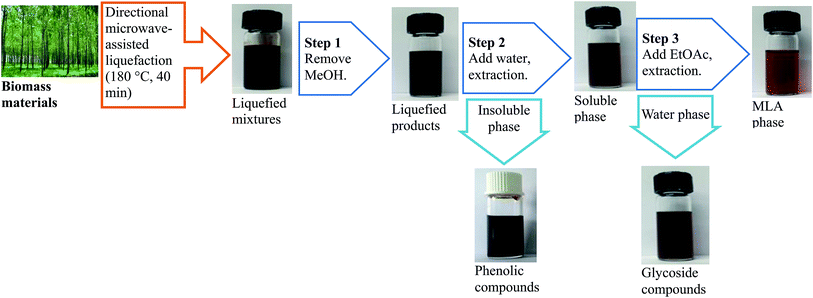
Selective catalytic conversion of waste lignocellulosic biomass for renewable value-added chemicals via directional microwave-assisted liquefaction†
Junfeng
Feng
 ab,
Jianchun
Jiang
a,
Chung-yun
Hse
b,
Zhongzhi
Yang
a,
Kui
Wang
ac,
Jun
Ye
a and
Junming
Xu
*a
ab,
Jianchun
Jiang
a,
Chung-yun
Hse
b,
Zhongzhi
Yang
a,
Kui
Wang
ac,
Jun
Ye
a and
Junming
Xu
*a
aInstitute of Chemical Industry of Forest Products, Chinese Academy of Forestry, Nanjing 210042, China. E-mail: jiangjc@icifp.cn
bUnited States Department of Agriculture (USDA) Forest Service, Southern Research Station, Pineville, Louisiana 71360, USA. E-mail: chse@fs.fed.us
cResearch Institute of Forestry New Technology, Chinese Academy of Forestry, Beijing 100091, China. E-mail: finechemistry@163.com
First published on 22nd February 2018
Abstract
Selective catalytic conversion of biomass waste for producing methyl levulinate (MLA) via directional microwave-assisted liquefaction was investigated. The goal of the study was to develop a directional liquefaction process using dielectric heating with microwave energy. The methanolysis of biomass into methyl levulinate was studied in the presence of several acid catalysts. The C6 sugar substrates in biomass were successfully converted into methyl levulinate under the optimized conditions (180 °C, 40 min) with a yield of 29.39 wt%. 5-Hydroxymethyl furfural, glucose, fructose, cellobiose, corn starch, and microcrystalline cellulose were selected as models for directional microwave-assisted liquefaction. Therefore, the possible reaction pathway of biomass to methyl levulinate could be investigated. The selective catalytic conversion of biomass was found to be highly efficient for the generation of MLA (reaching a maximum yield of approximately 30 wt%), higher than the levulinic acid yield (14 wt%) in aqueous solution under the same reaction conditions. The results suggested that directional microwave-assisted liquefaction is an effective method that can produce a high value-added fuel additive (methyl levulinate) from lignocellulosic biomass under designated reaction processes.
1. Introduction
Recently, renewable and abundant lignocellulosic biomass has attracted extensive attention for producing sustainable bio-fuels and bio-chemicals due to the depletion of fossil resources and the deterioration of the environment.1 Lignocellulosic biomass waste is one of the most abundant biomass sources and is available worldwide and mainly consists of hemicelluloses, cellulose and lignin. Biomass has the advantage of being renewable and can be used to produce hydrocarbons, high value-added chemicals and fine chemicals, such as alkanes, HMF, alditols, etc.2 Among these, one popular product is levulinic acid (LA) converted from carbohydrates, which has wide applications in food, medicine, agriculture, cosmetic, and fuel industries. However, the production of LA is limited by its ability to corrode most equipment used for its manufacture, and the necessary complexity of the separation equipment. As an alternative to LA, methyl levulinate (MLA) has shown better performance in the production and separation processes because it is non-corrosive and has a lower boiling point (about 190 °C), consuming less energy for the separation process. MLA is as useful as LA in many fields, such as medicine, solvents, organic chemistry, and the cosmetic industry.3 MLA contains short chain fatty esters with numerous potential applications such as a fuel additive to improve petroleum and diesel stability, and provide high lubricity, flashpoint stability, non-toxicity, low-temperature fluidity, and better flow properties under cold conditions.4 The U.S. Department of Energy and Pacific Northwest National Laboratory (PNNL)5 reported that levulinate esters are important chemicals and one of the recognized chemical building blocks that can be produced from carbohydrates in biomass.Some studies have reported catalytic methods for converting biomass models, such as furfuryl alcohol, fructose, glucose, cellobiose, starch, and cellulose into levulinate esters. Huang et al. researched the performance of Al2(SO4)3 for furfuryl alcohol alcoholysis, and obtained an 80.6% yield of MLA after several minutes under microwave irradiation.6 Hu et al. investigated an efficient catalyst for producing MLA from glucose, and achieved a maximum MLA yield of 52% using Amberlyst 70 catalyst in DMM/methanol solvent.7 Ding et al. studied the alcoholysis of cellulose into MLA with several acid catalysts and reached an MLA yield as high as 56%.8 The yields of levulinate esters have therefore improved, but the cost of the raw materials has also increased. Most studies have used biomass models as reactants rather than actual biomass with the recalcitrant crystalline structure of cellulose. However, biomass model studies were very important for establishing the optimal conditions and converting the lignocellulosic biomass into high-quality MLA. Therefore, the direct production of levulinate esters from the alcoholysis of real lignocellulosic biomass is of interest.
The conventional methods of producing MLA derived from lignocellulosic biomass involve three steps: producing glucose or 5-hydroxymethyl furfural (HMF) by the hydrolysis of cellulose in water,9 generating levulinic acid (LA) through dehydration and rearrangement of glucose or HMF,10 and producing MLA by esterification of LA with methanol. These reactions have some drawbacks such as low MLA yield, a long reaction route, and a considerable amount of energy needed to remove water during the LA separation process. There are many differences between models (cellulose, glucose, and fructose) and lignocellulosic biomass. Biomass has a complex lignocellulosic structure mainly consisting of strong chemical linkages between hemicellulose and lignin, and the recalcitrant crystalline structure of cellulose, which means that the conversion and separation process is more difficult than those of fructose, glucose, cellobiose, starch, and cellulose.
To overcome these problems, our study focuses on directional microwave-assisted liquefaction of lignocellulosic biomass into MLA by an acid-catalyzed reaction in subcritical methanol. In this paper, we studied the conversion of lignocellulosic biomass with highly recalcitrant cellulose and part hemicellulose to products with microwave irradiation and alcohol. Microwave irradiation is a dielectric heating process that has been used in heating and depolymerization of the biomass material in our previous study,11 accelerating the kinetics of organic synthesis.12 Microwave irradiation can directly cleave the hydrogen and glycosidic bonds in biomass, promoting the breakdown between its monomeric units such as methyl glucoside. Microwave energy can replace conventional thermal conversion due to its rapid and uniform heating13 which leads to better economic feasibility, shorter reaction time, and energy efficiency.14
Our previous studies found that using alcohols as solvents for biomass conversion has many advantages.15 For example, alcohols can suppress humin formation, minimize wastewater discharge, and allow high-quality products to be easily isolated by fractionation. Another advantage is that alcohols are able to readily dissolve high molecular-weight products that are derived from biomass,16 which could prevent the re-polymerization and re-condensation of liquefied products. The products formed in alcohols were more stable than the products formed in water, and the alcoholysis of biomass is more efficient than its hydrolysis.
The directional microwave-assisted liquefaction of bamboo to produce MLA is an alternative way that can effectively utilize lignocellulosic biomass. In this investigation, the influence of different process parameters, including catalyst type and amount, and reaction temperature and time, was studied so that we could optimize the degradation of bamboo in methanol and obtain the highest possible MLA yield. It was also found that high yields of MLA were generated from directional microwave-assisted liquefaction of bamboo using low concentrations of sulfuric acid in subcritical methanol. In addition to the directional liquefaction in methanol, the liquefaction of biomass and different models of carbohydrates in water has also been investigated to study the formation process of MLA. We believe that the liquefaction process could be efficiently promoted with microwave energy due to the direct conversion of electromagnetic energy into heat at the molecular level. Finally, the mechanisms of the cleavage of the molecular bonds in biomass using directional microwave-assisted liquefaction were proposed based on the results of GC-MS analysis. Overall, this selective catalytic strategy is an economical and efficient method for converting lignocellulosic biomass into high value-added chemicals.
2. Experimental
2.1. Chemicals
Bamboo was collected from the Kisatchie National Forest in Pineville, Louisiana, USA. Five other types of waste lignocellulosic biomass were used in this study: poplar, pine, eucalyptus, bagasse, and straw which were collected from a farm (Jiangsu, China). Methyl levulinate (MLA), 5-hydroxymethyl furfural (HMF), methyl glucoside (MLG), fructose, glucose, and cellobiose were purchased from Aladdin Reagents (99.9% purity). Corn starch and microcrystalline cellulose (MCC) were obtained from Sinopharm Chemical Reagents (Beijing, China). The materials (e.g. corn starch, microcrystalline cellulose and biomass) were kept at 105 °C overnight and screened for particles within the size range of 0.3–0.425 mm (40–50 mesh). The elemental and compositional properties of raw biomass samples are presented in Table 1. All the other chemicals in the study were of analytical grade, commercially available and used without further purification.| Materials | Elemental analysis (wt%) | Compositional analysis (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | Oa | N | S | Ashb | Extractivesc | Cellulosed | Lignine | Holocellulosef | Pentosang | |
| a O content was calculated by mass difference. b According to ASTM D 1102-84 (ASTM 2007). c According to ASTM D 1107-96 (ASTM 2007). d According to ASTM D 1102-84 (ASTM 2007). e According to ASTM D 1106-96 (ASTM 2007). f According to ASTM D 1103-60 (ASTM 2007). g According to ASTM D 1104-56 (ASTM 2007). | |||||||||||
| Bamboo | 48.46 | 4.23 | 46.94 | 0.08 | 0.29 | 1.20 | 3.73 | 43.69 | 24.58 | 71.23 | 26.09 |
| Eucalyptus | 48.50 | 4.20 | 47.07 | 0.03 | 0.20 | 0.35 | 2.54 | 41.70 | 23.39 | 72.94 | 19.21 |
| Straw | 45.71 | 4.88 | 48.96 | 0.10 | 0.35 | 5.21 | 2.63 | 43.45 | 21.46 | 70.89 | 21.92 |
| Bagasse | 40.09 | 5.02 | 54.70 | 0.06 | 0.12 | 2.32 | 2.58 | 44.23 | 23.01 | 72.01 | 19.65 |
| Poplar | 48.18 | 4.33 | 47.25 | 0.05 | 0.19 | 0.68 | 2.21 | 39.90 | 25.69 | 71.46 | 25.12 |
| Pine | 49.20 | 4.34 | 46.22 | 0.04 | 0.20 | 0.32 | 3.10 | 40.34 | 26.75 | 69.93 | 14.83 |
2.2. Directional microwave-assisted liquefaction
In a typical operation, liquefaction was carried out in a Milestone (Shelton, CT) microwave laboratory system equipped with 100 mL sealed Teflon reaction vessels. For each run, four vessels were put in the oven and the maximum microwave output power was set as 700 W. This microwave system was especially designed for microwave degradation and composed of delivered microwave power. The automatic temperature in this system was controlled with an internal temperature sensor (fiber optic sensor), and an ASM-400 magnetic stirrer for homogenous mixing of the sample. The operating pressure was 0–100 bar and the operating temperature was 0–300 °C. The temperature was increased from room temperature to the desired temperature within 5 min under 700 W as the starting microwave power. The temperature was monitored using an ATC-400FO automatic fiber optic temperature control system. The reaction temperature was controlled at 120–200 °C and then kept constant for a certain time (20–40 min) with the microwave power at 700 W. The reaction mixtures consisted of 2.0 g of biomass materials, 16.0 g of liquefied solvent, and 0.1–0.6 g of acid catalyst and were mixed with ultrasound and stirred before the liquefaction. After microwave-assisted liquefaction (with magnetic stirring), the vessels were cooled to room temperature in a water bath before opening. The gaseous products were vented because their yields were negligible. Then, the liquefied and solid products were filtered (Fig. S1†). The solid products were washed with 20 mL methanol several times, dried at 105 °C for 12 h, and weighed to calculate the yield of the residue. The filtrate was then evaporated at 40 °C under vacuum to remove the methanol from the liquefied product. The composition and content of liquefied products were analyzed by gas chromatography-mass spectrometry (GC-MS), GC, and high performance liquid chromatography (HPLC).2.3. Overall experimental design
Directional microwave-assisted liquefaction is a process focusing on the effective utilization of the cellulose and hemicellulose components in biomass. In this respect, we first demonstrated the possibility of acid catalysts for producing the target product under microwave energy. Different reaction parameters were studied to determine the influence of process variables on the conversion of biomass and the yield of the target product. After that, the different species of biomass were also tested for the adaptability of the microwave-assisted liquefaction process.The design of reaction parameters involved four factors (methanol amount, catalyst loading, reaction temperature and time). We used sulfuric acid in our previous study and found that it had favorable effects on the liquefaction of biomass.20 Therefore, we included sulfuric acid in this research. Several different acid catalysts including strong acids (i.e., HCl, HNO3, NH2SO3H, and C7H7SO3H), an organic acid (formic acid), solid acids (HZSM-5 and HBEA), ionic liquids ([BMIM]HSO4 and [HSO3-BMIM]HSO4) and heteropolyacids (H4SiW12O40 and H3PW12O40) were also investigated as catalysts in this microwave-assisted liquefaction process. Since 200 °C was the temperature employed in our previous research and it has been adequately proved that most of the biomass materials can be converted into liquefied products. We used a lower temperature of 120 °C as the lowest one in this investigation. We covered an 80 °C temperature range and performed experiments at 120, 140, 160, 180, and 200 °C in this study. We also used shorter reaction times such as 20, 30, 35, 40, 45, and 50 min in order to find the optimal reaction conditions. The design of the overall experiments in the directional microwave-assisted liquefaction is shown in Table 2.
| Materials | Catalysts | Solvents | Reaction conditions |
|---|---|---|---|
| a Other biomass included poplar, pine, eucalyptus, bagasse, and straw. b Biomass models included 5-hydroxymethyl furfural, glucose, fructose, methyl glucoside, cellobiose, corn starch, and microcrystalline cellulose. | |||
| Bamboo | H2SO4, C7H7SO3H, H3PW12O40, etc. | Methanol | Temperatures of 120, 140, 160, 180, and 200 °C. The reaction times were 20, 30, 35, 40, 45 and 50 min |
| Other biomassa | Only H2SO4, which is the most cost effective with good reactivity | Methanol | Repeated three times for each type of method using optimal reaction conditions from bamboo liquefaction |
| Biomass modelsb | Only H2SO4, which is the most cost effective with good reactivity | Methanol | Temperatures of 120, 140, 160, and 180 °C |
| Bamboo and models | H2SO4, C7H7SO3H, and H3PW12O40 | Water | Under optimal reaction conditions from bamboo liquefaction |
2.4. Analytical methods
Gas chromatography mass spectrometry (GC-MS) (Agilent 5975C VL MSD) analysis was undertaken using a capillary column with a 0.05 μm film thickness. Helium was used as the carrier gas at a flow rate of 1.5 mL min−1, and the following temperature program was applied: starting temperature of 30 °C for 5 min, and heating at a rate of 5 °C min−1 to a final temperature of 220 °C for 20 min. The MS detector was operated in the electron ionization mode (70 eV) with an ionization temperature of 200 °C. The mass spectra were recorded in electron ionization mode for m/z 50–550. Typically, 0.2 μL of sample was used.The levulinates and furfurals in the liquefied products were quantitatively analyzed by GC using a flame ionization detector. The levulinates and furfurals were separated on a capillary column (L 30 m, i. d. 0.32 mm, and film thickness 0.25 μm) with a programmed temperature range of 30–230 °C using nitrogen as the carrier gas. The internal standard was n-octanol. The quantitative analysis of glycosides was carried out using a HPLC instrument (Shimadzu LC-10ATVP) with an Aminex HPX-87H column and a RID-20A detector. The mobile phase was 0.005 mmol sulfuric acid (sonication and deaeration) in water with a flow rate of 0.5 mL min−1, and the column temperature was maintained at 45 °C.
The chemical structures of liquefied products were determined by GC-MS. GC was used to investigate the absolute contents of levulinates and furfurals in the liquefied product by comparison with an authentic n-octanol reference sample. The corresponding peak area ratio of MLA and n-octanol reflected the content ratio according to the standard curve (y = 2.06543x − 0.02107, coefficient of correlation (R2) = 0.99998). And the corresponding 5-MMF and n-octanol peak area ratio reflected the content ratio according to the standard curve (y = 1.9657x + 0.0612, coefficient of correlation (R2) = 0.9996).
Eqn (1) was used to calculate the liquefaction product yield from bamboo (on a weight basis), and eqn (2) was used to calculate the MLA yield from bamboo. Eqn (3) was used to measure the main byproduct (5-methoxymethyl furfural: 5-MMF and methyl glucoside: MLG) yields.
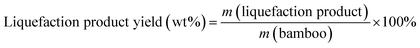 | (1) |
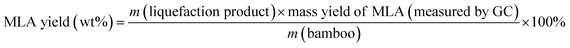 | (2) |
Byproduct yield (wt%):
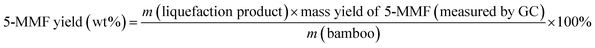 | (3) |
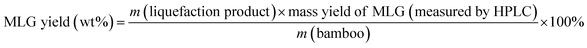 | (4) |
3. Results and discussion
The catalytic dehydration of non-edible carbohydrates to methyl levulinate is a particularly attractive approach for carbohydrate-based biomass conversion. Lignocellulosic biomass usually has poor reactivity and solubility in traditional organic solvents because of the high-degree of polymerization. This difficulty arises because the highly crystalline microfiber structure of cellulose is linked via hemicellulosic tethers to form the hemicellulose–cellulose network structure. During the liquefaction, methanol was essential for the decomposition of carbohydrates. Methanol was expected to readily dissolve the polar products derived from biomass due to their low dielectric constants, which could efficiently prevent the re-condensation and re-polymerization of liquefied products. The hydroxyl function as the nucleophile in methanol can attack the electrophilic C adjacent to the glycosidic bonds with the electron moving to the oxonium ion, which creates good leaving groups and neutral hydroxyl groups by breaking the C–O bonds. The acid catalyst can provide reactive protons (hydrogen ions (H+)) which could activate the oxygen atoms in the glycosidic bonds. Therefore, we introduced methanol and various kinds of acid catalysts into the directional microwave-assisted liquefaction of lignocellulosic biomass.The effects of different process parameters, including different catalysts, catalyst amounts, reaction temperatures, and times, were investigated to determine the influence of process variables on the yields of MLA, and optimize the degradation of biomass in subcritical methanol medium at the optimal catalyst concentration. In general, high temperature may contribute to the acceleration of the liquefaction reaction rate and the enhancement of biomass conversion efficiency. In the present experiment, the reaction temperature was varied in 20 °C increments from 120 °C to 200 °C. In order to find the optimal reaction conditions, the effects of different reaction times on the liquefaction process were investigated. Afterwards, different biomass materials (bamboo, bagasse, eucalyptus, pine, poplar, and straw) were tested for the adaptability of the microwave-assisted liquefaction process. In order to propose the liquefaction pathway for acid-catalyzed conversion of biomass to MLA in methanol, a series of biomass carbohydrates such as fructose, glucose, cellobiose, corn starch, and microcrystalline cellulose were selected as experimental substrates.
3.1. Effect of various acid catalysts
The acid catalyst can provide protons for the cleavage of glycosidic bonds that are the dominant linkages between the monosaccharide units of cellulose and hemicellulose in biomass. We used sulfuric acid in our previous studies and found that it had a good effect on the conversion of liquefied biomass. Our previous results suggested that most linkages can be methanolyzed in an acid catalyst and release monosaccharide derivatives such as methyl glucoside.15Different acidic catalysts including some liquid strong acids (i.e., HNO3, HCl, and H2SO4), several solid strong acids (C7H7SO3H and NH2SO3H), heteropolyacids (H4SiW12O40 and H3PW12O40), solid acidic zeolites (HBEA and HZSM-5), and an organic acid (HCOOH) were identified as catalysts at an amount of 0.4 g. According to the color of the residue, we determined the degree of the liquefaction reaction of the bamboo. The little yields of methyl levulinate (0.45 wt% and 2.57 wt%) over HBEA and HZSM-5 were reasonable due to their small pore size (less than 1 nm), which will lead to mass transfer limitations and coke formation. The results of C7H7SO3H, H3PW12O40, and H4SiW12O40 showed that they are also strong enough to depolymerize the holocellulose into MLA and byproducts (MLG and furfurals) in subcritical methanol with microwave-assistance. The performance of H2SO4 was much better than these heteropolyacids. Table 3 shows that H2SO4 is the most efficient catalyst with a high conversion of 85.67 wt% and an MLA yield of 24.95 wt%. This result indicates that H2SO4 could provide the strongest acid sites during the directional microwave-assisted liquefaction process. It is speculated that the higher boiling point and stronger acidity of sulfuric acid contribute to its efficiency. Other liquid acids (HCl and HNO3) performed poorer, and HCOOH was the poorest performing catalyst in this liquefaction experiment.
| Entry | Catalysts | Liquefied product color | Residue colorb | Conv. (wt%) | MLA yieldc (wt%) | Byproduct yield (wt%) | |
|---|---|---|---|---|---|---|---|
| Furfuralsd | MLGe | ||||||
| a Reaction conditions: bamboo 2.0 g, methanol 16.0 g, acid catalyst 0.4 g, 180 °C, 30 min. Biomass samples were irradiated for 5 min under 700 W. b The residue was from the liquefied solid that was washed with methanol for several times and dried at 105 °C for 12 h. c MLA: methyl levulinate (MLA yields were based on GC analysis with an internal standard method). d Furfurals: 5-methoxymethyl furfural and furfural (furfural yields were based on GC analysis with an internal standard method). e MLG: methyl pentose glucoside and methyl hexose glycoside (MLG yields were based on HPLC analysis with an external standard method). | |||||||
| 1 | Blank | Pale brown | Gray | 6.45 | — | 0.67 | 1.34 |
| 2 | HCl | Pale brown | Gray | 24.35 | 2.47 | 3.18 | 4.22 |
| 3 | HNO3 | Pale brown | Pale brown | 50.02 | 7.59 | 10.24 | 9.32 |
| 4 | H2SO4 | Brown | Brown | 85.67 | 24.95 | 23.31 | 20.75 |
| 5 | HCOOH | Pale brown | Brown | 13.22 | 0.32 | 0.47 | 0.53 |
| 6 | H3PW12O40 | Brown | Brown | 75.28 | 15.30 | 13.20 | 16.46 |
| 7 | H4SiW12O40 | Brown | Brown | 82.30 | 17.22 | 15.41 | 17.09 |
| 8 | NH2SO3H | Pale brown | Pale brown | 48.27 | 6.57 | 11.30 | 13.04 |
| 9 | C7H7SO3H | Brown | Brown | 76.49 | 19.02 | 20.18 | 18.32 |
| 10 | [BMIM]HSO4 | Pale brown | Brown | 60.67 | 14.33 | 12.17 | 14.90 |
| 11 | [HSO3-BMIM]HSO4 | Brown | Brown | 82.82 | 19.23 | 14.22 | 19.02 |
| 12 | HBEA | Pale brown | Pale brown | 19.35 | 0.45 | 3.21 | 5.42 |
| 13 | HZSM-5 | Brown | Gray | 37.49 | 2.57 | 2.90 | 8.45 |
Using sulfuric acid at low concentrations is a highly promising strategy for synthesizing the target product from methanolysis of biomass: H2SO4 can offer enough hydrogen ions (H+) to complete the reaction and promote the conversion of byproducts (MLG and furfurals) to MLA. The use of low concentrations of H2SO4 results in negligible quantities of dimethyl-ether, an undesirable byproduct of the dehydration of methanol. The remaining sulfuric acid also undergoes neutralization (with NaOH) of the liquefied mixture, thus minimizing the subsequent polymerization. The highly active protons in H2SO4 can effectively activate the oxygen atoms in the structural bonds of cellulose, hemicellulose and lignin. Using low concentrations of H2SO4 as an inexpensive catalyst is a highly promising strategy for producing MLA from methanolysis of lignocellulosic biomass. These results indicated that most of the C6 sugar substrates in cellulose (glucose units) and hemicellulose (galactose and mannose units) were decomposed using H2SO4 catalysts.
3.2. Effects of reaction parameters on the microwave-assisted liquefaction of bamboo
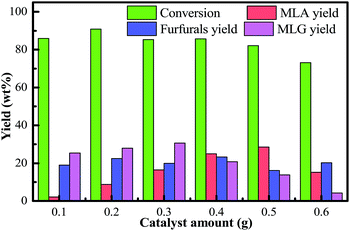
The higher sulfuric acid concentration (more than 0.5 g) could not significantly increase the formation of MLA. At higher acid concentrations (0.6 g), the yield of the main byproducts (furfurals) increased by about 4 wt%; however, the yield of MLA fell by approximately 13 wt%. This indicated that byproducts undergo side reactions at higher acid concentrations which increases the byproduct contents in the liquefied product. It should also be mentioned that an undesirable feature of the system is the acid-catalyzed side-reaction of the methanol to yield diethyl ether. Higher sulfuric acid concentration may promote the condensation of the methanol. Meanwhile, the usage of higher acid concentration requires that the reactor is corrosion-resistant which will increase the cost of the reaction equipment. Also, the addition of excess sulfuric acid has a negative effect on the reaction and more spent acid needs to be neutralized after the reaction. Therefore, lower acid concentration (0.5 g) is recommended for obtaining high MLA yields in this process.
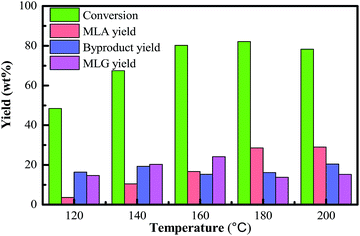
It was known that the operating temperature plays an important role in the control of thermochemical reactions. The reaction temperature influences the conversion of the raw material and the yields of products. In general, elevated temperature could promote the enhancement of biomass conversion efficiency and accelerate the rate of the liquefaction process. In this process, methanolysis of bamboo can result in a variety of products, with MLA being the main soluble product. Other products such as furfurals were also detected, but in a low amount, which may be formed from the methanolysis of hemicellulose in the bamboo. 5-methoxymethylfurfural is presumed to be an intermediate in the formation of methyl levulinate via cleavage of the aldehyde group on the furfural intermediate in a several-step sequence. Therefore, a suitable reaction temperature (180 °C) can prevent most side-reactions, which can benefit the fractionation process of high purity products.
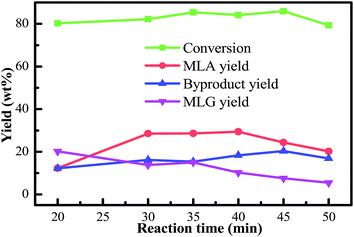
As the reaction time extended, the yields of MLG derived from the primary degradation reactions of cellulose and hemicellulose showed a decreasing trend. The yields of furfurals derived from the secondary degradation reactions of cellulose and hemicellulose generated an increasing trend. Therefore, it should be mentioned that the further methanolysis of glycoside compounds can produce furfurals and MLA. MLG and furfurals are the immediate products in the conversion of bamboo into MLA. Furthermore, as the reaction time was extended, the conversion of bamboo gradually declined over time. Extending the reaction time may also cause the re-condensation of the liquefied products. In general, considering the conversion of biomass, the yield of MLA, and the purity of MLA, we selected the optimal reaction time to be 40 min. Under these circumstances, a higher yield of MLG was achieved at a moderate reaction time.
3.3. Mass balance and purification of MLA by extraction
This study not only focused on the conversion of biomass, but also paid attention to the yield of MLA after the directional microwave-assisted liquefaction. With different liquefaction parameters, the conversion of biomass could be increased from 80 wt% to 85 wt% using methanol with an acid catalyst. However, many complex mixtures of oxygenated and active compounds were produced at the same time, mainly from the decomposition of cellulose and hemicellulose. During the liquefaction, cellulose and hemicellulose were first dehydrated and decomposed into monosaccharide derivatives (MLG including methyl pentose glucoside and methyl hexose glycoside). These monosaccharide derivatives can be further converted into other byproducts, such as furfural, methoxy-2-furanmethanol, 5-methoxymethyl furfural and furan. The methanolysis of some byproducts can produce methyl levulinate (MLA) with a longer reaction time, higher acid catalyst concentration or reaction temperature. The best liquefaction product yield and phenol yield conditions were a sulfuric acid catalyst at a loading of 0.5 g dissolved in 16 g solvent, a reaction time of 40 min, and a reaction temperature of 200 °C.| Materials | Conv. (wt%) | MLA yieldb (wt%) | Furfural yieldc (wt%) | MLG yieldd (wt%) |
|---|---|---|---|---|
| a Reaction conditions: biomass materials 2.0 g, methanol 16.0 g, acid catalyst 0.5 g, 180 °C, 40 min. Biomass samples were irradiated for 5 min under 700 W. b MLA: methyl levulinate (MLA yields were based on GC analysis with an internal standard method). c Furfurals: 5-methoxymethyl furfural and furfural (furfural yields were based on GC analysis with an internal standard method). d MLG: methyl pentose glucoside and methyl hexose glycoside (MLG yields were based on HPLC analysis with an external standard method). | ||||
| Bamboo | 84.11 | 29.39 | 18.34 | 10.22 |
| Eucalyptus | 81.25 | 23.72 | 19.21 | 17.32 |
| Poplar | 81.14 | 24.56 | 14.90 | 18.45 |
| Pine | 80.02 | 25.67 | 15.33 | 19.03 |
| Bagasse | 85.02 | 28.12 | 17.32 | 12.27 |
| Straw | 79.25 | 21.67 | 20.17 | 17.49 |
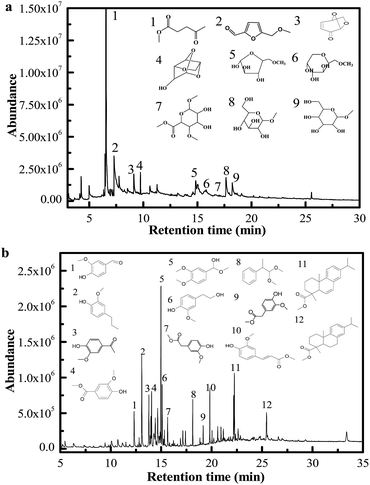
The procedures to produce and extract MLA from the directional microwave-assisted liquefaction of biomass are shown in Fig. 5. It was found that the majority of soluble phase products consisted of MLG, furfurals, and MLA that originated from cellulose and hemicellulose (Fig. 4(a)). The total content of MLG, furfurals, and MLA in soluble phase products was 80.23% (area). Only 2.04% (area) of phenolic compounds were identified in soluble phase products. Therefore, we speculated the monosaccharide derivatives and degradation products (MLG, furfurals, and MLA) derived from hemicellulose and cellulose were successfully separated. Then, the high-purity MLA can be further separated from soluble phase products via extraction with EtOAc. The content of the extracted MLA reached a maximum of 86.3% (measured by GC). The insoluble phase products were mostly composed of phenolic compounds that are decomposed from lignin (Fig. 4(b)). Due to their similar physical and chemical properties within each fraction, the platform chemicals make it possible to design high value added chemical products, and have great commercial potential for producing high-quality fine chemicals and biofuels using mild upgrading conditions.
3.4. Proposed reaction pathway for the alcoholysis conversion of lignocellulosic biomass
Cellulose and hemicellulose are the main components in biomass, accounting for approximately 70 wt% of biomass. Methyl monosaccharides, such as methyl glucosides, are important intermediate chemicals derived from holocellulose. They are first-step products that result from direct cleavage of holocellulose by solvent attack for producing MLA from biomass. Therefore, an investigation into the methanolysis of methyl monosaccharides can provide a reference for proposing the reaction pathway for the alcoholysis conversion of biomass. In order to explore the reaction pathway for the acid-catalyzed conversion of bamboo into MLA, a series of carbohydrates such as 5-hydroxymethyl furfural (HMF), glucose, fructose, cellobiose, corn starch, and microcrystalline cellulose (MCC) were selected as the model substrates for microwave-assisted liquefaction under their optimal reaction conditions. The most significant differences between these materials are their internal microstructure. Single sugars present a regular and uniform morphology. Corn starch and MCC contain a large number of monosaccharide units that are joined by glycosidic bonds, and MCC has a higher degree of crystallinity than corn starch. The bamboo biomass contained highly crystallized microfibrils (cellulose), complicated three-dimensional networks (lignin), and hemicelluloses.| Material | Temperature (°C) | Conv. (wt%) | MLA yieldb (wt%) | Furfural yieldc (wt%) | MLG yieldd (wt%) |
|---|---|---|---|---|---|
| a Reaction conditions: biomass carbohydrates 2.0 g, methanol 16.0 g, acid catalyst 0.5 g, 40 min. Biomass samples were irradiated for 5 min under 700 W. b MLA: methyl levulinate (MLA yields were based on GC analysis with an internal standard method). c 5-MMF: 5-methoxymethyl furfural (5-MMF yields were based on GC analysis with an internal standard method). d MLG: methyl hexose glycoside (MLG yields were based on HPLC analysis with an external standard method). e MCC: microcrystalline cellulose. | |||||
| HMF | 140 | 98.27 | 68.69 | 10.21 | — |
| 120 | 96.21 | 64.20 | 13.74 | — | |
| Glucose | 160 | 98.29 | 52.21 | 14.29 | 4.56 |
| 140 | 92.09 | 39.24 | 20.31 | 15.29 | |
| Fructose | 160 | 97.49 | 67.29 | 4.33 | 10.48 |
| 140 | 99.18 | 53.29 | 12.17 | 2.34 | |
| Methyl glucoside | 160 | 98.54 | 56.32 | 30.26 | 1.46 |
| 140 | 99.04 | 47.18 | 23.19 | 0.96 | |
| Cellobiose | 180 | 95.24 | 48.37 | 13.21 | 25.13 |
| 140 | 90.41 | 44.29 | 15.39 | 18.32 | |
| Corn starch | 180 | 92.28 | 46.53 | 11.29 | 14.73 |
| 160 | 94.17 | 32.18 | 6.79 | 14.43 | |
| 140 | 96.22 | 19.34 | 13.21 | 34.28 | |
| MCCe | 180 | 89.63 | 37.20 | 13.31 | 13.48 |
| 160 | 85.34 | 21.33 | 15.22 | 19.32 | |
| 140 | 80.14 | 2.42 | 13.19 | 28.35 | |
The MLA yield from microcrystalline cellulose (37.20 wt%) was dramatically lower than that from corn starch (46.53 wt%) or cellobiose (48.37 wt%) under the same reaction conditions. In addition, microcrystalline cellulose was insoluble in the subcritical methanol solvent and this caused poor substrate accessibility to the acid catalyst. Therefore, the highly crystallized cellulose was hard to depolymerize and less MLA was produced from microcrystalline cellulose in the microwave-assisted liquefaction. The yield of MLA from corn starch (46.53 wt%) was almost the same as the yield from cellobiose (48.37 wt%). This result was probably due to the solubility state of cellobiose and corn starch in subcritical methanol medium. During the microwave-assisted methanolysis process, cellobiose and corn starch are easily transferred to methyl glucosides and 5-methoxymethylfurfural as the intermediate chemicals in the presence of an acid catalyst with a methanol solvent. In addition, with MLG as the model compound, a substantially higher yield (56.32 wt%) of MLA was found compared with glucose (52.21 wt%). It may be concluded that the reaction pathway from microcrystalline cellulose, corn starch, and glucose to MLA involves the intermediate formation of methyl glucosides.
| Materials | Temperature (°C) | In water | In methanol | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Con. (wt%) | Yieldb (wt%) | Conv. (wt%) | Yieldc (wt%) | ||||||
| LA | HMF | Glucose | MLA | 5-MMF | MLG | ||||
| a Reaction conditions: biomass carbohydrates 2.0 g, methanol 16.0 g, acid catalyst 0.5 g, 40 min. Biomass samples were irradiated for 5 min under 700 W. b LA and HMF: levulinic acid and hydroxymethyl methyl furfural. c MLA: methyl levulinate, 5-MMF: 5-methoxymethyl furfural, MLG: methyl hexose glycoside. d MCC: microcrystalline cellulose. | |||||||||
| Glucose | 160 | 91.27 | 53.12 | 6.47 | — | 98.21 | 52.21 | 14.29 | 4.56 |
| Fructose | 140 | 93.29 | 60.44 | 5.18 | — | 99.18 | 67.29 | 4.33 | 10.48 |
| Corn starch | 180 | 76.39 | 42.10 | 8.42 | 12.69 | 92.28 | 46.53 | 11.29 | 14.73 |
| MCCd | 180 | 40.19 | 32.18 | 10.21 | 9.23 | 87.63 | 37.20 | 13.31 | 13.48 |
| Bamboo | 180 | 25.21 | 13.50 | 16.32 | 8.94 | 84.11 | 29.39 | 18.34 | 10.22 |
Bamboo was investigated in both microwave-assisted hydrolysis and methanolysis reactions. Approximately 84 wt% of the bamboo biomass was converted into liquefied products at 180 °C with methanol; however, only 25 wt% of bamboo was converted with water medium. Methanol showed high reaction activity for the decomposition of bamboo. During the reaction, subcritical methanol could provide good solubility for the liquefied products, which promoted the conversion of solid biomass to liquefied products. Methanol was expected to readily dissolve the high molecular-weight polar products derived from biomass due to their low dielectric constants, which could efficiently prevent the re-polymerization and re-condensation of liquefied products. Under these conditions, the diffusion and reactivity of the solvent can be significantly improved. The yields of the target product and byproducts in the methanolysis of bamboo are higher than those in the hydrolysis reaction. Such significant differences may lead to different end products due to the process, although bamboo is the common starting point for both hydrolysis and methanolysis. The structures of intermediate chemicals in methanol are different from those generated in water. For example, in water the reaction follows a cellulose–glucose–HMF–LA pathway, while in methanol, methyl glucoside and 5-methoxymethyfurfural (5-MMF) are formed sequentially, and then converted into methyl levulinate. The present experiment demonstrated that methanol is a more efficient reaction medium than water for the degradation of bamboo to obtain MLA.
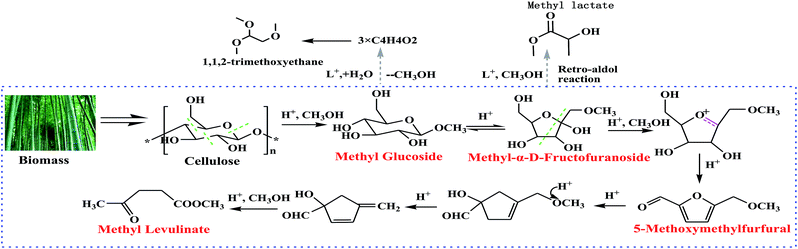 | ||
| Fig. 6 The proposed liquefaction pathway for the acid-catalyzed conversion of biomass into methyl levulinate in methanol. | ||
The conversion from 5-MMF to MLA is not a one-step reaction, and there are several active intermediate chemicals existing between 5-MMF and MLA. We believe that the active intermediates existing between 5-MMF and methyl levulinate in methanol are different from those in water, and it is the reactivity of these active intermediates that controls the rate of 5-MMF conversion with an effective acid. A CH3OH molecule in 5-MMF can be released into the solution with enough acid sites through the dealcoholization reaction leading to the formation of MLA.
3.5. Directional liquefaction and conventional liquefaction
Directional microwave-assisted liquefaction focuses on the production of a high value-added fuel additive (methyl levulinate) from lignocellulosic biomass. Compared with other conventional liquefaction studies,19 the composition and distribution of products were significantly different. There are hundreds of oxygen-containing compounds in liquefied products; however, each product accounts for only a small part of the products. These oxygenated derivatives are mainly derived from the secondary alcoholysis of sugar compounds, or the polymerization and condensation (side-reactions) of phenolic compounds.In our designated microwave-assisted liquefaction, the distribution of liquefied products was relatively concentrated, mainly into three categories of products (methyl levulinate, phenolics, and glycosides). The selectivity of the target product (methyl levulinate) has been significantly improved. In this study, methanol was used as the liquefaction solvent. During the reaction, subcritical methanol could provide good solubility for the liquefied products, which promoted the conversion of solid biomass into liquefied products. Methanol was expected to readily dissolve the high molecular-weight products derived from biomass due to their low dielectric constants, which could efficiently prevent re-polymerization and re-condensation of liquefied products.
The main components of liquefied bamboo using conventional liquefaction and microwave-assisted liquefaction processes are shown in Table 7. The contents of methyl levulinate after conventional liquefaction and microwave-assisted liquefaction were significantly different, 33.19% and 64.32%, respectively. The furfurals in the conventional liquefaction products were higher than those in microwave-assisted liquefaction products. The results indicated that side reactions may inevitably occur in the liquefaction process; however, the main liquefied products can be promoted by carefully adjusting the reaction conditions. With the appropriate choice of reagents that absorb microwave irradiation, rapid heating throughout the entire vessel can be achieved. In both conventional liquefaction and directional liquefaction processes, there are many oxygenated derivatives in liquefied products. In the conventional liquefaction products, the distribution of each product is dispersed and accounts for only a small part of the liquefied products. The oxygenated compounds such as 5-methoxymethyl furfural, furfural, and methyl levulinate were the main products of the conventional liquefaction process. Although there doesn't seem to be a significant change between conventional liquefaction and directional microwave-assisted liquefaction in the complexity of the liquefied mixtures, the target products from directional liquefaction were concentrated and their yields were significantly improved. It was found that methyl levulinate, phenolics, and glycosides are the major products of directional microwave-assisted liquefaction, while the oxygenated compounds were mainly found in the conventional liquefaction. In the designated microwave-assisted liquefaction, the purities and yields of the target product (methyl levulinate) were also greatly improved. Therefore, the directional microwave-assisted liquefaction can make the oriented conversion of the three components of biomass into the target product possible.
| Time (min) | Compound name | Area percentage (%) | |
|---|---|---|---|
| Directional | Conventional | ||
| a Conditions: all experiments used catalyst 0.5 g, bamboo 2 g, methanol 16 g. A conventional liquefaction process was carried out at 200 °C for 20 min, and the reaction conditions for directional liquefaction were 180 °C for 40 min. | |||
| — | Esters | 69.20 | 36.10 |
| 2.12 | Methyl formate | 1.58 | 0.59 |
| 2.76 | Dimethyl ether | 2.02 | 1.87 |
| 8.94 | Methyl levulinate | 64.32 | 33.19 |
| 18.66 | Pentanoate | 0.23 | — |
| 20.10 | 4-Methylpentanoate | 1.05 | 0.45 |
| — | Furans | 4.51 | 11.59 |
| 7.33 | Furfural | 1.23 | 2.21 |
| 7.83 | 2,5-Dimethylfuran | — | 1.35 |
| 12.32 | 2-Furanmethanol | 0.41 | 2.56 |
| 15.45 | 5-Methoxymethyl furfural | 2.87 | 5.45 |
| — | Glycosides | 9.42 | 16.69 |
| 13.26 | Methyl-β-D-arabinopyranoside | — | 2.23 |
| 17.87 | Methyl-α-D-xylofuranoside | 2.49 | 5.14 |
| 23.04 | Methyl-α-D-glucoside | 5.21 | 6.43 |
| 25.37 | Methyl-α-D-glucopyranoside | 1.15 | 2.89 |
| 27.38 | 4-Methoxy-α-D-glucopyranoside | 0.57 | — |
| — | Phenols | 14.89 | 25.78 |
| 14.23 | 2-Methoxyphenol | 4.23 | 6.34 |
| 15.83 | 4-Methyl guaiacol | 6.34 | 11.23 |
| 16.65 | Vanillin | 4.29 | 8.21 |
| — | Others | 1.98 | 4.90 |
| 6.75 | Tripropylene glycol methyl ether | 0.75 | 2.69 |
| 29.48 | 2,3-Dihydroxy acid | 1.23 | 2.21 |
4. Conclusion
This study presented an efficient catalytic process for producing methyl levulinate from biomass in the presence of sulfuric acid by using microwave dielectric heating. To obtain high yields of MLA from bamboo, various parameters need to be optimized in this directional microwave-assisted liquefaction, including various catalysts, acid catalyst loading, reaction temperature and time. A high yield of methyl levulinate of 29 wt% was achieved from the conversion of biomass obtained from bamboo at 200 °C with a reaction time of 40 min in methanol, which is higher than the yield of LA in water. The reaction time was significantly reduced by using microwave irradiation as a more effective heating method compared to conventional heating. Different model carbohydrates were used to study the biomass to MLA reaction pathway. The formation of methyl levulinate through the methanolysis of bamboo followed the sequence: cellulose → methyl glucoside → 5-MMF → methyl levulinate; methyl glucoside and 5-MMF are important intermediates and their further conversion is affected by various parameters. This process is very stable at laboratory levels and provides a potential approach for the preparation of high quality chemical feedstocks from liquefied products.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank National Nonprofits Institute Research Grant (CAFYBB2018QC001) and the Forestry Industry Research Special Funds for Public Welfare Projects (201504602) for supported our study. The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (31530010 and 31600590) and Research Grant of Jiangsu Province Biomass Energy and Materials Laboratory (JSBEM-S-201701) for this investigation.Notes and references
- (a) P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538 RSC; (b) H. Li, Z. Xu, P. Yan and Z. C. Zhang, Green Chem., 2017, 19, 1751–1756 RSC.
- (a) N. Shi, Q. Liu, Q. Zhang, T. Wang and L. Ma, Green Chem., 2013, 15, 1967–1974 RSC; (b) X. Zhang, Q. Zhang, T. Wang, L. Ma, Y. Yu and L. Chen, Bioresour. Technol., 2013, 134, 73–80 CrossRef PubMed; (c) Y. Liao, Q. Liu, T. Wang, J. Long, L. Ma and Q. Zhang, Green Chem., 2014, 16, 3305–3312 RSC; (d) Y. Liu, L. Chen, T. Wang, Q. Zhang, C. Wang, J. Yan and L. Ma, ACS Sustainable Chem. Eng., 2015, 3, 1745–1755 CrossRef CAS.
- L. Yang, X. Yang, E. Tian, V. Vattipalli, W. Fan and H. Lin, J. Catal, 2016, 333, 207–216 CrossRef CAS.
- (a) H. Li, Z. Fang, J. Luo and S. Yang, Appl. Catal., B, 2017, 200, 182–191 CrossRef CAS; (b) F. D. Pileidis and M. M. Titirici, ChemSusChem, 2016, 9, 562–582 CrossRef CAS PubMed.
- T. Werpy and G. Petersen, Top value added chemicals from biomass volume I, 2004, pp. 45–48 Search PubMed.
- Y. Huang, T. Yang, M. Zhou, H. Pan and Y. Fu, Green Chem., 2016, 18, 1516–1523 RSC.
- X. Hu, S. Jiang, L. Wu, S. Wang and C. Li, Chem. Commun., 2017, 53, 2938–2941 RSC.
- D. Ding, J. Xi, J. Wang, X. Liu, G. Lu and Y. Wang, Green Chem., 2015, 17, 4037–4044 RSC.
- (a) X. Zhang, P. Murria, Y. Jiang, W. Xiao, H. I. Kenttämaa, M. M. Abu-Omar and N. S. Mosier, Green Chem., 2016, 18, 5219–5229 RSC; (b) J. Song, H. Fan, J. Ma and B. Han, Green Chem., 2013, 15, 2619–2635 RSC; (c) Z. Zhang, J. Song and B. Han, Chem. Rev., 2016, 117(10), 6834–6880 CrossRef PubMed.
- B. Chen, F. Li and Z. Huang, ChemSusChem, 2014, 7, 202–209 CrossRef CAS PubMed.
- J. Xu, J. Jiang, C. Hse and T. F. Shupe, Green Chem., 2012, 14, 2821–2830 RSC.
- (a) Y. Qu, Q. Wei, H. Li, P. Oleskowicz, C. Huang and J. Xu, Bioresour. Technol., 2014, 162, 358–364 CrossRef CAS PubMed; (b) Á. Szabolcs, M. Molnár, G. Dibó and L. T. Mika, Green Chem., 2013, 15, 439–445 RSC.
- H. Li, Y. Qu, Y. Yang, S. Chang and J. Xu, Bioresour. Technol., 2016, 199, 34–41 CrossRef CAS PubMed.
- A. Motevali, S. Minaei, A. Banakar, B. Ghobadian and M. Khoshtaghaza, Energy Convers. Manage., 2014, 87, 711–725 CrossRef.
- J. Xu, X. Xie, J. Wang and J. Jiang, Green Chem., 2016, 18, 3124–3138 RSC.
- (a) H. Huang and X. Yuan, Prog. Energy Combust. Sci., 2015, 49, 59–80 CrossRef; (b) S. Brand, R. F. Susanti, S. K. Kim, H. S. Lee, J. Kim and B. I. Sang, Energy, 2013, 59, 173–182 CrossRef CAS.
- J. Feng, J. Jiang, J. Xu, Z. Yang, K. Wang, Q. Guan and S. Chen, Appl. Energy, 2015, 154, 520–527 CrossRef CAS.
- (a) J. Fan, M. De-Bruyn, V. L. Budarin, M. J. Gronnow, P. S. Shuttleworth, S. Breeden, D. J. Macquarrie and J. H. Clark, J. Am. Chem. Soc., 2013, 135, 11728–11731 CrossRef CAS PubMed; (b) A. Shrotri, H. Kobayashi and A. Fukuoka, ChemCatChem, 2016, 8, 1059–1064 CrossRef CAS; (c) Y. B. Huang and Y. Fu, Green Chem., 2013, 15, 1095–1111 RSC.
- (a) Q. Bu, H. Lei, L. Wang, G. Yadavalli, Y. Wei, X. Zhang, L. Zhu and Y. Liu, J. Anal. Appl. Pyrolysis, 2015, 112, 74–79 CrossRef CAS; (b) M. Climent, A. Corma and S. Iborra, Green Chem., 2014, 16, 516–547 RSC.
- J. Feng, J. Jiang, J. Xu and Z. Yang, RSC Adv., 2015, 5(48), 38783–38791 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00579b |
| This journal is © The Royal Society of Chemistry 2018 |