The impacts of PbI2 purity on the morphology and device performance of one-step spray-coated planar heterojunction perovskite solar cells†
Jiaxu
Yao
,
Liyan
Yang
,
Feilong
Cai
,
Yu
Yan
,
Robert S.
Gurney
,
Dan
Liu
and
Tao
Wang
 *
*
School of Materials Science and Engineering, Wuhan University of Technology, Wuhan, 430070, China. E-mail: twang@whut.edu.cn
First published on 27th November 2017
Abstract
We have fabricated planar heterojunction perovskite solar cells by one-step ultrasonic spray-coating of the perovskite precursor solution in air. Uniform perovskite films with high surface coverage can be prepared after optimization of the precursor solution and spray-coating parameters. We found that the purity of PbI2, although varying only from 98 to 99.9%, can significantly affect the crystallinity, grain size and boundaries of MAPbI3 films that were fabricated via one-step spray-coating, and ultimately determined the power conversion efficiency (PCE) of perovskite devices. PbI2 with a purity of 98% resulted in a low conversion of precursors to perovskites, whilst a high purity of 99.9% led to perovskites with a high crystallinity, large grain size and narrow grain boundaries. Our p–i–n type, planar heterojunction solar cell ITO/PEDOT:PSS/MAPbI3/PCBM/Ag made from MAI and 99.9% purity PbI2 achieved a maximum PCE of 12.6% without hysteresis, whereas the 98% purity PbI2-based device showed a low PCE of only 4.9% with the presence of hysteresis. However, the impacts of PbI2 purity on the device efficiency can be minimized by changing the deposition method from one-step spray-coating to a two-step spin casting approach.
1. Introduction
Solar cells convert the energy of sunlight directly into electricity which is regarded as an important method of generating renewable energy to meet the global energy demands. Efforts are devoted to the synthesis and discovery of new semiconductors for the photoactive layer of solar cells, among which organometal halide perovskites are an exceptional candidate to generate high power conversion efficiency (PCE) in photovoltaic devices.1–5 Organometal halide perovskites CH3NH3PbX3 (X = Br, Cl, I) were first introduced in photovoltaics in 2009 as a sensitizer in dye-sensitized solar cells which demonstrated a PCE of 3.8%,6 and then as the photoactive layer of perovskite solar cells7,8 which achieved the highest efficiency of 22.1% recently.9However, the majority of studies that address the fabrication of perovskite solar cells heavily rely on spin-casting the photoactive materials, a method that consumes a lot of raw materials and is also not compatible with the large-area manufacturing processing required for the commercialization of this promising photovoltaic product. Consequently, various approaches have been explored such as bar-coating,10 doctor-blading,11 roll-to-roll production12 and spray-coating13 to attempt to achieve high performance and lower the manufacturing cost from mass production. Among these film deposition techniques, spray-coating has demonstrated great potential for the production of uniform organic and perovskite thin films towards the fabrication of high efficiency spray-coated solar cells.14–17 For example, Meng et al.18 reported a two-step ultrasonic spray method to fabricate centimeter-scale uniform and smooth perovskite MAPbI3 films for efficient and large-area solar cells, with the mesoscopic solar cell achieving an efficiency of 16.0%. Lidzey et al.19 reported p–i–n type, planar perovskite solar cell devices in which all of the solution-processable layers, including PEDOT:PSS, perovskite, and PCBM, were deposited by ultrasonic spray-casting in air, and the all-spray-cast devices achieved a pinnacle PCE of 9.9%. Bishop et al. utilised spray-coating under ambient conditions to sequentially deposit compact-TiO2, mesoporous-TiO2, CH3NH3PbI(3−x)Clx perovskite and doped spiro-OMeTAD layers, creating a mesoporous perovskite solar cell having an average PCE of 9.2% and a peak PCE of 10.2%, values that compare favourably with those of control-devices fabricated by spin-casting that had an average efficiency of 11.4%.20 A two-step deposition method, with the first step involving spraying lead iodide (PbI2) solution and then immersing the sample in methylammonium iodide (MAI) solution, led to a maximum PCE of 13% (average PCE of 10.2%) in n–i–p type MAPbI3 devices with compact and mesoporous TiO2 as the electron transport layer.21 Das et al.22 fabricated uniform and highly crystalline CH3NH3PbI3−xClx films via ultrasonic spray-coating, and the as-fabricated solar cells based on these perovskite films on glass and polyethylene terephthalate (PET) substrates exhibited PCEs as high as 13% and 8.1%, respectively.
In this work, we have used a spray-assisted one-step deposition method to deposit uniform perovskite films with good surface coverage after optimization of the spray-coating process parameters, including the nozzle-substrate distance, humidity, substrate temperature and so on. Other layers of our devices were deposited via spin-casting or thermal evaporation. We found that the purity of the precursor PbI2, although varying only from 98 to 99.9%, significantly affected the conversion of precursors to perovskites during this one-step spray-coating and the subsequent thermal-annealing process. PbI2 with a purity of 98% resulted in a low conversion of precursors to perovskites and a low device efficiency. PbI2 with a high purity of 99.9% led to perovskites with a high crystallinity, large grain size and narrow grain boundaries. Our p–i–n type, planar heterojunction solar cell ITO/PEDOT:PSS/MAPbI3/PCBM/Ag incorporating this improved perovskite layer achieved a maximum PCE of 12.6%, with an open-circuit voltage (VOC) of 0.87 V, a short-circuit current density (JSC) of 22.5 mA cm−2, and a fill factor (FF) of 0.63, and was hysteresis free. Precursor purity is therefore an important factor that determines the perovskite morphology and device efficiency during one-step spray-coating of MAPbI3 solar cells.
2. Materials and methods
Methylammonium iodide (MAI) was synthesized following our previous report. Briefly, 30 ml of methylamine (33 wt% in methanol, Aladdin) and 27.5 ml of hydroiodic acid (45 wt% in water, Macklin) were mixed in a round-bottom flask at 0 °C for 2 h with stirring. The precipitates were collected by evaporating the solvent by vacuum distillation at 45 °C for 1 h. The precipitates were then dissolved in ethanol and recrystallized in the presence of diethyl ether, and these steps were repeated three times to improve the purity. Finally, the products were dried at 60 °C in a vacuum for more than 24 h. Lead iodide (PbI2) of different purities (99.9, 99 and 98%) was used as received from Macklin. Phenyl-C60-butyric acid methyl ester (PC61BM) was purchased from Luminescence Technology. Poly(3,4-ethylenedio-xythiophene):poly(styrenesulfonate) (PEDOT:PSS) (Al4083) was purchased from Nichem.The glass substrates (20 × 15 mm2) with pre-patterned ITO were cleaned by ultrasonication in deionized water, ethanol, acetone and isopropanol, respectively, then dried with a stream of nitrogen and treated with UV Ozone cleaning for 15 min. The hole transport layer was prepared by spin-coating a 40 nm thick film of PEDOT:PSS onto the ITO substrate at 5000 rpm for 30 s and annealing at 135 °C for 30 min in air. To prepare the perovskite precursor solution, the MAI powder was mixed with PbI2 of different purities (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio), dissolved in dimethyl sulfoxide (DMSO) and γ-butyrolactone (GBL) (1
1 mole ratio), dissolved in dimethyl sulfoxide (DMSO) and γ-butyrolactone (GBL) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio) with 2.5% (volume ratio) 2-propanol (IPA) mixed solvent solution to a concentration of 300 mg ml−1 and then stirred at room temperature until completely dissolved. Here, we have added anti-solvent IPA to generate a supersaturated condition to accelerate the nucleation and crystallization to create smooth, uniform and high-quality perovskite films for high device efficiency. For the two-step spin coating, 50 μl of the mixed precursor containing PbI2 (605 mg ml−1) and MAI (50 mg ml−1) was first dropped onto the PEDOT:PSS film substrate spinning at 6000 rpm for 15 s, and then 75 μl of MAI in IPA solution (35 mg ml−1) was cast on top at 4000 rpm for 45 s. The substrate was then annealed at 100 °C for 30 min to form the MAPbI3 film.
1 volume ratio) with 2.5% (volume ratio) 2-propanol (IPA) mixed solvent solution to a concentration of 300 mg ml−1 and then stirred at room temperature until completely dissolved. Here, we have added anti-solvent IPA to generate a supersaturated condition to accelerate the nucleation and crystallization to create smooth, uniform and high-quality perovskite films for high device efficiency. For the two-step spin coating, 50 μl of the mixed precursor containing PbI2 (605 mg ml−1) and MAI (50 mg ml−1) was first dropped onto the PEDOT:PSS film substrate spinning at 6000 rpm for 15 s, and then 75 μl of MAI in IPA solution (35 mg ml−1) was cast on top at 4000 rpm for 45 s. The substrate was then annealed at 100 °C for 30 min to form the MAPbI3 film.
Spray-coating of the precursor solution was performed using a Siansonic UC342 spray-coater. The ultrasonic tip was held at 60 mm above the device substrate and set at a moving speed of 600 mm min−1. The solution delivery rate was set at 0.7 ml min−1via a well-controlled syringe pump. The precursor solution was sprayed onto the PEDOT:PSS-coated ITO substrate held at 60 °C under atmospheric conditions with a controlled humidity of 40%, and the pressure of the nitrogen gas flow was 0.5 MPa. The spray droplets reached the device substrates and merged to form a wet film, which was further heated at a temperature of 115 °C for several seconds to form a dense perovskite film of around 500 nm thickness, and then again annealed at 100 °C for 30 min for further crystallization. The perovskite films were then spin-cast with a PC61BM electron-extraction layer in a nitrogen-filled glovebox. The solution of PCBM was prepared at 20 mg ml−1 in chlorobenzene (CB, Sigma Aldrich) and spin-cast at 1000 rpm m−1 for 30 s to form a 50 nm thick film. Finally, a 150 nm Ag layer was deposited by thermal evaporation under a vacuum of at least 2 × 10−6 Torr through a shadow mask to form the cathode. The area of the active layer of the device defined by the size of the cathode layer is 4 mm2.
The device performance was tested under ambient conditions using a 3A Newport solar simulator (AM1.5). The light intensity was calibrated to 100 mW cm−2 using an NREL certified, KG5 filtered silicon reference cell. The temperature of the laboratory during device testing was approximately 25 °C. External quantum efficiency (EQE) measurements were carried out using a Zolix IPCE measurement system (Zolix SCS10-X150-DSSC) with a scanning step of 5 nm. Optical images were obtained using an Olympus BX51 microscope. Photoluminescence (PL) mapping images were obtained using a PL microscopic spectrometer (Flex One, Zolix, China) with mapping ability. The excitation source of PL utilizes a 532 nm CW laser, and the mapping was performed with a step size of 0.005 mm. The absorption spectra of the perovskite were obtained by using a UV-vis spectrophotometer (Hitachi U-3900H, Japan). X-ray diffraction spectra were measured with an X-ray diffractometer (D8 Advance, Bruker, Germany) using Cu Kα radiation at 40 kV and 40 mA. Impedance measurements were carried out using the ModuLab XM electrochemical workstation (AMETEK, UK) under a bias of 0.6 V with an amplitude of 50 mV from 1 MHz to 100 Hz under dark conditions. Equivalent circuit simulations were conducted using the software package ZView (Scribner Associate, Inc.).
3. Results and discussion
Spray-coating of the precursor solution was performed using a Siansonic UC342 spray-coater, with Fig. 1a outlining the scheme of this one-step spray casting process using the precursor solution made of PbI2 and MAI at a blending mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in dimethyl sulfoxide (DMSO)
1 in dimethyl sulfoxide (DMSO)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) γ-butyrolactone (GBL)
γ-butyrolactone (GBL)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol (IPA) (1
2-propanol (IPA) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.025). During the ultrasonic spray-coating process, the precursor solution was delivered to the tip through the nozzle, and was broken into a spray of tens of microns in diameter with the assistance of ultrasonic (with a fixed power of 1.65 watts). Then the spray was directed to the device substrate (glass/ITO/PEDOT:PSS) with the help of a nitrogen flow from a gas pipe alongside the tip (see Fig. 1a). The quality of the spray-cast film can be affected by many parameters of the spray-coating system including the solid concentration and flow rate of the precursor solution, the nozzle-to-substrate distance, substrate temperature, humidity, tip-moving speed and nitrogen flow rate, leaving a wide space for the optimization of the morphology and device performance. Pre-optimization of these parameters was carried out in order to cast films where spray droplets can reach the device substrate and merge to form a uniform wet film before drying. Efforts have been made to avoid situations where spray droplets partially dried before they reached the substrate, or the droplets dried too quickly preventing necessary coalescence. In both cases, either pinholes or the presence of micron and nanometer-sized particles with a huge number of interfaces would exist, either shortening the device or reducing the carrier transport mobility within the film. We emphasize here that there is a wide window rather than one sole method that exists to achieve uniform films by spray-coating. It is therefore trivial to find an optimized processing condition for each single operating parameter. We have employed the processing parameters as detailed in the Experimental section to cast uniform wet precursor films, and then baked the films at a temperature of 115 °C for several seconds to form a dense perovskite film, with the color of the wet film gradually changing from yellow to brown-black within several seconds which indicates the formation of the perovskite.23 We then continued the annealing at 100 °C for an additional 30 min to allow a sufficient conversion from precursors to perovskites. The device substrates were then transferred into a glovebox to spin-cast a PCBM layer of 50 nm. Finally, a 150 nm Ag layer was deposited by thermal evaporation through a shadow mask to form the cathode, thus finishing the fabrication of perovskite solar cells with a typical structure of ITO/PEDOT:PSS/MAPbI3/PCBM/Ag (see Fig. 1b). The solid content of the precursor solution and the tip-moving speed were adjusted to vary the thickness of the active layer, and the optimum film thickness was found to be around 500 nm through device efficiency studies.
0.025). During the ultrasonic spray-coating process, the precursor solution was delivered to the tip through the nozzle, and was broken into a spray of tens of microns in diameter with the assistance of ultrasonic (with a fixed power of 1.65 watts). Then the spray was directed to the device substrate (glass/ITO/PEDOT:PSS) with the help of a nitrogen flow from a gas pipe alongside the tip (see Fig. 1a). The quality of the spray-cast film can be affected by many parameters of the spray-coating system including the solid concentration and flow rate of the precursor solution, the nozzle-to-substrate distance, substrate temperature, humidity, tip-moving speed and nitrogen flow rate, leaving a wide space for the optimization of the morphology and device performance. Pre-optimization of these parameters was carried out in order to cast films where spray droplets can reach the device substrate and merge to form a uniform wet film before drying. Efforts have been made to avoid situations where spray droplets partially dried before they reached the substrate, or the droplets dried too quickly preventing necessary coalescence. In both cases, either pinholes or the presence of micron and nanometer-sized particles with a huge number of interfaces would exist, either shortening the device or reducing the carrier transport mobility within the film. We emphasize here that there is a wide window rather than one sole method that exists to achieve uniform films by spray-coating. It is therefore trivial to find an optimized processing condition for each single operating parameter. We have employed the processing parameters as detailed in the Experimental section to cast uniform wet precursor films, and then baked the films at a temperature of 115 °C for several seconds to form a dense perovskite film, with the color of the wet film gradually changing from yellow to brown-black within several seconds which indicates the formation of the perovskite.23 We then continued the annealing at 100 °C for an additional 30 min to allow a sufficient conversion from precursors to perovskites. The device substrates were then transferred into a glovebox to spin-cast a PCBM layer of 50 nm. Finally, a 150 nm Ag layer was deposited by thermal evaporation through a shadow mask to form the cathode, thus finishing the fabrication of perovskite solar cells with a typical structure of ITO/PEDOT:PSS/MAPbI3/PCBM/Ag (see Fig. 1b). The solid content of the precursor solution and the tip-moving speed were adjusted to vary the thickness of the active layer, and the optimum film thickness was found to be around 500 nm through device efficiency studies.
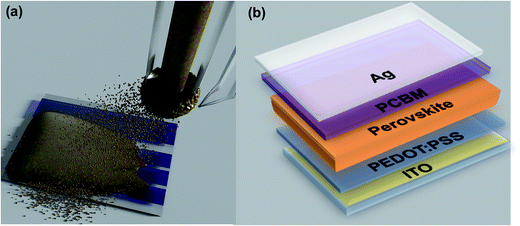 | ||
| Fig. 1 (a) Scheme of perovskite film casting via one-step spray-coating; (b) structure of the perovskite devices. | ||
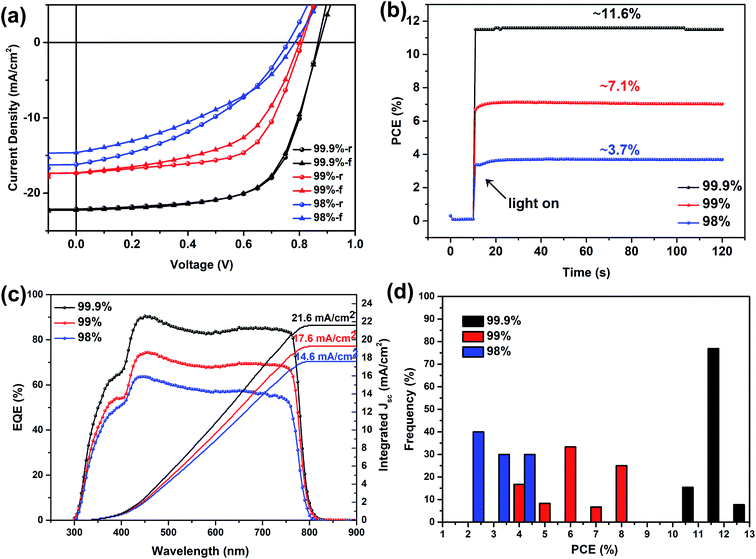
While other parameters were kept similar, our device study suggests that the purity of the precursor PbI2, which contains byproducts and other metal residues (mainly Na, K, Ca, Si and Fe revealed by elemental analysis) during PbI2 production, can significantly affect the device performance as shown in Fig. 1 and Table 1. For solar cells incorporating the perovskite layer spray-cast from the precursor made of MAI and PbI2 with a purity of 99.9%, average PCE values of 11.3% and 10.6% were achieved during reverse (1.1 V to −0.1 V) and forward (−0.1 V to 1.1 V) scans, respectively. A maximum efficiency of 12.6% was achieved from the reverse scan, with an associated open-circuit voltage (VOC) of 0.87 V, short-circuit current density (JSC) of 22.5 mA cm−2 and a fill factor (FF) of 0.63. The maximum PCE from the forward scan was 12.5%, therefore demonstrating negligible hysteresis. The average and maximum PCEs achieved in these planar heterojunction spray-coated MAPbI3 solar cells are very close to our previously reported efficiency of spin-cast MAPbI3 solar cells, while both use PEDOT:PSS and PCBM as hole and electron extracting materials, respectively.24 However, the photovoltaic metrics, i.e. PCE, VOC, JSC and FF, were all reduced when the purity of the PbI2 during the one-step spray-casting was reduced (as summarized in Table 1) and obvious hysteresis appeared as well (see Fig. 2a). When the PbI2 purity was 99%, the average PCE reduced abruptly to 6.6% with a maximum achieved PCE of only 8.8%, from the reverse scan. When the PbI2 purity was further reduced to 98%, although a marginal reduction, the average PCE further deteriorated to 3.5% (with the maximum PCE of 4.9% during the reverse scan). In order to confirm the photo-response of such solar cells, the external quantum efficiency (EQE) was measured. As shown in Fig. 2c, the integrated current density was 21.6, 17.6 and 14.6 mA cm−2 in the perovskite spray-cast from MAI and PbI2 with purities of 99.9, 99 and 98% respectively, and these values are around 4% lower than the JSC recorded from the J–V scans. Considering that EQE measurement adopts monochromatic illumination with a typical wavelength ranging from 300 to 800 nm, while a J–V scan utilizes a solar simulator that better represents the solar spectrum, this discrepancy between the EQE and JSC of 5–10% has been reported in the literature and is acceptable.25 The decrease in the EQE efficiency suggests that a low-purity precursor may reduce light absorption, increase charge recombination and reduce charge transport in the final perovskite device,26,27 effects that we will discuss in later sections. Such a change is consistent with the stabilized power output measured at the maximum power point (MPP) as shown in Fig. 2b, where the reduction of the PbI2 purity from 99.9% to 99% and further to 98% results in the decrease of the stabilized power output from 11.6% to 7.1% and further to 3.7%, respectively. Fig. 2d illustrates the statistical distribution of PCEs of devices prepared using MAI and PbI2, with different purities. PCEs of perovskite solar cells spray-cast using PbI2 of 99% purity or less barely achieved an efficiency over 10%. These results clearly reveal the feasibility of controlling the precursor purity to enhance the PCEs of spray-cast perovskite solar cells. In a previous work investigating the impact of PbI2 purity on the photovoltaic performance using a one-step spin casting film fabrication method revealed significantly reduced PCE even when the purity of PbI2 was reduced from 99.999% to 99%.26 We have found that the impacts of PbI2 purity on the device efficiency can be minimized by changing the deposition method from one-step spray-coating to a two-step spin casting approach.24Table 2 summarizes the photovoltaic metrics of spin-coated perovskite solar cells using PbI2 of three different purities The maximum PCEs from reverse scans are 11.9%, 12.1% and 12.2%, respectively, when the PbI2 purity is 98, 99 and 99.9%. X-ray diffraction and scanning electron microscopy characterizations (see Fig. S1†) show that perovskite crystals with less defects can be formed while using PbI2 of three different purities. This highlights that the impacts of precursor purity on the device performance rely heavily on the perovskite casting method and specific processing approaches.27
| PbI2 purity | Scan direction | PCEave (PCEmax) | FFave (%) (max) | J SC ave (mA cm−2) (max) | V OC ave (V) (max) |
|---|---|---|---|---|---|
| 99.9% | RS | 11.3 ± 0.5(12.6) | 61.2 ± 2.0(63.4) | 21.7 ± 0.4(22.5) | 0.85 ± 0.01(0.87) |
| FS | 10.6 ± 0.9(12.5) | 58.0 ± 3.3(60.2) | 21.9 ± 0.4(22.3) | 0.83 ± 0.03(0.87) | |
| 99% | RS | 6.6 ± 1.4(8.8) | 52.4 ± 7.1(57.5) | 16.3 ± 1.3(17.8) | 0.77 ± 0.04(0.81) |
| FS | 5.9 ± 1.1(7.6) | 47.9 ± 5.5(55.6) | 16.2 ± 1.4(17.5) | 0.75 ± 0.04(0.80) | |
| 98% | RS | 3.5 ± 0.8(4.9) | 36.0 ± 3.0(43.6) | 14.2 ± 0.9(15.2) | 0.68 ± 0.08(0.74) |
| FS | 3.0 ± 0.8(4.4) | 33.0 ± 4.2(42.6) | 12.4 ± 1.2(13.6) | 0.62 ± 0.07(0.76) |
| PbI2 purity | Scan direction | PCEave (PCEmax) | FFave (%) (max) | J SC ave (mA cm−2) (max) | V OC ave (V) (max) |
|---|---|---|---|---|---|
| 99.9% | RS | 11.5 ± 0.6(12.2) | 70.6 ± 2.6(73.2) | 19.2 ± 0.3(19.6) | 0.85 ± 0.01(0.85) |
| FS | 11.4 ± 0.5(12.0) | 69.4 ± 2.1(71.4) | 19.3 ± 0.3(19.7) | 0.85 ± 0.02(0.85) | |
| 99% | RS | 11.3 ± 0.6(12.1) | 69.8 ± 2.8(72.5) | 18.9 ± 0.3(19.3) | 0.85 ± 0.02(0.86) |
| FS | 11.1 ± 0.5(11.7) | 69.7 ± 2.9(71.2) | 18.9 ± 0.3(19.2) | 0.84 ± 0.02(0.86) | |
| 98% | RS | 10.4 ± 1.0(11.9) | 66.1 ± 3.4(69.9) | 17.5 ± 0.9(19.0) | 0.90 ± 0.01(0.90) |
| FS | 10.1 ± 0.9(11.7) | 65.9 ± 2.8(69.8) | 17.3 ± 1.0(18.8) | 0.88 ± 0.01(0.89) |
In order to investigate how the purity of PbI2 would determine the device performance to such a great extent during the spray-coating process, we have examined the morphology and crystallinity of these perovskite films. First of all, the PbI2 purity led to a notable difference in the precursor blend solutions. Fig. 3a shows the photos of the precursor MAI blended with PbI2 of different purities, with the same solid content in the solution with a mixture of DMSO and GBL solvents (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The solutions with 99.9% and 99% purity of PbI2 are transparent yellow, while the one with 98% purity PbI2 is opaque although no sediments were observed. We suspect that this is due to light scattering from metal residues that the low purity PbI2 contains. The absorption spectra of these perovskite films spray-cast on glass/PEDOT:PSS are shown in Fig. 3b, and it is obvious that the perovskite film made from 99.9% purity PbI2 has the strongest light absorption. The perovskite film made from 99% purity PbI2 showed a small reduction of absorption in the longer wavelength range (from 650 to 780 nm), but the film made from 98% purity PbI2 showed significant absorption reduction from 500 to 780 nm. The reduced light absorption ability would lead to reduced EQE values, as our EQE measurements have proved. X-ray diffraction (XRD) was employed to investigate the crystallinity of our spray-cast perovskite films, as shown in Fig. 3c. Two diffraction peaks at the 2θ of 20.6 and 40.6° correspond to the (112)/(220) and (224)/(440) planes of MAPbI3 crystals.28 The intensities of these two peaks are strongest for the perovskite films cast from MAI and 99.9% purity PbI2, and are much lower for the perovskite films cast from MAI and 99% purity PbI2. These two peaks of the perovskite film cast from MAI and 98% purity PbI2 have the lowest intensity, indicating the lowest crystallinity and quality of the perovskite in the film. Furthermore, we found that for perovskite films spray-cast using low purity PbI2, numerous diffraction peaks appeared in the 2θ range from 25 to 35° in the XRD spectra, in addition to the two primary diffraction peaks, although their intensities are much lower. By comparing them to the XRD spectra of the MAI powder,29 we regarded the peaks with low intensity as those of the precursor MAI, which has not been converted to MAPbI3 during the film fabrication process. We can actually observe the unconverted MAI on the surfaces of the perovskite films made using 99% and 98% purity PbI2 (see the white spots in Fig. 3d), while they are absent on the perovskite film surface prepared from MAI and the highest purity PbI2. This suggests that the purity of PbI2 affected the conversion and growth of MAPbI3 during this one-step spray-casting process.
1). The solutions with 99.9% and 99% purity of PbI2 are transparent yellow, while the one with 98% purity PbI2 is opaque although no sediments were observed. We suspect that this is due to light scattering from metal residues that the low purity PbI2 contains. The absorption spectra of these perovskite films spray-cast on glass/PEDOT:PSS are shown in Fig. 3b, and it is obvious that the perovskite film made from 99.9% purity PbI2 has the strongest light absorption. The perovskite film made from 99% purity PbI2 showed a small reduction of absorption in the longer wavelength range (from 650 to 780 nm), but the film made from 98% purity PbI2 showed significant absorption reduction from 500 to 780 nm. The reduced light absorption ability would lead to reduced EQE values, as our EQE measurements have proved. X-ray diffraction (XRD) was employed to investigate the crystallinity of our spray-cast perovskite films, as shown in Fig. 3c. Two diffraction peaks at the 2θ of 20.6 and 40.6° correspond to the (112)/(220) and (224)/(440) planes of MAPbI3 crystals.28 The intensities of these two peaks are strongest for the perovskite films cast from MAI and 99.9% purity PbI2, and are much lower for the perovskite films cast from MAI and 99% purity PbI2. These two peaks of the perovskite film cast from MAI and 98% purity PbI2 have the lowest intensity, indicating the lowest crystallinity and quality of the perovskite in the film. Furthermore, we found that for perovskite films spray-cast using low purity PbI2, numerous diffraction peaks appeared in the 2θ range from 25 to 35° in the XRD spectra, in addition to the two primary diffraction peaks, although their intensities are much lower. By comparing them to the XRD spectra of the MAI powder,29 we regarded the peaks with low intensity as those of the precursor MAI, which has not been converted to MAPbI3 during the film fabrication process. We can actually observe the unconverted MAI on the surfaces of the perovskite films made using 99% and 98% purity PbI2 (see the white spots in Fig. 3d), while they are absent on the perovskite film surface prepared from MAI and the highest purity PbI2. This suggests that the purity of PbI2 affected the conversion and growth of MAPbI3 during this one-step spray-casting process.
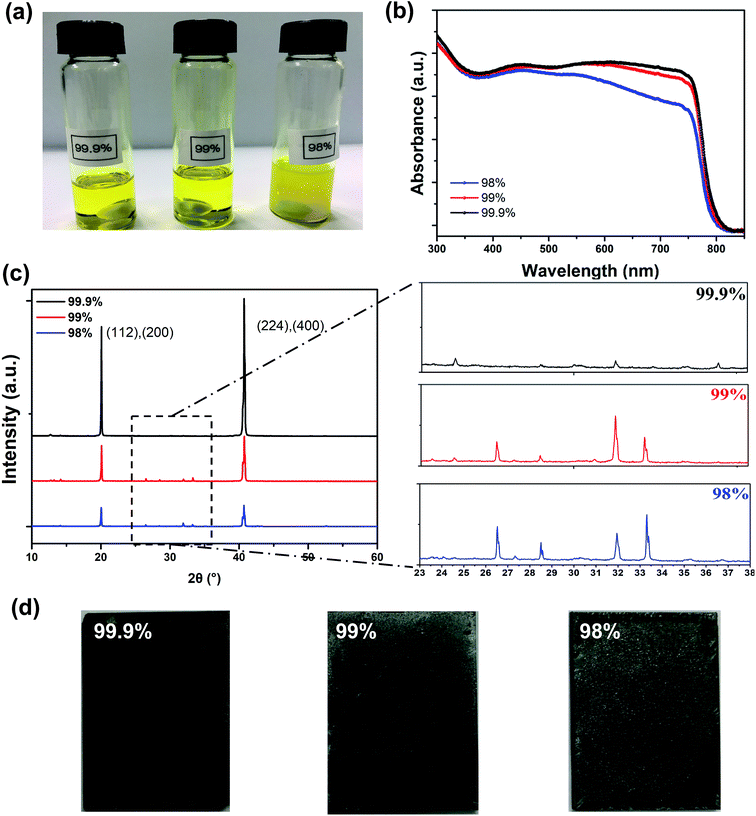 | ||
| Fig. 3 (a) Pictures of different purity precursor solutions; (b) UV-vis absorption spectra; (c) XRD patterns and (d) film photographs based on different purity samples. | ||
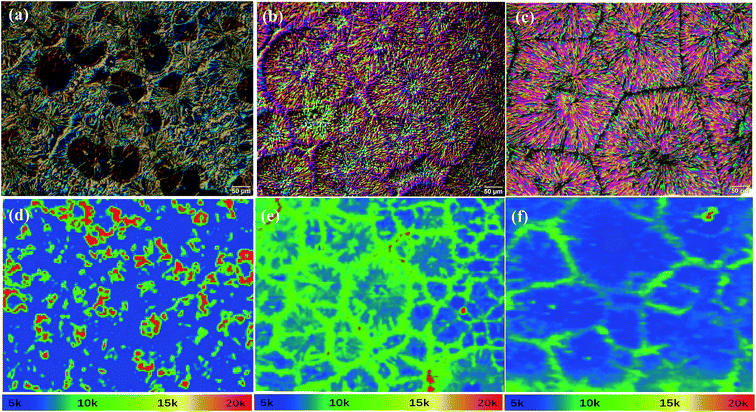
The quality of the perovskite films prepared using PbI2 with different purities can be further characterized by optical imaging and photoluminescence (PL) mapping as the grain sizes in these spray-cast perovskite films are much larger than those of their spin cast counterparts.23 As can be seen from the optical image in Fig. 4a, the perovskite film prepared from 98% purity PbI2 has a rough surface, and non-uniform distribution of few circular perovskite crystals on the film surface. This is better revealed via the PL mapping presented in Fig. 4d, in which irregular patterns made of precursors and perovskite crystals with a less defined shape are observed.10 In Fig. 4d–f, the blue area has the lowest PL intensity, and the red area has the highest PL intensity, while the green area lies in the middle. In Fig. 4d, the blue and red areas are both continuous and we believe they correspond to the un-converted precursors MAI and PbI2, respectively. Our results therefore suggest that the impurities in 98% purity PbI2 significantly hinder the growth of MAPbI3, leading to an extremely low conversion of precursors to perovskites and is consistent with the XRD testing; therefore, low device efficiency and hysteresis were obtained. When the purity of PbI2 was increased from 98 to 99%, the conversion of precursors to perovskites was greatly enhanced. However, both big and small perovskite crystals can be observed from the optical (Fig. 4b) and PL (Fig. 4e) images, and the presence of the small crystals introduces a large number of grain boundaries within the perovskite films. These grain boundaries correspond to the green lines in Fig. 4e and have a higher PL intensity due to serious charge recombination in the boundaries.10 The presence of these grain boundaries with high charge recombination rates will significantly reduce the FF and JSC of the perovskite devices, as shown in Table 1 and Fig. 2. The perovskite films cast from 99.9% purity PbI2 were uniform, and the perovskite crystals were large (200–400 μm) with much fewer grain boundaries (see Fig. 4c and f), characteristics that would lead to enhanced charge transport and reduced recombination, and eventually contribute to an enhanced device efficiency as our device study has demonstrated. Based on these results, we can conclude that the variations in the device performance are caused by the PbI2 purity through its impacts on the crystallinity and morphology of the spray-cast perovskite photoactive layer.
The charge recombination in the spray-cast perovskite devices using MAI and PbI2 precursors of different purities was evaluated via the light intensity dependent VOC. In the VOCvs. light intensity plot shown in Fig. 5a, a slope value of unity kT/q suggests bimolecular recombination in the device, whilst the deviation of the slope from unity kT/q suggests a combination of trap-assisted and bimolecular recombination in the device.30–32 For the spray-cast perovskite using 98% purity PbI2, a slope of 1.89kT/q is obtained and suggests a severe energy loss as a result of trap-assisted recombination. This slope decreases to 1.26kT/q and then 1.05kT/q as the purity increases, suggesting reduced charge traps due to improved morphology and crystallinity. The charge recombination in these devices was further examined via electrochemical impedance spectroscopy (EIS) measurements of the corresponding devices, under a bias of 0.6 V with an amplitude of 10 mV from 1 MHz to 100 Hz under dark conditions. Fig. 5b shows the Nyquist plots of perovskite devices measured near the open-circuit voltage under dark conditions, and can be well fitted using the equivalent circuit model shown in the inset. The circuit includes a series resistance (Rs), a contact resistance (Rco) and a recombination resistance (Rrec)33. The Rrec extracted from data fitting of the EIS spectra increased from 1618 to 2895 and eventually 4706 Ω with the increased PbI2 purity from 98 to 99 and 99.9% respectively, suggesting a continuously reduced charge recombination rate. The EIS measurements are therefore consistent with the conclusion from the measurements of VOC as a function of light intensity.
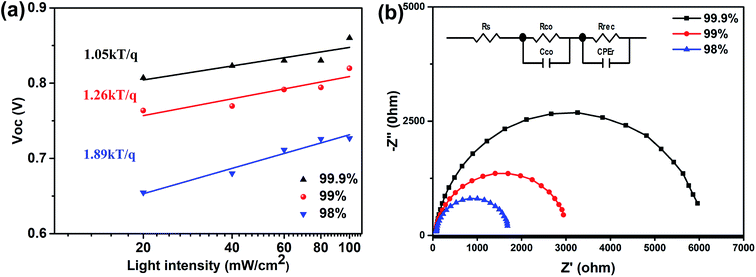 | ||
| Fig. 5 (a) VOC as a function of light intensity in a semi-log plot, and (b) Nyquist plots of the solar cells incorporating perovskite layers spray-cast from MAI and PbI2 having different purities. | ||
4. Conclusion
In summary, we have demonstrated that the purity of the precursor PbI2 can significantly affect the device efficiency through its impact on the morphology and crystallinity of the perovskite photoactive layer prepared via one-step spray-casting. While the PbI2 with a high purity of 99.9% leads to a high conversion of the precursors to perovskites, producing uniform crystals with the grain size of 200–400 μm and fewer grain boundaries, the purity of 99% introduces a large number of grain boundaries through the existence of small crystals among the big ones, and the purity of 98% results in an extremely low conversion of precursors to perovskites and therefore the lowest device efficiency. By incorporating the spray-cast perovskite layer using MAI and the 99.9% PbI2 into a planar heterojunction device ITO/PEDOT:PSS/MAPbI3/PCBM/Ag, we achieved the maximum PCE of 12.6% (JSC = 22.5 mA cm−2, VOC = 0.87 V, and FF = 63%) without hysteresis, efficiency that is similar to that of the spin-cast counterpart. These results suggest that the purity of precursors should be strongly considered when preparing high performance perovskite solar cells using a scalable spray-coating approach.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Mr Qiuquan Cai for preliminary investigation on the spray-coated perovskite solar cells. We acknowledge funding support from the National Natural Science Foundation of China (Grant No. 21504065, 21774097), and the Fundamental Research Funds for the Central Universities (WUT: 2015III018 and 2015III029) of China. TW also acknowledges support from the Recruitment Program of Global Experts (1000 Talents Plan) of China.References
- H. S. Jung and N.-G. Park, Small, 2015, 11, 10 CrossRef CAS PubMed.
- Q. Lin, A. Armin, P. L. Burn and P. Meredith, Acc. Chem. Res., 2016, 49, 545 CrossRef CAS PubMed.
- L. Yang, A. T. Barrows, D. G. Lidzey and T. Wang, Rep. Prog. Phys., 2016, 79, 026501 CrossRef PubMed.
- J. Seo, J. H. Noh and S. Seok II, Acc. Chem. Res., 2016, 49, 562 CrossRef CAS PubMed.
- J. S. Manser, M. I. Saidaminov, J. A. Christians, O. M. Bakr and P. V. Kamat, Acc. Chem. Res., 2016, 49, 330 CrossRef CAS PubMed.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050 CrossRef CAS PubMed.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643 CrossRef CAS PubMed.
- H.-S. Kim, C.-R. Lee, J.-H. Im, K.-B. Lee, T. Moehl, A. Marchioro, S.-J. Moon, R. Humphry-Baker, J.-H. Yum, J. E. Moser, M. Grätzel and N.-G. Park, Sci. Rep., 2012, 2, 591 CrossRef PubMed.
- W. S. Yang, B.-W. Park, E. H. Jung, N. J. Jeon, Y. C. Kim, D. U. Lee, S. S. Shin, J. Seo, E. K. Kim, J. H. Noh and S. Seok II, Science, 2017, 356, 1376 CrossRef CAS PubMed.
- X. Liu, X. Xia, Q. Cai, F. Cai, L. Yang, Y. Yan and T. Wang, Sol. Energy Mater. Sol. Cells, 2017, 159, 412 CrossRef CAS.
- Z. Yang, C. Chueh, F. Zuo, J. H. Kim, P. Liang and A. K. Y. Jen, Adv. Energy Mater., 2015, 5, 1500328 CrossRef.
- K. Hwang, Y. Jung, Y. Heo, F. H. Scholes, S. E. Watkins, J. Subbiah, D. J. Jones, D. Kim and D. Vak, Adv. Mater., 2015, 27, 1241 CrossRef CAS PubMed.
- Z. Liang, S. Zhang, X. Xu, N. Wang, J. Wang, X. Wang, Z. Bi, G. Xu, N. Yuan and J. Ding, RSC Adv., 2015, 5, 60562 RSC.
- T. Wang, N. W. Scarratt, H. Yi, A. D. F. Dunbar, A. J. Pearson, D. C. Watters, T. S. Glen, A. C. Brook, J. Kingsley, A. R. Buckley, M. W. A. Skoda, A. M. Donald, R. A. L. Jones, A. Iraqi and D. G. Lidzey, Adv. Energy Mater., 2013, 3, 505 CrossRef CAS.
- Y. Zhang, J. Griffin, N. W. Scarratt, T. Wang and D. G. Lidzey, Prog. Photovoltaics, 2016, 24, 275 CAS.
- Y. Zhang, N. W. Scarratt, T. Wang and D. G. Lidzey, Vacuum, 2017, 139, 154 CrossRef CAS.
- M. Habibi, M.-R. Ahmadian-Yazdi and M. Eslamian, J. Photonics Energy, 2017, 7, 22003 CrossRef.
- H. Huang, J. Shi, L. Zhu, D. Li, Y. Luo and Q. Meng, Nano Energy, 2016, 27, 352 CrossRef CAS.
- D. K. Mohamad, J. Griffin, C. Bracher, A. T. Barrows and D. G. Lidzey, Adv. Energy Mater., 2016, 6, 1600994 CrossRef.
- J. E. Bishop, D. K. Mohamad, M. Wong-Stringer, A. Smith and D. G. Lidzey, Sci. Rep., 2017, 7, 7962 CrossRef PubMed.
- M. Remeika, S. R. Raga, S. Zhang and Y. Qi, J. Mater. Chem. A, 2017, 5, 5709 CAS.
- S. Das, B. Yang, G. Gu, P. C. Joshi, I. N. Ivanov, C. M. Rouleau, T. Aytug, D. B. Geohegan and K. Xiao, ACS Photonics, 2015, 2, 680 CrossRef CAS.
- W. Zhang, M. Saliba, D. T. Moore, S. K. Pathak, M. T. Hörantner, T. Stergiopoulos, S. D. Stranks, G. E. Eperon, J. A. Alexander-Webber, A. Abate, A. Sadhanala, S. Yao, Y. Chen, R. H. Friend, L. A. Estroff, U. Wiesner and H. J. Snaith, Nat. Commun., 2015, 6, 6142 CrossRef CAS PubMed.
- L. Yang, Y. Yan, F. Cai, J. Li and T. Wang, Sol. Energy Mater. Sol. Cells, 2017, 163, 210 CrossRef CAS.
- A. T. Barrows, A. J. Pearson, C. K. Kwak, A. D. F. Dunbar, A. R. Buckley and D. G. Lidzey, Energy Environ. Sci., 2014, 7, 2944 CAS.
- J. Chang, H. Zhu, B. Li, F. Isikgor, Y. Hao, Q. Xu and J. Ouyang, J. Mater. Chem. A, 2016, 4, 887 CAS.
- N. Guo, T. Zhang, G. Li, F. Xu, X. Qian and Y. Zhao, J. Semicond., 2017, 38, 14004 CrossRef.
- W. Chang, D. Lan, K. M. Lee, X. Wang and C. Liu, ChemSusChem, 2017, 10, 1405 CrossRef CAS PubMed.
- X. Wang, X. Li, G. Tang, L. Zhao, W. Zhang, T. Jiu and J. Fang, Org. Electron., 2015, 24, 205 CrossRef CAS.
- H. Kim, J. Lee, N. Yantara, P. P. Boix, S. A. Kulkarni, S. Mhaisalkar, M. Grätzel and N. Park, Nano Lett., 2013, 13, 2412 CrossRef CAS PubMed.
- G. Xing, N. Mathews, S. Sun, S. S. Lim, Y. M. Lam, M. Grätzel, S. Mhaisalkar and T. C. Sum, Science, 2013, 342, 344 CrossRef CAS PubMed.
- T. Wang, N. W. Scarratt, H. Yi, I. F. Coleman, Y. Zhang, R. T. Grant, J. Yao, M. W. A. Skoda, A. D. F. Dunbar, R. A. L. Jones, A. Iraqi and D. G. Lidzey, J. Mater. Chem. C, 2015, 3, 4007 RSC.
- G. A. H. Wetzelaer, M. Scheepers, A. M. Sempere, C. Momblona, J. Vila and H. J. Bolink, Adv. Mater., 2015, 27, 1837 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00536a |
| This journal is © The Royal Society of Chemistry 2018 |