Electrospray synthesis of nano-Si encapsulated in graphite/carbon microplates as robust anodes for high performance lithium-ion batteries†
Wen
Liu
a,
Yongming
Zhong
a,
Siyuan
Yang
a,
Shengsen
Zhang
a,
Xiaoyuan
Yu
a,
Hongqiang
Wang
 *b,
Qingyu
Li
b,
Jun
Li
*b,
Qingyu
Li
b,
Jun
Li
 c,
Xin
Cai
c,
Xin
Cai
 *a and
Yueping
Fang
a
*a and
Yueping
Fang
a
aCollege of Materials and Energy, South China Agricultural University, Guangzhou, 510642, China. E-mail: caixin2015@scau.edu.cn
bGuangxi Key Laboratory of Low Carbon Energy Materials, School of Chemistry & Pharmaceutical Sciences, Guangxi Normal University, Guilin 541004, P. R. China
cDepartment of Chemistry, Kansas State University, Manhattan, KS 66506, USA
First published on 10th January 2018
Abstract
Developing efficient Si-based anode materials for new-generation lithium-ion batteries (LIBs) has drawn extensive attention. Here, an electrosprayed Si/graphite/carbon (Si/G/C) composite is explored as a prominent anode material for LIBs. The designed Si/G/C composite possesses a reasonable structure with nano-Si encapsulated in the conductive graphite flake/amorphous carbon framework. The Si/G/C composite achieves superior reversible Li+ storage capability, showing a considerable discharge capacity of 832 mA h g−1 at 200 mA g−1. Moreover, it realizes an encouraging capacity of ca. 400 mA h g−1 under a high current density of 500 mA g−1 after 200 cycles. The excellent capacity and rate performance can be attributed to the structural benefits of the Si/G/C composite: (i) the highly conductive graphite flakes serve as good dispersive scaffolds and electronic conductors, allowing for fast charge transfer and favorable ion diffusion; (ii) the amorphous carbon layer acts as a protective coating to bind/fix nano-Si onto graphite and reduce the formation of unstable solid electrolyte interphase (SEI) film; and (iii) both the layered graphite and amorphous carbon layer introduce adequate buffer space or voids to alleviate the volume changes of Si during the Li+ insertion/extraction cycles. This high-capacitive and robust Si/graphite-based hybrid is attractive as an alternative anode material for practical rechargeable LIBs.
Introduction
Modern society has witnessed the widespread adoption of rechargeable lithium-ion batteries (LIBs) that are green, compact and efficient. Yet, the ever-increasing growth of various mobile electronics, electrical vehicles/hybrid vehicles and even grid-scale energy supply systems requires more advanced LIBs with higher energy/power densities, longer life spans and lower mass-production costs.1 To further improve the capacity performance of current LIBs, it is urgently necessary to develop new-generation anode materials, since the graphite anode used in commercial LIBs has almost reaching its capacity limit, i.e., a theoretical electrochemical capacity of 372 mA h g−1 for LiC6.2 As a promising alternative to replace graphite, Si has received tremendous attention in terms of its suitable lithiation potential (<0.5 V vs. Li+/Li), natural abundance, non-toxicity and especially high theoretical specific capacity (3572 mA h g−1 for the Li15Si4 phase).3,4 However, Si always suffers from severe volume deformation of over 300% during electrochemical alloying and dealloying with Li+.5,6 The extreme mechanical stress and deterioration induced by the volume variations can bring several problems, such as the loss of particle interconnectivity and electrical contacts, the fracture/pulverization of the electrode, or even electrode breakdown, along with the formation of an unstable solid electrolyte interphase (SEI), and can finally result in poor reversibility or rapid capacity decay in the device.7,8In order to relieve the drastic volume expansion/contraction of Si-based anode materials during Li+ insertion/extraction cycles, some useful strategies have been developed, including (1) downsizing Si and building accessible nanostructures (nanowires, nanotubes, 3D porous Si, etc.), so as to reduce the dimensional cracking to a critical size as possible;9–12 (2) the establishment of suitable geometrical structures containing enough buffer/void space for the active Si to release the large strains generated during electrochemical cycles, such as core–shell structures, yolk–shell structures and pomegranate structures;13–16 (3) modification, coating or compositing with other stable or conductive materials, with the purpose of forming a kinetically stable SEI on the surface of Si, and introducing effective electrical paths or shortened Li-ion diffusion distances for reversible Li+ storage;17–19 and (4) other methods, such as the use of matching binders or morphological control to increase the structural stability of Si.20,21 Among these strategies, Si/C nanocomposites are recognized as one of the most efficient routes to enhancing the electrochemical performance of Si.22 On one hand, the incorporated carbon component can act as a conductive framework to promote carrier transport, or act as a protective/passivation layer to accommodate the interfacial stress from Si particles.23 On the other hand, the availability of diverse forms/types of carbonaceous materials with different functionalities can facilitate the construction of desirable Si/C architectures.24 Nevertheless, the columbic efficiency and initial cycling efficiency of common Si/C composites are far from satisfactory, which is detrimental to the ultimate capacity performance. To address this issue, the incorporation of conventional graphite materials with excellent columbic efficiency into nano-Si could be a favorable choice.25 For instance, some graphite-containing Si anode materials have been prepared via chemical vapor deposition (CVD) or simple mechanical milling.26–33 Though the obtained results are encouraging, to achieve a robust Si/graphite composite without sacrificing the specific capacity still remains a challenge.
Apart from the good electrical conductivity and mechanical strength afforded by the highly conductive graphite component, the ultimate electrochemical performance of a Si/graphite composite still relies on the good distribution of Si particles in the graphite matrix. Hence, size compatibility between the Si nanoparticles and the micro-graphite must be considered.34 Also, minimizing the direct exposure of Si to the electrolyte is equally important to reduce side reactions with the electrolyte and enhance the cycling tolerance of Si-based anodes. In this regard, a combined strategy including the above two aspects is promising to further improve the structural stability and high-rate capacity of Si/graphite composites. For instance, it is feasible to suitably disperse and support Si particles using conductive graphite scaffolds, along with a protective coating layer, to help the formation of more stable SEI film upon cycling. Besides, designing a rational structure for the Si-carbon composite, associated with a scalable preparation process, is essential. As an easily handled and template-free approach, electrospraying has been proven efficient for fabricating porous nano/microscale assemblies with tunable size.35,36 Herein, mesoporous Si/graphite/C (Si/G/C) microplates were prepared through electrospray synthesis combined with ball milling. Thanks to a layered graphite dispersion medium and a polyacrylonitrile (PAN)-derived amorphous carbon coating, nano-Si can be well encapsulated in the carbon-coated graphite matrix. The Si/G/C composite is quite beneficial for enhancing the structural integrity and cycling durability of a nano-Si anode. It obtained a considerable discharge capacity of 832 mA h g−1 at a current density of 200 mA g−1, and maintained a stable cycling capacity of 523 mA h g−1 after 200 cycles.
Experimental section
Synthesis of the Si/G composite
Both Si and the graphite flakes were pretreated before ball milling. In a typical synthesis, 1.0 g of commercial silicon nanoparticles (Si NPs, <70 nm, 99%, HWNANO) was dispersed in 100 mL of xylene via ultrasonic treatment for 1 h, followed by the addition of 1 mL of 3-aminopropyltriethoxysilane (KH-550). The mixture was heated up to 80 °C and refluxed for 12 h under inert gas protection. The resulting dispersion was filtered, washed with anhydrous ethanol repeatedly and dried at 80 °C under vacuum to obtain amino group-functionalized nano-Si. Commercial graphite flakes (∼0.4 μm, XFNANO) were refluxed in 3 M nitric acid for 6 h and then rinsed with deionized water several times to obtain oxygen-containing group-functionalized graphite flakes.Afterwards, 0.5 g of amino-functionalized nano-Si was ultrasonically dispersed in 50 mL of anhydrous ethanol for 30 min before 2.0 g of functionalized graphite flakes were added. The suspension was homogenized with ultrasound for another 30 min and filtered to prepare the initial Si/G powder, which was transferred into a high-energy planetary ball mill (PM2L, DROIDE, Shanghai) and ground at 300 rpm for 12 h. The weight ratio of powder to ball was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30. The collected solids were heated in a tube furnace at a heating rate of 3 °C min−1 and carbonized at 650 °C under N2 flow for 3 h to afford the Si/graphite flake composite (Si/G).
30. The collected solids were heated in a tube furnace at a heating rate of 3 °C min−1 and carbonized at 650 °C under N2 flow for 3 h to afford the Si/graphite flake composite (Si/G).
Preparation of the Si/G/C composite
0.6 g of Si/G and 0.6 g of PAN (Mw: ∼150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Macklin) were dissolved in 20.0 g of dimethyl formamide (DMF) with vigorous stirring at room temperature for 12 h to form a stable and homogenous precursor solution. As for the electrostatic spray process, a working voltage of 20 kV was supplied by a commercial machine (TL-01, TLWNT). The precursor solution was injected into a syringe (22-gauge size needle) at a flow speed of 0.5 mL h−1. Electrosprayed particles were collected on a homemade aluminum collector that was 10–15 cm away from the needle. The as-formed powder was stabilized at 250 °C in an oven in air for 3 h, and then moved to a tube furnace for carbonization at 650 °C under N2 flow for 3 h. The obtained product was the final Si/G/C composite. For comparison, a Si/C composite was prepared under the same conditions, except that 0.6 g of Si/G was replaced by 0.12 g of Si.
000, Macklin) were dissolved in 20.0 g of dimethyl formamide (DMF) with vigorous stirring at room temperature for 12 h to form a stable and homogenous precursor solution. As for the electrostatic spray process, a working voltage of 20 kV was supplied by a commercial machine (TL-01, TLWNT). The precursor solution was injected into a syringe (22-gauge size needle) at a flow speed of 0.5 mL h−1. Electrosprayed particles were collected on a homemade aluminum collector that was 10–15 cm away from the needle. The as-formed powder was stabilized at 250 °C in an oven in air for 3 h, and then moved to a tube furnace for carbonization at 650 °C under N2 flow for 3 h. The obtained product was the final Si/G/C composite. For comparison, a Si/C composite was prepared under the same conditions, except that 0.6 g of Si/G was replaced by 0.12 g of Si.
Material characterization
The morphologies and microstructures of the silicon/carbon composites were imaged using a field-emission scanning electron microscope (SEM, Merlin, Zeiss) equipped with an energy-dispersive spectrometer (EDS, Oxford). High-resolution transmission electron microscope (HR-TEM) analysis of the Si/G/C composite was conducted on a JEOL-2010 microscope with a 200 kV accelerating voltage. The crystalline phases of the samples were examined using an X-ray diffractometer (XRD, Rigaku) operated with Cu Kα radiation (λ = 1.54 Å). Thermogravimetric (TG) curves were recorded using a thermogravimetric analyzer (TG, STA449C, NETZSCH) in air, from 40 °C to 900 °C at a rate of 10 °C min−1. The elemental compositions were analyzed via X-ray photoelectron spectroscopy (XPS, VG ESCALAB250), adopting a monochromatized Al Kα X-ray source at 300 W under an acceleration of 15 kV. Raman spectra were obtained using a micro-Raman spectrometer (LabRAM Aramis, France), using a 532 nm laser source.Device assembly and electrochemical measurements
Silicon/carbon composites were used as the active material in anodes. Their electrochemical performances were examined using CR2025 coin-type cells that were assembled in an argon-filled glove box. Specifically, the active material (Si/G, Si/C or Si/G/C, respectively), conducting agent (acetylene black) and binder (Na–alginate) were well mixed in deionized water at a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (wt%). The as-prepared slurry was then uniformly cast onto copper foil to fabricate the film electrodes. All the electrodes were dried at 80 °C under vacuum overnight to acquire working electrodes. All the specific capacities were calculated according to the total weight of the active mass, except for the acetylene black and binder. Meanwhile, lithium foil was used as the counter electrode. A piece of microporous membrane (Celgard 2400, thickness of ca. 20 μm) was adopted as a separator between the working electrode and the counter electrode. The electrolyte was 1 M LiPF6 in a mixed organic solvent containing ethylene carbonate (EC), dimethyl carbonate (DMC) (vEC/vDMC = 1
10 (wt%). The as-prepared slurry was then uniformly cast onto copper foil to fabricate the film electrodes. All the electrodes were dried at 80 °C under vacuum overnight to acquire working electrodes. All the specific capacities were calculated according to the total weight of the active mass, except for the acetylene black and binder. Meanwhile, lithium foil was used as the counter electrode. A piece of microporous membrane (Celgard 2400, thickness of ca. 20 μm) was adopted as a separator between the working electrode and the counter electrode. The electrolyte was 1 M LiPF6 in a mixed organic solvent containing ethylene carbonate (EC), dimethyl carbonate (DMC) (vEC/vDMC = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and a moderate amount of fluoroethylene carbonate (FEC). All the electrochemical tests were performed at room temperature, unless otherwise specified. The galvanostatic charge/discharge measurements, cycling tests and rate capabilities were all characterized using a Land battery system (LAND CT-2001A). The cut-off potential ranged from 0.05 V to 3.0 V (vs. Li/Li+). Cyclic voltammetry (CV) was conducted between 0.01 and 1.2 V (vs. Li/Li+) at a scanning rate of 0.1 mV s−1. The Si/G/C electrode was used as the working electrode and lithium foil was adopted as the counter electrode. Electrochemical impedance spectroscopy (EIS) was carried out using an electrochemical workstation (Zahner Im6/6ex, German), covering the frequency range of 0.01 Hz to 100 kHz with an AC amplitude of 5 mV.
1) and a moderate amount of fluoroethylene carbonate (FEC). All the electrochemical tests were performed at room temperature, unless otherwise specified. The galvanostatic charge/discharge measurements, cycling tests and rate capabilities were all characterized using a Land battery system (LAND CT-2001A). The cut-off potential ranged from 0.05 V to 3.0 V (vs. Li/Li+). Cyclic voltammetry (CV) was conducted between 0.01 and 1.2 V (vs. Li/Li+) at a scanning rate of 0.1 mV s−1. The Si/G/C electrode was used as the working electrode and lithium foil was adopted as the counter electrode. Electrochemical impedance spectroscopy (EIS) was carried out using an electrochemical workstation (Zahner Im6/6ex, German), covering the frequency range of 0.01 Hz to 100 kHz with an AC amplitude of 5 mV.
Results and discussion
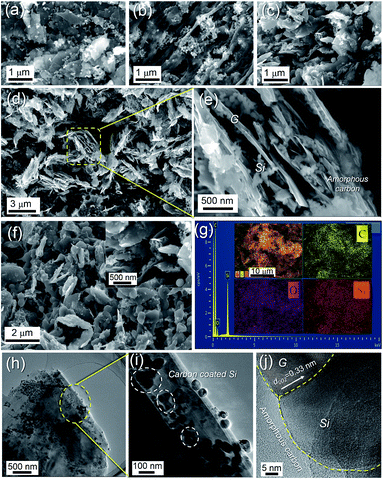
The preparation of Si/G/C mainly involves ball milling and electrospraying processes, which are schematically shown in Fig. 1. To improve the ball milling, nano-Si was chemically functionalized with the silane coupling agent KH-550 to prevent the particles from self-aggregation. It is noteworthy that the positively charged nano-Si, following KH-550 modification, will electrostatically graft onto the negatively charged graphite oxide flakes,37 benefiting the adhesion of nano-Si to the graphite microsheets. As demonstrated in Fig. 2a, the graphite flakes, possessing a size of 0.5–3 μm, are smooth and densely packed. They maintained the flake-like morphology after mechanical milling. Amounts of Si NPs, with particle sizes of less than 100 nm, are randomly dispersed/attached to the graphite substrate; also, some nano-Si is embedded in the thin graphite flakes (Fig S1a and b†). Subsequently, Si/G particles were dispersed in PAN solution for electrospraying, followed by the pyrolysis of PAN to form a carbon coating on the outside of the Si/G hybrid. Images of the as-prepared Si/G/C composite are shown in Fig. 2c–f; it is commonly in the form of microplates with irregular edges, and shares almost similar particle sizes with Si/G. Most of the nano-Si is no longer exposed on the surface and is effectively covered by the pyrolyzed carbon layer. From a side-view of the Si/G/C microplates (Fig. 2d and e), it is clear that many nano-Si clusters are located in the interspaces between graphite flakes. As nano-Si was well attached to the 2D graphite microflakes, the random stacking of micro/nano-Si–graphite assemblies generated a porous morphology and void spaces that could be helpful in defending against the expansion of Si during Li+ storage. Interestingly, several short nanofibrils were formed in certain places on the surface of Si/G/C (Fig S1c and d†), which may be caused by locally concentrated PAN solution during the electrospray synthesis. These carbon nanofibrils deriving from PAN can connect some of the Si/G/C microplates and may help retain the structural integrity of Si/G/C. For Si/C, many nano-Si agglomerates are anchored on the grooved surface of the unidirectional and porous carbon nanofibril substrates (Fig. 2b, S1e and f†). Therefore, it is necessary to disperse and encapsulate nano-Si in the graphite/carbon matrix through ball milling and the electrospraying procedure, respectively. Fig. 2g shows the EDX spectrum of the Si/G/C composite. The atomic content values for C, Si and O in Si/G/C are 81.55%, 8.09% and 10.36%, respectively. On the basis of the elemental mapping analysis, nano-Si is evenly distributed over the entire carbon matrix, showing the good homogeneity of the Si/G/C hybrid.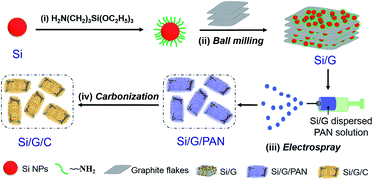 | ||
| Fig. 1 Schematic illustration of the “ball milling-electrospray” process for the silicon nanoparticles-embedded graphite flakes/carbon composite (Si/G/C). | ||
To further identify the microstructure of the Si/G/C composite, TEM characterization was conducted. As shown in Fig. 2h, numerous nano-Si particles are evidently embedded in the regular and laminar graphite. It can be seen that some Si NPs are coated with disordered carbon layers (Fig. 2i). According to the HR-TEM image of Si/G/C (Fig. 2j), the interfaces of the Si/G/C hybrid are distinct. Interplanar spacing of 0.33 nm between the lattice fringes is attributed to the (002) plane of the crystalline graphite.38 Interestingly, both the crystalline zone and the amorphous zone are observed in the Si particles (Fig. S2†). This is reasonable, since prolonged ball milling might lead to amorphized Si particles.28 Previous studies have found that amorphous Si is more structurally and kinetically stable than crystalline Si, which always endures anisotropic expansion at different crystallographic planes.7 It can be concluded that the Si/G/C composite shows a well-designed structure, with nano-Si embedded in the graphite/carbon matrix. Graphite flakes in Si/G/C can serve as an effective conductive matrix to support nano-Si, but also facilitate the dispersion of nano-Si, preventing aggregation. The good electrical conductivity and considerable specific area of the graphite flakes favor charge transport and increase interfacial contact between the electrode and electrolyte.29 Moreover, the amorphous carbon layer derived from PAN acts as a good agent to fix and encapsulate nano-Si in the graphite scaffolds, while forming a protective and elastic coating on the outside of the hybrid.33 Si/G/C is expected to relax the mechanical stress and volume deformation of active nano-Si during reversible Li+ storage.
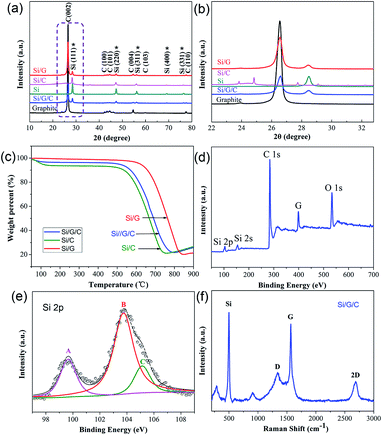
Fig. 3a shows powder X-ray diffraction (XRD) patterns of pristine graphite flakes, Si NPs, Si/C, Si/G and the Si/G/C composite. The diffraction peaks of Si/G/C, with 2θ values of 28.48°, 47.34° 56.2°, 69.16° and 76.44°, can be well indexed to the (111), (220), (311), (400) and (331) planes of cubic phase Si (JCPDS 27-1402).39 The intensity of the characteristic peak at 28.48° is apparently weak, due to the mixing of Si with graphite and the amorphous carbon component. Meanwhile, the peak at 26.58°, corresponding to the (002) plane of graphite in Si/G/C, is still prominent after the combined ball milling-electrospraying process. Also, no apparent shift in this characteristic peak was observed, based on the enlarged view presented in Fig. 3b. This reveals that the original structure and high crystallinity of the graphite flakes were well maintained in the final Si/G/C hybrid. In addition, no other impurity peaks are shown. To determine the exact content of active Si in the composites, thermogravimetric analysis (TGA) was used and the results are shown in Fig. 3c. The slight increase in weight at 750–900 °C was probably caused by the high temperature oxidation of Si. Based on the weight loss and the residual weight percentages, the Si content values in Si/G, Si/C and Si/G/C are estimated to be 20.22%, 21.13% and 21.79%, respectively.
To study the chemical states on the surface of the Si/G/C composite, X-ray photoelectron spectroscopy (XPS) was applied. The wide XPS spectrum, including Si, C and O elements, is provided (Fig. 3d). The high-resolution Si 2p XPS spectrum is presented in Fig. 3e, and three main bonding types can be derived. The peak featured at 99.7 eV (marked A) is ascribed to the Si0 component (Si–Si), confirming the presence of elemental Si in the Si/G/C composite. The major peak featured at 103.8 eV (peak B) and the shoulder peak at 105.1 eV (peak C) correspond to Si3+ and Si4+ species (Si–O) on the surface, which originate from silicon oxides, as fresh Si NPs could be partially oxidized during synthesis.40 In addition, the O 1s XPS spectrum (Fig. S3†) shows a dominant peak located at 533.3 eV (peak E), which can be assigned to the O–Si bond.41Fig. 3f shows the Raman spectrum of the as-synthesized Si/G/C composite. The sharp peak at 498 cm−1 and two nearby small peaks below 1000 cm−1 can be attributed to crystalline nano-Si.42 Two characteristic peaks centered at 1339 cm−1 and 1568 cm−1 reflect the amorphous carbon component (D band) and ordered graphite carbon (G band) in the composite. An intensity ratio (R value: ID/IG) of 0.37 is calculated, revealing a significant amount of graphite flakes, which is likely to benefit the electrical conductivity of the Si/G/C composite. It is notable that an obvious 2D peak centered at 2683 cm−1 was detected, which is highly correlated with the graphene layers, possibly resulting from partly exfoliated graphite flakes during mechanical milling.43 The Si/G/C composite demonstrated a considerable BET surface area of ca. 152 m2 g−1, with plenty of pores with an average pore size of 21.68 nm distributed mainly in the mesoporous region (Fig. S4†), which was expected to promote ion diffusion in the porous composite.
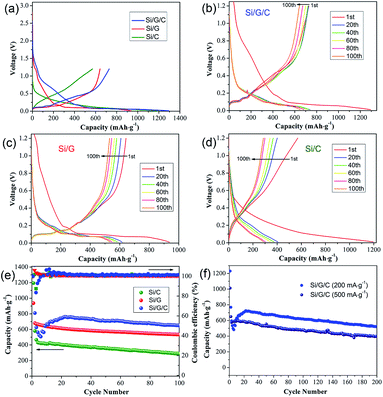
The electrochemical performances of the silicon/carbon composites were examined in coin-type cells. All the specific capacities were calculated based on the total weight of the active mass, i.e., the total weight of Si and carbon species in the Si/G/C, Si/G and Si/C composites, respectively. Fig. 4a shows the galvanostatic charge/discharge profiles of the three composites over the voltage range of 0.05–3.0 V (vs. Li/Li+) at a current density of 50 mA g−1. Relatively broad voltage plateaus are observed between 0.15 V and 0.5 V, revealing the typical Li+ insertion/extraction reaction of Si. The initial discharge capacities of Si/G/C, Si/G and Si/C were 1295 mA h g−1, 937 mA h g−1 and 1194 mA h g−1, giving rising to initial cycling efficiencies of 56.3%, 68.5% and 47.7%, respectively. The capacity losses are mainly ascribed to irreversible structural damage to the electrode and the unavoidable formation of a SEI layer on the surface of Si, which is in accordance with the potential slopes ranging from 1.2 V to 0.6 V in the discharge profiles. Despite the significant efficiency losses, the introduction of graphite is clearly favorable for higher initial cycling efficiency. Aside from the first charge/discharge profile, several subsequent charge/discharge profiles from Si/G/C, Si/G and Si/C, at the 20th, 40th, 60th, 80th and 100th cycle, are provided (Fig. 4b–d). Compared to Si/G and Si/C, variations in the charge/discharge profiles of Si/G/C are largely reduced, especially for those after the first 20 cycles. Usually, more fractures of Si occur in the initial crystalline-to-amorphous transformation cycles, rather than in subsequent cycles.7,44 The columbic efficiencies of Si/G/C during the following cycles reached approximately 100%, suggesting the good reversibility of the composite. This demonstrates that Si/G/C possesses the best durability characteristics and that it can withstand the huge volume changes during Li+ insertion/extraction cycles. Once the SEI layer steadily forms after the first few cycles, both the conductive graphite and the carbon coating layer in the Si/G/C composite will exert positive effects. They can provide useful buffer space to separate nano-Si from the electrolyte and release the mechanical strain on the surface induced by volume variations during lithiation/delithiation cycles.
The cycling capacities and columbic efficiencies of Si/G/C, Si/G and Si/C under 200 mA g−1 are demonstrated in Fig. 4e. As the cycle number increases, the discharge capacities of the three composites exhibit a dramatic decline over the first few cycles, which is primarily due to irreversible capacity loss as the SEI layer forms on the surface of the Si particles. Afterwards, the established SEI film tends to be stabilized. Both Si/G and Si/C demonstrate a similar and gradual drop in capacity values, and they retain discharge capacities of 533 mA h g−1 and 289 mA h g−1 after 100 cycles, respectively. This suggests that both graphite and amorphous carbon are beneficial for improving the cycling durability of Si, since individual Si particles often undergo capacity failure after only several cycles.45 On the other hand, the capacity of Si/G/C experienced a slight rise during the second ten cycles, which may be a result of the activation process and the gradual infiltration of nano-Si by the electrolyte.4,28 Owing to synergistic effects from the graphite flakes and the amorphous carbon coating, Si/G/C achieved greatly enhanced cycling stability. It maintained a high specific capacity of 652 mA h g−1 after 100 cycles with acceptable columbic efficiency. Also, a specific capacity of 523 mA h g−1 was obtained, even after 200 cycles. As displayed in Fig. 4f, the cycling performance of Si/G/C under high-rate charging/discharging was also examined. It can still maintain a considerable capacity of about 400 mA h g−1 after 200 cycles under a high current density of 500 mA g−1. The impressive cycling capacity and high-rate tolerance of Si/G/C are superior to most reported silicon/graphite counterparts (see Table S1†). This suggests that the Si/G/C composite can effectively suppress intrinsic volume expansion/contraction and maintain structural integrity over cycling. It is thus necessary and effective to incorporate nano-Si with conductive graphite flakes and amorphous carbon, so as to reduce surface side reactions and the local stress of Si during the cycling process.
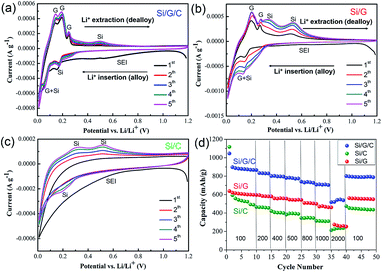
To further investigate the electrochemical alloying–dealloying process, CV curves from the Si/G/C, Si/G and Si/C electrodes were measured between 0.01 and 1.2 V (vs. Li/Li+) at a scan rate of 0.1 mV s−1, respectively. Shown in Fig. 5a, for Si/G/C, as for the first cathodic scan, the insertion of Li+ into Si is accompanied by a transition from crystalline Si into an amorphous LixSi phase. A broad peak located in the voltage range of 0.4 to 1.0 V is derived from the reductive decomposition of the organic electrolytes and subsequent SEI formation on the surface of the active electrode. This peak basically weakened or even disappeared during the following cycles, due to some irreversible change in the active materials. The reduction peak centered at 0.15 V and an obvious peak near 0 V result from the lithiation of Si and graphite to form LixSi and LixC6 species, respectively. For the anodic scan, the oxidation peaks below 0.2 V and two featured peaks (0.25 V and ∼0.5 V) correspond to the extraction of Li+ (delithiation) from graphite and LixSi, respectively.29 As the cycle number increases from 1 to 5, these reduction and oxidation peaks gradually become stronger, probably due to lithium ion diffusion and the activation of the electrode materials.27 According to Fig. 5b, the dominant cathodic/anodic peaks due to Li+ insertion/extraction behavior in Si/G were roughly similar to those in Si/G/C, which further reveals that the overall lithium storage is attributed to Si and the graphite species in the Si/G composite. However, during the first cathodic scan, the branch at 0.4 to 0.9 V belonging to the formation of the SEI film in the Si/G composite does not show a distinct broad peak like that in Si/G/C. This implies that Si/G/C possesses relatively slower lithiation kinetics than Si/G, because of protection from the amorphous carbon coating.28 In contrast to the two graphite-containing composites (Si/G/C and Si/G), the Si/C composite did not exhibit any obvious lithiation/delithiation peaks in the 1st sweep cycle, as displayed in Fig. 5c. In later cycles, the lithiation of Si particles and the Li–Si alloy delithiation process are clarified by the cathodic peak centered at 0.19 V and the broadened anodic peaks of low-potential delithiation (0.31 V) and high-potential delithiation (0.51 V), respectively.46 This phenomenon was correlated with the intensive interface reaction that occurred between the electrolyte and Si during the 1st cycle, and the active material exhibits poor lithiation abilities before the formation of a stable SEI film. Therefore, it can be speculated that the Si nanoparticles suffered from more structural damage and poor activity during the first cycle without the existence of strong and conductive graphite scaffolds, which is in accordance with the low initial columbic efficiency of Si/C mentioned above. It is noted that the overlap and repeatability of the CV curves for the Si/G/C electrode are the best among the three. Also, the area change of the CV curves of Si/G/C during cycling is significantly less than those of Si/G and Si/C. These findings confirm the considerable reversible activity and cycling ability of Si/G/C toward Li+ storage. The results agree well with previous charge/discharge profiles and the corresponding cycling performance in Fig. 4.
The rate capabilities of the Si/G/C, Si/G and Si/C composites were further evaluated to explore their practical potential. Fig. 5d demonstrates the rate performances under a series of current densities, that is, from 100 mA g−1 to 2000 mA g−1, with each sustained for several cycles. Compared to Si/G and Si/C, Si/G/C exhibited much higher specific capacities and enhanced stability under the same current density. Even under a high current density of 2000 mA g−1, Si/G/C achieved a high capacity of 538 mA h g−1, which is more than twice that of Si/G and Si/C. When the current density returned to 100 mA g−1, Si/G/C showed a specific capacity of 795 mA h g−1, which is over 90% retention of its initial capacity performance. The superior capacity and cycling performance of the Si/G/C composite are reasonable, and are related to its well-designed structure, associated with conductive 2D graphite supports and the elastic carbon coating layer protecting against the volume variation of Si. Table S1† illustrates the preparation and electrochemical performances of recently developed Si/graphite-based anode materials. Compared with previously reported results, our Si/G/C composite is one of the best Si/graphite-based anode materials, delivering high specific capacity as well as robust cycling durability. We expect that the reversible capacity and cycling performance of the Si/G/C composite can be further optimized by adjusting the relative content of graphite and amorphous carbon.
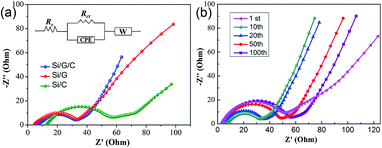
The charge transport behavior at the electrode/electrolyte interface was further elucidated using EIS analysis. Generally, EIS plots consist of a semicircle in the high-frequency range and a sloped line in the lower-frequency range. The former is usually derived from the charge transfer resistance (Rct) and the corresponding chemical capacitance at the electrode/electrolyte interface. The latter is often related to the impedance of the electrolyte and ion diffusion into the electrode, i.e., the Warburg impedance (as shown in the inset of Fig. 6a).47,48Fig. 6a displays the EIS curves of Si/G/C, Si/G and Si/C. Since Si has poor electronic conductivity, the introduction of PAN-derived amorphous carbon or graphite flakes into the composite can significantly improve the electronic conductivity of Si-based electrodes. This can be confirmed by the relatively low ohmic resistance (Rs: 5–15 Ω) of the three composites, especially for the graphite-containing ones. Graphite flakes possess high electrical conductivity and a considerable specific surface area. They can act as a conductive framework to accelerate charge transport as well as ion diffusion at electrode/electrolyte interfaces. Apparently, Si/G/C and Si/G showed better diffusion properties than Si/C. Also, the simulated Rct values for Si/G/C and Si/G were determined to be 33 Ω and 30 Ω, respectively, which are much lower than that (55 Ω) of Si/C, revealing the enhanced charge transfer kinetics of Si–G composites. This agrees well with the superior capacity performance of Si/G/C and Si/G, discussed above. In addition, the EIS characteristics of the Si/G/C composite during cycling were studied. The impedance of ion diffusion and Rct for the first charge/discharge cycle (1st) are relatively significant because of SEI formation at the Si/electrolyte interface.49Rct for subsequent cycles (10th and 20th) obviously decreased because of the gradual activation and penetration of the Si particles by the electrolyte. However, Rct for prolonged charge/discharge cycles (50th and 100th) began to rise, though the shape of the EIS curve and, thereby, the basic interfacial properties were preserved. The performance degradation basically results from the repeated formation of SEI film and the inevitable structural deterioration of Si during continued cycling.6
High-capacity active materials are more likely to endure drastic volume changes or structural variations during Li+ insertion/extraction reactions.3,50–52 The consequence is the damaging or breaking of the electrode on an extended scale, accompanied by capacity fading. Despite the excellent capacity performance and favorable interfacial behavior of the Si/G/C composite, it is still essential to investigate the electrode morphology and structural expansion/evolution in a more direct and visual way. Fig. 7 presents SEM images of the Si/G/C, Si/G and Si/C electrodes before and after an intensive 200 cycles. According to the cross-sectional view (Fig. 7a, c, and e), the electrode film of Si/G/C expanded by about 97% in thickness and good electrical contacts were maintained. As a comparison, the electrode films of Si/G and Si/C expanded by 210% and 174%, respectively, concomitantly with electrode disconnections and distinct fractures along the vertical side. As observed in the top-view images (Fig. 7b, d, f), some minor cracks were produced in the Si/G/C and Si/G electrode films. It was found that Si/G/C or Si/G could retain their basic microscopic structures, even after 200 lithiation/delithiation cycles. However, the Si/C film showed a nearly pulverized morphology after 200 cycles. Graphite flakes are mechanically strong with enough buffer space. The PAN-derived carbon layer further immobilizes/adheres nano-Si onto the graphite scaffolds and offers extra buffer space. Therefore, Si/G/C demonstrated good structural integrity. The volume expansion of Si upon cycling can be greatly inhibited, due to the addition of graphite flakes and the amorphous carbon coating. These findings are in accordance with the above cycling capacity results and rate performances of the three composites. It is worth noting here that the film fabrication technique for active electrodes is important from a practical viewpoint so as to ensure the macroscopic mechanical strength of the electrode materials during electrochemical cycling, a point which has not often been mentioned. In this respect, the adoption of improved film fabrication processes or the use of suitable binders for our Si/graphite composites in future work is both feasible and essential.
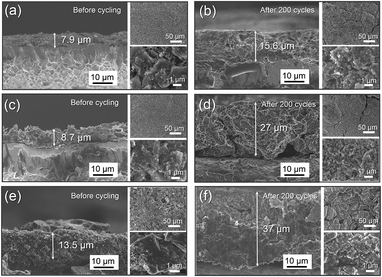 | ||
| Fig. 7 SEM images of the Si/G/C (a, b), Si/G (c, d) and Si/C (e, f) electrodes on copper foil before and after cycling for 200 cycles at a current density of 200 mA g−1. | ||
Conclusions
Mesoporous Si/G/C microplates were synthesized via electrospraying, in conjunction with ball milling. Due to good dispersion by the graphite flakes and the fixation effects of the amorphous carbon coating, nano-Si can be well encapsulated in the conductive graphite/carbon matrix. The layered graphite scaffolds and the PAN-derived amorphous carbon layer in the Si/G/C composite can improve structural integrity, providing sufficient buffer space to relieve the volume expansion of nano-Si during repeated charging/discharging cycles. Moreover, the conductive graphite and amorphous carbon coating can also enhance the electrical conductivity of the Si-based composite, leading to favorable ion diffusion and charge transfer kinetics in the Si/G/C composite. Benefiting from improved structural stability and electronic conductivity, the Si/G/C composite achieved high capacity and exceptional rate performance. It exhibits superior capacity performance, with a stable cycling capacity of 652 mA h g−1 after 100 cycles, and even an impressive discharge capacity of 538 mA h g−1 was maintained at 2 A g−1. Our high-capacity robust Si/G/C hybrid is promising as an alternative anode material for new-generation rechargeable LIBs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Natural Science Foundation of China (NSFC 51602109, NSFC 21673083), the Natural Science Foundation of Guangdong Province (No. 2017A030313283), the Guangdong Provincial Science and Technology Project (No. 2016A050502048) and the Guangzhou Science and Technology Planning Project (No. 201704030022).Notes and references
- R. Van Noorden, Nature, 2014, 507, 26–28 CrossRef CAS PubMed.
- L. Ji, Z. Lin, M. Alcoutlabi and X. Zhang, Energy Environ. Sci., 2011, 4, 2682–2699 CAS.
- R. Teki, M. K. Datta, R. Krishnan, T. C. Parker, T. M. Lu, P. N. Kumta and N. Koratkar, Small, 2009, 5, 2236–2242 CrossRef CAS PubMed.
- J. R. Szczech and S. Jin, Energy Environ. Sci., 2011, 4, 56–72 CAS.
- H. Kim, M. Seo, M. H. Park and J. Cho, Angew. Chem., Int. Ed., 2010, 49, 2146–2149 CrossRef CAS PubMed.
- X. Su, Q. Wu, J. Li, X. Xiao, A. Lott, W. Lu, B. W. Sheldon and J. Wu, Adv. Energy Mater., 2014, 4, 1300882 CrossRef.
- M. T. McDowell, S. W. Lee, W. D. Nix and Y. Cui, Adv. Mater., 2013, 25, 4966–4984 CrossRef CAS PubMed.
- X. Zhou, Y. X. Yin, L. J. Wan and Y. G. Guo, Chem. Commun., 2012, 48, 2198–2200 RSC.
- C. K. Chan, H. Peng, G. Liu, K. McIlwrath, X. F. Zhang, R. A. Huggins and Y. Cui, Nat. Nanotechnol., 2008, 3, 31–35 CrossRef CAS PubMed.
- C. K. Chan, R. N. Patel, M. J. O'Connell, B. A. Korgel and Y. Cui, ACS Nano, 2010, 4, 1443–1450 CrossRef CAS PubMed.
- T. Song, J. Xia, J. H. Lee, D. H. Lee, M. S. Kwon, J. M. Choi, J. Wu, S. K. Doo, H. Chang, W. Il Park, D. S. Zang, H. Kim, Y. Huang, K. C. Hwang, J. A. Rogers and U. Paik, Nano Lett., 2010, 10, 1710–1716 CrossRef CAS PubMed.
- M. Ge, J. Rong, X. Fang and C. Zhou, Nano Lett., 2012, 12, 2318–2323 CrossRef CAS PubMed.
- T. H. Hwang, Y. M. Lee, B. S. Kong, J. S. Seo and J. W. Choi, Nano Lett., 2012, 12, 802–807 CrossRef CAS PubMed.
- N. Liu, H. Wu, M. T. McDowell, Y. Yao, C. Wang and Y. Cui, Nano Lett., 2012, 12, 3315–3321 CrossRef CAS PubMed.
- N. Liu, Z. Lu, J. Zhao, M. T. McDowell, H. W. Lee, W. Zhao and Y. Cui, Nat. Nanotechnol., 2014, 9, 187–192 CrossRef CAS PubMed.
- C. J. Tang, J. X. Zhua, X. J. Wei, L. He, K. N. Zhao, C. Xu, L. Zhou, B. Wang, J. Z. Sheng and L. Q. Mai, Energy Storage Materials, 2017, 7, 152–156 CrossRef.
- S. Jing, H. Jiang, Y. Hu, J. Shen and C. Li, Adv. Funct. Mater., 2015, 25, 5395–5401 CrossRef CAS.
- D. M. Piper, J. J. Travis, M. Young, S. B. Son, S. C. Kim, K. H. Oh, S. M. George, C. Ban and S. H. Lee, Adv. Mater., 2014, 26, 1596–1601 CrossRef CAS PubMed.
- Z. F. Li, H. Zhang, Q. Liu, Y. Liu, L. Stanciu and J. Xie, ACS Appl. Mater. Interfaces, 2014, 6, 5996–6002 CAS.
- B. Koo, H. Kim, Y. Cho, K. T. Lee, N. S. Choi and J. Cho, Angew. Chem., Int. Ed., 2012, 51, 8762–8767 CrossRef CAS PubMed.
- Y. Yao, M. T. McDowell, I. Ryu, H. Wu, N. Liu, L. Hu, W. D. Nix and Y. Cui, Nano Lett., 2011, 11, 2949–2954 CrossRef CAS PubMed.
- L. F. Cui, Y. Yang, C. M. Hsu and Y. Cui, Nano Lett., 2009, 9, 3370–3374 CrossRef CAS PubMed.
- S. Chen, M. L. Gordin, R. Yi, G. Howlett, H. Sohn and D. Wang, Phys. Chem. Chem. Phys., 2012, 14, 12741–12745 RSC.
- B. Wang, X. Li, X. Zhang, B. Luo, M. Jin, M. Liang, S. A. Dayeh, S. T. Picraux and L. Zhi, ACS Nano, 2013, 7, 1437–1445 CrossRef CAS PubMed.
- J. H. Lee, W. J. Kim, J. Y. Kim, S. H. Lim and S. M. Lee, J. Power Sources, 2008, 176, 353–358 CrossRef CAS.
- M. Murase, N. Yabuuchi, Z. J. Han, J. Y. Son, Y. T. Cui, H. Oji and S. Komaba, Chemsuschem, 2012, 5, 2307–2311 CrossRef CAS PubMed.
- X. Zhu, H. Chen, Y. Wang, L. Xia, Q. Tan, H. Li, Z. Zhong, F. Su and X. S. Zhao, J. Mater. Chem. A, 2013, 1, 4483–4489 CAS.
- C. H. Yim, F. M. Courtel and Y. Abu-Lebdeh, J. Mater. Chem. A, 2013, 1, 8234–8243 CAS.
- Y. H. Huang, C. T. Chang, Q. Bao, J. G. Duh and Y. L. Chueh, J. Mater. Chem. A, 2015, 3, 16998–17007 CAS.
- H. Wang, J. Xie, S. Zhang, G. Cao and X. Zhao, RSC Adv., 2016, 6, 69882–69888 RSC.
- Z. Wang, Z. Mao, L. Lai, M. Okubo, Y. Song, Y. Zhou, X. Liu and W. Huang, Chem. Eng. J., 2017, 313, 187–196 CrossRef CAS.
- B. Lu, B. Ma, R. Yu, Q. Lu, S. Cai, M. Chen, Z. Wu, K. Xiang and X. Wang, ChemistrySelect, 2017, 2, 3479–3489 CrossRef CAS.
- S. Y. Kim, J. Lee, B. H. Kim, Y. J. Kim, K. S. Yang and M. S. Park, ACS Appl. Mater. Interfaces, 2016, 8, 12109–12117 CAS.
- M. Ko, S. Chae, J. Ma, N. Kim, H. W. Lee, Y. Cui and J. Cho, Nat. Energy, 2016, 1, 16113 CrossRef CAS.
- Y. X. Yin, S. Xin, L. J. Wan, C. J. Li and Y. G. Guo, J. Phys. Chem. C, 2011, 115, 14148–14154 CAS.
- Y. Xu, Y. Zhu, F. Han, C. Luo and C. Wang, Adv. Energy Mater., 2015, 5, 1400753 CrossRef.
- M. Zhou, F. Pu, Z. Wang, T. Cai, H. Chen, H. Zhang and S. Guan, Phys. Chem. Chem. Phys., 2013, 15, 11394–11401 RSC.
- G. Hou, B. Cheng, Y. Cao, M. Yao, B. Li, C. Zhang, Q. Weng, X. Wang, Y. Bando, D. Golberg and F. Yuan, Nano Energy, 2016, 24, 111–120 CrossRef CAS.
- T. Zhang, J. Gao, L. J. Fu, L. C. Yang, Y. P. Wu and H. Q. Wu, J. Mater. Chem., 2007, 17, 1321–1325 RSC.
- C. Martin, M. Alias, F. Christien, O. Crosnier, D. Belanger and T. Brousse, Adv. Mater., 2009, 21, 4735–4741 CrossRef CAS.
- J. Yi, X. He, Y. Sun and Y. Li, Appl. Surf. Sci., 2007, 253, 4361–4366 CrossRef CAS.
- M. Su, Z. Wang, H. Guo, X. Li, S. Huang, W. Xiao and L. Gan, Electrochim. Acta, 2014, 116, 230–236 CrossRef CAS.
- J. H. Lee, D. W. Shin, V. G. Makotchenko, A. S. Nazarov, V. E. Fedorov, Y. H. Kim, J. Y. Choi, J. M. Kim and J.-B. Yoo, Adv. Mater., 2009, 21, 4383–4387 CrossRef CAS PubMed.
- S. W. Lee, H. W. Lee, I. Ryu, W. D. Nix, H. J. Gao and Y. Cui, Nat. Commun., 2015, 6, 7533 CrossRef CAS PubMed.
- D. J. Lee, H. Lee, M. H. Ryou, G. B. Han, J. N. Lee, J. Song, J. Choi, K. Y. Cho, Y. M. Lee and J. K. Park, ACS Appl. Mater. Interfaces, 2013, 5, 12005–12010 CAS.
- H. D. Chen, Z. L. Wang, X. H. Hou, L. J. Fu, S. F. Wang, X. Q. Hua, H. Q. Qin, Y. P. Wu, Q. Ru, X. Liu and S. J. Hu, Electrochim. Acta, 2017, 249, 113–121 CrossRef CAS.
- R. Ruffo, S. S. Hong, C. K. Chan, R. A. Huggins and Y. Cui, J. Phys. Chem. C, 2009, 113, 11390–11398 CAS.
- H. Tian, X. Tan, F. Xin, C. Wang and W. Han, Nano Energy, 2015, 11, 490–499 CrossRef CAS.
- H. Wu, G. Zheng, N. Liu, T. J. Carney, Y. Yang and Y. Cui, Nano Lett., 2012, 12, 904–909 CrossRef CAS PubMed.
- Y. Oumellal, N. Delpuech, D. Mazouzi, N. Dupre, J. Gaubicher, P. Moreau, P. Soudan, B. Lestriez and D. Guyomard, J. Mater. Chem., 2011, 21, 6201–6208 RSC.
- C. Erk, T. Brezesinski, H. Sommer, R. Schneider and J. Janek, ACS Appl. Mater. Interfaces, 2013, 5, 7299–7307 CAS.
- Y. Yao, N. Xu, D. D. Guan, J. T. Li, Z. C. Zhuang, L. Zhou, C. W. Shi, X. Liu and L. Q. Mai, ACS Appl. Mater. Interfaces, 2017, 9, 39425–39431 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00542c |
| This journal is © The Royal Society of Chemistry 2018 |