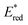A red to blue series of push–pull dyes for NiO based p-DSSCs†
R.
Brisse
a,
C.
Praveen
 b,
V.
Maffeis
ac,
T.
Bourgeteau
a,
D.
Tondelier
d,
T.
Berthelot
b,
V.
Maffeis
ac,
T.
Bourgeteau
a,
D.
Tondelier
d,
T.
Berthelot
 a,
B.
Geffroy
ad,
T.
Gustavsson
c,
J. M.
Raimundo
b and
B.
Jousselme
a,
B.
Geffroy
ad,
T.
Gustavsson
c,
J. M.
Raimundo
b and
B.
Jousselme
 *a
*a
aLaboratory of Innovation in Surface Chemistry and Nanosciences (LICSEN), NIMBE, CEA, CNRS, Université Paris-Saclay, CEA Saclay, 91191 Gif-sur-Yvette Cedex, France. E-mail: bruno.jousselme@cea.fr; Tel: +33 169089191
bCentre Interdisciplinaire de Nanoscience de Marseille (CINaM), CNRS UMR 7325, Aix Marseille Université, 13288 Marseille Cedex 09, France
cLIDYL, CEA, CNRS, Université Paris-Saclay, CEA-Saclay, 91191 Gif-sur-Yvette, France
dLaboratoire de Physique des Interfaces et Couches Minces (LPICM), CNRS UMR 7647, Ecole Polytechnique, Palaiseau F-91128, France
First published on 18th December 2017
Abstract
Finding efficient dyes for NiO photocathodes either for inverse or tandem DSSCs is essential to developing these promising low-cost solar cells. This paper reports the design, synthesis and physical property characterization of four new triphenylamine–bithiophene push–pull dyes with acceptors of increasing electronic affinity, in order to shift their absorption to the red region of the visible spectrum. The dyes were tested in a p-type DSSC configuration with 850 nm NiO ink-jet printed photocathodes and their performances were compared with that of the reference dye P1. With an iodine electrolyte, one of the dyes, possessing a 1,3-diethyl-2-thiobarbituric acceptor, shows superior performance to P1, with PCE reaching 0.124% and a JSC of 4.32 mA cm−2.
Introduction
With theoretical PCE reaching 40%, Tandem Dye Sensitized Solar Cells (T-DSSCs), which are composed of two dye-sensitized photoelectrodes, a photocathode and a photoanode, sandwiching a redox electrolyte, could represent a cost-effective and promising alternative to silicon based solar cells.1 Moreover, this field of research has several connections with the work concerning Dye Sensitized PhotoElectrosynthetic Cells (DSPECs), for low-cost and solar hydrogen production.2 However, the performance of such devices has been hampered by several hurdles, the most critical certainly being the very fast recombination of the NiO-reduced dye at the surface of the semiconducting electrode, when it is irradiated.3,4 Either geminate5 or not,6 this recombination is restricted to the most commonly used p-type material in this field of research, NiO, and more precisely to the unusually high density of intra-band-gap states on the top of its valence band, as recently reported.5,6 However, since no better electrode material has been found up to now, important research has concentrated on dye design. For that purpose, different strategies have been revealed to outperform others. The first one consists of appending a secondary electron accepting group on the sensitizer, as reported by Odobel and coworkers, using a naphthalene diimide electron acceptor.7,8 The second strategy involves the use of molecular dyads, such as PMI-6T–TPA9 and BH4,10,11 which promote charge separation at the surface of NiO. A third class of dyes gives among the best current p-type solar cells and consists of the so-called “push–pull” dyes. These dyes, widely used in the field of photoactive devices, are donor–acceptor entities (i.e. they consist of one electron rich and one electron poor part), for which light absorption induces an “immediate” movement of the electronic density from the donor to the acceptor part, generating an intramolecular charge-transfer state. In 2008, the group of Sun and coworkers successfully implemented a triphenylamine–dicyanovinylidene dye in a NiO-based p-type DSSC, resulting in the so-called “P1” dye, characterized by an “unbeatable” IPCE of 64%.12,13 The success of this class of dyes was later confirmed with other compounds, such as CAD3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 and QT1
14 and QT1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, which displayed record short-circuit currents for a p-type device above 8 mA cm−2.
15, which displayed record short-circuit currents for a p-type device above 8 mA cm−2.
However, the exact rationale for push–pull dyes to be efficient is not well understood and a contradiction exists between the usually short lifetime of photo-reduced push–pull dyes at the surface of NiO, in the range of tens of picoseconds, and their superior DSSC performances.16,17 Subtle mechanisms may exist to explain the efficiency of push–pull dyes, linked to their arrangement at the level of the electrode surface, the way their orbitals are coupled to the NiO valence band, or the way they orientate the interaction of the (iodine) electrolyte species with the surface of NiO.18,19 In order to understand better the reason for the efficiency of push–pull compounds, it is important to develop new ones. An important point is to design and synthesize efficient dyes that do not compete with the optical absorption of the photoanode, for efficient T-DSSCs. As exemplified by recent studies there exist very efficient photoanodes absorbing a narrow portion of the blue part of the visible spectrum.20,21 Therefore, research has focused on finding suitable dyes for photocathodes that absorb the red/IR part of the solar spectrum (at wavelengths longer than 600 nm). Such dyes have been relatively scarce, however, due to the limited number of available functional groups.14,22–24 To reach that goal, one possibility is to use push–pull dyes with a fixed electron rich moiety and to increase the electronic affinity (or “strength”) of the acceptor part.16
In this work, we designed and synthesized four new push–pull dyes (Fig. 1) for NiO-based photocathodes. They are composed of a “classical” triphenylamine-bithiophene moiety, for efficient dye-to-NiO hole injection and are flanked by two carboxylic acid groups, for grafting onto NiO. The dyes incorporate four different acceptors with a gradually increasing electron affinity: naphthalimide, 1,3-indandione, 1,3-diethyl-2-thiobarbituric acid and 2-(3-oxo-indan-1-ylidene)-malonitrile respectively. These acceptors are different from the ones used in the past in the field of p-type DSSCs (except the 1,3-diethyl-2-thiobarbituric acid one16,23,25). Moreover, concerning the naphthalimide acceptor, its structure is very different from the perylene-monoimide structure that has been used in other studies,9,10 as the naphthalimide entity does not absorb in the visible region (perylene-monoimide strongly absorbs at around 500 nm). The increase of the acceptor's electron affinity allowed for a gradual modulation of the optical absorption window of the photocathode in order to reach the red part of the spectrum. These new dyes were tested in a p-type DSSC configuration and their performances were compared with that of the P1 dye. Their efficiencies were also correlated with their structures and their physical properties. The influence of the geometry of the dye on the molar absorption coefficient of the optical charge transfer absorption band is also presented.
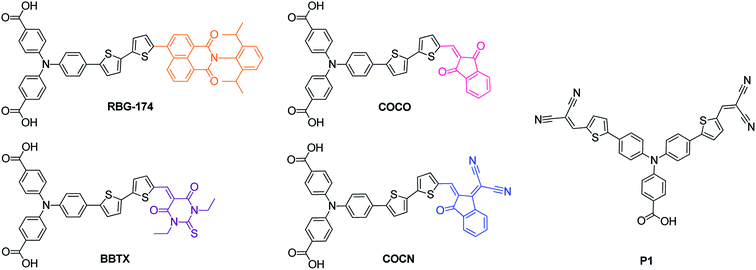 | ||
| Fig. 1 Chemical structures of the synthesized push–pull dyes (RBG-174, COCO, BBTX, COCN and the reference dye P1). | ||
Results and discussion
Synthesis of the chromophores
The synthesis of the different dyes was executed in a modular manner (Fig. 2 and 3); the experimental details can be found in the ESI.† Initially, a triphenylamine-boronic-ester moiety 3 was prepared in three steps.26 In particular, the key Buchwald–Hartwig coupling reaction was performed under strict anhydrous conditions. This provided the triphenylamine entity via a one step reaction with a good yield, which is novel considering previous reports.22,26 Concerning the acceptors, 1,3-indandione, 1,3-diethyl-2-thiobarbituric acid and 2-(3-oxo-indan-1-ylidene)-malonitrile were commercially available. The naphthalimide accepting group 4 was synthesized according to previous protocols.27 All the accepting groups were conjugated to a bithiophene entity via Knoevenagel condensation of the indane-derivatives and a bithiophene carboxaldehyde or through a microwave assisted Suzuki–Miyaura cross coupling of the bithiophene boronic pinacol–ester and naphthalimide acceptor 4. A Suzuki–Miyaura microwave assisted cross-coupling reaction finally ensured that all the bithiophene–acceptors were efficiently coupled to triphenylamine-boronic-pinacol–ester 3 (Fig. 3). The final products were obtained after deprotection of the carboxylic acid with trifluoroacetic acid.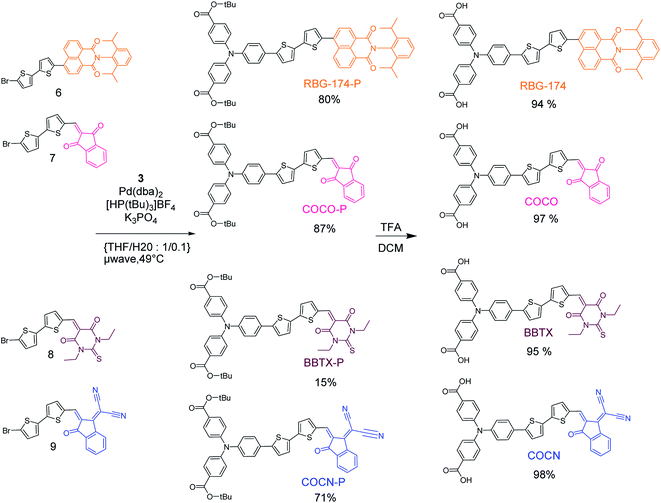 | ||
| Fig. 3 Microwave-assisted Suzuki–Miyaura coupling for the synthesis of the tert-butyl ester protected version of the dyes and their subsequent deprotection in trifluoroacetic acid. | ||
Physical characterisation and quantum calculation
Because of the carboxylic acid functionalities and absence of alkyl chains, it was not possible to solubilize COCO and COCN dyes in common and low boiling point organic solvents. Thus, their physical properties were investigated using their tert-butyl ester protected analogues (COCO-P and COCN-P respectively). In order to have comparable results, the physical properties of RBG-174 and BBTX were also assessed from their tert-butyl ester protected versions (i.e.RBG-174-P and BBTX-P). The properties of dye P1, which was used in this publication as a reference dye, are also recalled in this paper (Table 1).16| Dye | λ abs, max, CT (nm) | ε max (L mol−1 cm−1) | E 0−0 (eV) | E ox (V vs. Fc+/Fc) | E red (V vs. Fc+/Fc) | (V vs. Fc+/Fc) | ΔGinjd (eV) | ΔGregd (eV) |
|---|---|---|---|---|---|---|---|---|
a
E
0−0 energy was calculated using the equation E0−0 = 1240/λ0−0, where λ0−0 is the intersection wavelength between the normalized absorption and emission spectra for the lowest energy band. The absorption spectrum had been normalized versus the CT band.
b For reversible processes the reported potential is the half-wave potential. For irreversible processes the peak potential was used.
c
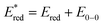 .
d .
d
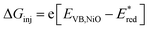 , with EVB,NiO ∼ −0.12 V vs. Fc+/FcACN,14 , with EVB,NiO ∼ −0.12 V vs. Fc+/FcACN,14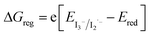 , with , with 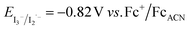 .14 It should be noted however that in ref. 14, the redox potentials were determined by differential pulse voltammetry.
e
P1 data were taken from ref. 16. .14 It should be noted however that in ref. 14, the redox potentials were determined by differential pulse voltammetry.
e
P1 data were taken from ref. 16.
|
||||||||
| RBG-174-P | 442 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2.38 | 0.50 | −1.68 | 0.7 | −0.84 | −0.80 |
| COCO-P | 519 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 660 660 |
2.05 | 0.57 | −1.45 | 0.6 | −0.74 | −0.57 |
| BBTX-P | 542 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 530 530 |
1.97 | 0.58 | −1.28 | 0.7 | −0.83 | −0.40 |
| COCN-P | 590 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 390 |
1.81 | 0.57 | −1.11 | 0.7 | −0.84 | −0.23 |
| P1 | 481 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
2.25 | 0.62 | −1.53 | 0.72 | −0.86 | −0.65 |
The absorption spectrum and molar absorption coefficient of each dye in methylene chloride were determined from their steady-state absorption spectra (Fig. 4). Then, in order to evaluate the ability of the compounds to inject a hole into the NiO valence band (VB) (EVB ∼ 0.12 V vs. Fc+/FcACN)14 and to be regenerated by the iodine based electrolyte 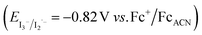 ,14 the ground-state redox levels of the dyes were determined via cyclic voltamperometry, using Fc+/Fc as a pseudo-reference for comparison with the literature (Fig. S11†) and the E0−0 energy was determined optically, from the intersection of the normalized absorption and fluorescence spectra (Fig. S9†). In order to assess the orbital composition of the optical transitions and to corroborate the evolution of the redox levels with the structure of the molecules, quantum chemistry calculations with Gaussian 03 Software were performed for every final component (on the carboxylic acid version). Therefore, geometry optimization, orbital energy as well as shape, and a simulation of the absorption spectrum were established (see the ESI†).
,14 the ground-state redox levels of the dyes were determined via cyclic voltamperometry, using Fc+/Fc as a pseudo-reference for comparison with the literature (Fig. S11†) and the E0−0 energy was determined optically, from the intersection of the normalized absorption and fluorescence spectra (Fig. S9†). In order to assess the orbital composition of the optical transitions and to corroborate the evolution of the redox levels with the structure of the molecules, quantum chemistry calculations with Gaussian 03 Software were performed for every final component (on the carboxylic acid version). Therefore, geometry optimization, orbital energy as well as shape, and a simulation of the absorption spectrum were established (see the ESI†).
Optical properties
The UV visible absorption spectra of the four bithiophene dyes are presented in Fig. 4. All the dyes display two main and intense absorption bands. The highest energy band is centred at approximately 360 nm, and its intensity varies between 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 50
000 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mol−1 L cm−1. Judging from the quantum calculations, this band orbital composition varies from one dye to another, but always comprises a contribution centred on the donor part (see the ESI†). In agreement with the theoretical calculation of the orbital's energy, when the electron affinity of the acceptor is increased, the lowest energy band shifts toward higher wavelengths: 442 nm for RBG-174-P, 519 nm for COCO-P, 542 nm for BBTX-P and 590 nm for COCN-P. In particular, the strong 2-(3-oxo-indan-1-ylidene)-malonitrile acceptor enables reaching the red part of the spectrum (absorption up to ca. 680 nm). As expected, quantum chemistry calculations attribute this band to a push–pull transition. Its molar absorption coefficient (at the peak wavelength) varies from 27
000 mol−1 L cm−1. Judging from the quantum calculations, this band orbital composition varies from one dye to another, but always comprises a contribution centred on the donor part (see the ESI†). In agreement with the theoretical calculation of the orbital's energy, when the electron affinity of the acceptor is increased, the lowest energy band shifts toward higher wavelengths: 442 nm for RBG-174-P, 519 nm for COCO-P, 542 nm for BBTX-P and 590 nm for COCN-P. In particular, the strong 2-(3-oxo-indan-1-ylidene)-malonitrile acceptor enables reaching the red part of the spectrum (absorption up to ca. 680 nm). As expected, quantum chemistry calculations attribute this band to a push–pull transition. Its molar absorption coefficient (at the peak wavelength) varies from 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 mol−1 cm−1 L for RBG-174-P to 43
100 mol−1 cm−1 L for RBG-174-P to 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 mol−1 cm−1 L for the dye COCO-P, 64
700 mol−1 cm−1 L for the dye COCO-P, 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 mol−1 cm−1 L for the dye BBTX-P and finally 53
500 mol−1 cm−1 L for the dye BBTX-P and finally 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 mol−1 cm−1 L for the dye COCN-P. The very high value for BBTX-P is advantageous to compete with the NiO self-absorption.3 One interpretation for the evolution of the molar absorption coefficient values across the dye series is to consider that most of the HOMO/LUMO overlapping occurs on the bithiophene part of the molecules. Keeping in mind that a push–pull transition is a displacement of the electronic density from the donor part to the acceptor part of the dye, we correlated the intensity of the push–pull transitions with the dihedral angle between the two thiophene rings of the dyes. These angles can be seen as a “brake” to the electronic motion through the thiophene bridge triggered by light irradiation. As can be seen, there is a clear linear relationship between the inter-thiophene angle and the molar absorption coefficient (Fig. 5). BBTX-P is a strongly absorbing dye, owing to its perfectly flat geometry (inter-thiophene angle close or equal to 1.58°).
400 mol−1 cm−1 L for the dye COCN-P. The very high value for BBTX-P is advantageous to compete with the NiO self-absorption.3 One interpretation for the evolution of the molar absorption coefficient values across the dye series is to consider that most of the HOMO/LUMO overlapping occurs on the bithiophene part of the molecules. Keeping in mind that a push–pull transition is a displacement of the electronic density from the donor part to the acceptor part of the dye, we correlated the intensity of the push–pull transitions with the dihedral angle between the two thiophene rings of the dyes. These angles can be seen as a “brake” to the electronic motion through the thiophene bridge triggered by light irradiation. As can be seen, there is a clear linear relationship between the inter-thiophene angle and the molar absorption coefficient (Fig. 5). BBTX-P is a strongly absorbing dye, owing to its perfectly flat geometry (inter-thiophene angle close or equal to 1.58°).
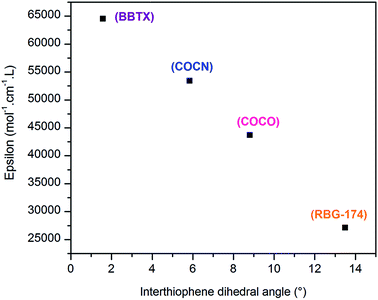 | ||
| Fig. 5 Evolution of the molar absorption coefficient as a function of the inter-thiophene angle across the synthesized dyes series. | ||
Redox properties
All the dyes displayed two oxidation waves between 0.50 V and 0.58 V vs. Fc+/FC and one reduction wave at −1.68 V, −1.45 V, −1.28 and −1.11 V vs. Fc+/FC, respectively for RBG-174-P, COCO-P, BBTX-P and COCN-P.The values of the oxidation potentials are typical for TPA-thiophene based push–pull compounds.26 For RBG-174-P, the first oxidation potential is slightly more positive than for the other dyes (70 mV difference). This is in agreement with the steric hindrance existing between the bithiophene and the naphthalimide which induces a large dihedral angle between these two entities (43.7°, see Fig. S12†) and obviously prevents the HOMO of RBG-174-P from spanning over the naphthalimide moiety, in contrast to the other dyes, which are flatter. The first oxidation wave is reversible in all cases, indicating that the radical-cation of all dyes is a stable species.
Concerning the reduction wave, it is observed that its potential shifts towards more negative values, with the increase of the accepting strength. This observation is in agreement with the shift of the optical absorption and with previous studies.12,28 The reduction process is reversible for dye RBG-174-P and irreversible for the other dyes. One difference between COCO, BBTX, COCN and RBG-174 is the presence of an ethylene bond for the first three ones. As suggested in a study by Oliva and co-workers, it is possible that for push–pull dyes with an accepting group bearing an ethylene functionality, the radical anion, corresponding to the reduced form of the dye, has a short lifetime and undergoes dimerization, giving an irreversible reduction wave.29
The dye-to-NiO hole injection driving force was calculated along with the dye regeneration driving force (see Table 1). As expected, the hole injection driving force is around 800 mV for all dyes, which, for the P1 dye, strongly favors hole-injection from the dye to the NiO VB, upon irradiation. The driving force for the dye regeneration by the iodine electrolyte decreases with the strength of the acceptor, from 800 mV for the RBG-174 dye to 230 mV for the COCN dye. Regarding recent literature, this driving force should be high enough for all dyes to perform well, especially for the COCN dye, which shows a regeneration driving force superior by 50 mV to one of the current best performing dyes, CAD3 (ΔGreg,CAD3 = 170 mV![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14).
14).
PV results
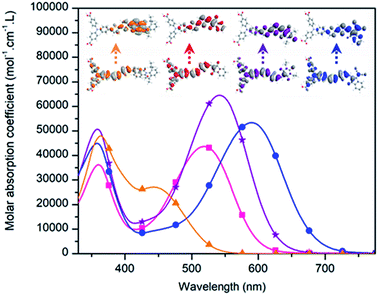
The performances of the dyes in NiO-based p-DSSCs were investigated with 850 nm NiO films, fabricated according to a previously reported procedure, i.e. inkjet-printing 4 layers of a pluronic–NiCl2-based sol–gel ink, with sequential annealing at 450 °C.30 We initially intended to sensitize NiO with RBG-174 in EtOH. Literature traditionally reports the use of dye solution in a concentration range of 0.1 mM.9,13 Therefore, a 5 × 10−4 M sensitizing solution of RBG-174 was prepared. However, this trial was unsuccessful and no sensitization was observed. When changing the solvent from EtOH to MeOH, keeping the same dye loading (i.e. 5 × 10−4 mol of compound per liter of solvent) effective sensitization occurred. Deeper investigations should be undertaken in order to understand this, but a possible explanation is that the MeOH solution was saturated, in contrast to the EtOH solution, in which RBG-174 was totally soluble. Indeed, saturated solutions for the sensitization of NiO with the three other dyes of the series were therefore employed. MeOH was also used for BBTX and concerning COCO and COCN, which were not soluble at all in common organic solvents as said above, they could be solubilised in a 1/1 mixture of MeOH and methylene chloride. A picture of the sensitization solutions is presented in Fig. S24.†p-Type DSSCs, with an iodine-based electrolyte, were eventually constructed and characterised under one sun illumination, with the AM 1.5 standard. All the experimental details concerning device fabrication and tests can be found in the ESI.† The different photovoltaic characteristics of these photocathodes, under illumination, are displayed in Fig. 6 (for the dark-current trace, see Fig. S26†). The performances are summarized in Table 2 and are compared with the P1 dye. The restricted error bars obtained in this work allowed for a good level of comparison of the performance of the different dyes.
| RBG-174 | COCO | BBTX | COCN | P1 | |
|---|---|---|---|---|---|
| PCE (%) | 0.096 ± 0.008 | 0.080 ± 0.010 | 0.126 ± 0.010 | 0.038 ± 0.003 | 0.117 ± 0.008 |
| J SC (mA cm−2) | 2.88 ± 0.23 | 2.45 ± 0.30 | 4.32 ± 0.32 | 1.53 ± 0.10 | 3.15 ± 0.15 |
| V OC (mV) | 90 ± 4 | 91 ± 5 | 88 ± 2 | 77 ± 1 | 108 ± 4 |
| FF (%) | 36.7 ± 0.1 | 35.9 ± 0.6 | 33.0 ± 0.5 | 32.3 ± 0.7 | 34.5 ± 0.9 |
All dyes gave working devices with PCE ranging from 0.038% for COCN to 0.126% for BBTX. Except for COCN, the short-circuit currents (between 2.45 mA cm−2 and 4.3 mA cm−2) are in the range of efficient p-type DSSCs usually found in recent literature.8,31,32 The obtained VOC values, between 77 mV and 91 mV (compared to 108 mV for P1), are also typical of iodine based electrolytes and NiO electrodes, as well as the FF, which was interestingly found to decreases from 36.7% to 32.3% across the bithiophene dye series, with the electron affinity of the accepting group.
BBTX outperforms all the other dyes including the reference dye P1, owing to a high JSC value of 4.32 mA cm−2. This result is promising, since it equalizes the JSC delivered by CAD3, at a similar level of NiO thickness (850 nm, see ref. 18). Since all dyes display favorable energetics, the good performance of BBTX can certainly be attributed to its greater molar extinction coefficient and the important width of its charge transfer band. Its flat geometry may also favor better electronic communication with the NiO and moreover, the aromaticity of the dye-anion form of the thiobarbituric ring could provide greater stability to the BBTX-NiO charge separated state and thus enhance its lifetime. RBG-174 and COCO (as well as P1) display similar JSC values (around 3 mA cm−2) but P1 shows a higher VOC (108 mV) than the two other dyes (90 mV). P1 displays the lowest dark-current across the dye studied in this paper and this could explain its superior VOC (see Fig. S26†). However, it should be noted that NiO//COCO electrodes also gave low dark currents; therefore, P1 may favor the photocurrent generation process compared with COCO. Importantly, it is also to be noted that despite its low absorption in the visible region and its lower molar extinction value, RBG-174 gives satisfactory performance. This is possibly linked to a better decoupling of the LUMO orbital from the NiO surface, thanks to the torsion angle between the bithiophene and the naphthalimide unit. The limited performance of COCN is more challenging to explain as this dye should have favourable energetic (see above). One possible explanation for the lower performance of COCN is that it is aggregated in an unfavourable manner onto the NiO surface. The solid state spectrum of the NiO//COCN electrode, displayed in Fig. S10,† is in favour of this explanation as hypso and hypochromic shifts and an enlargement of the dye spectrum compared with the solution spectrum are observed. Actually, the physical properties of COCN resemble the ones of CAD3; however, the solar cell performance of COCN is very low. One difference between these two dyes is the long alkyl chains, absent on COCN; these alkyl chains may help in preventing dye aggregation and boost solar cell efficiency. Additionally, in the case of COCN, the affinity of the cyano groups for oxide surfaces may also cause COCN to bind with the NiO via these groups rather than via the two carboxylic acid functionalities, therefore preventing efficient hole injection.
Conclusion
We have presented the design and synthesis of a series of four new push–pull, triphenylamine-bithiophene dyes with accepting groups of increasing strength. We have isolated compounds which absorb specific parts of the visible spectrum, ranging from 400 nm up to 700 nm. NiO-based p-type DSSCs were successfully built with all the dyes and tested under simulated sunlight, giving promising performance. In particular, this paper reports a very efficient dye, BBTX, having a thiobarbituric acid acceptor, which provides a photocurrent up to 4.3 mA cm−2, for a NiO electrode with a thickness of 850 nm. We are now exploring the origin of the dyes' performance with the help of ultrafast transient optical techniques.Conflicts of interest
There are no conflicts to declare.Acknowledgements
R. B. thanks the DSM Energy Program for the PhD thesis grant. This work was supported by the FCH Joint Undertaking (ArtipHyction Project, Grant Agreement No. 303435).References
- A. Nattestad, I. Perera and L. Spiccia, J. Photochem. Photobiol., C, 2016, 28, 44–71 CrossRef CAS.
- N. Kaeffer, J. Massin, C. Lebrun, O. Renault, M. Chavarot-Kerlidou and V. Artero, J. Am. Chem. Soc., 2016, 138, 12308–12311 CrossRef CAS PubMed.
- T. Daeneke, Z. Yu, G. P. Lee, D. Fu, N. W. Duffy, S. Makuta, Y. Tachibana, L. Spiccia, A. Mishra, P. Bäuerle and U. Bach, Adv. Energy Mater., 2015, 5, 1401387 CrossRef.
- A. Morandeira, G. Boschloo, A. Hagfeldt and L. Hammarström, J. Phys. Chem. B, 2005, 109, 19403–19410 CrossRef CAS PubMed.
- L. D'Amario, L. J. Antila, B. Pettersson Rimgard, G. Boschloo and L. Hammarström, J. Phys. Chem. Lett., 2015, 6, 779–783 CrossRef PubMed.
- R. J. Dillon, L. Alibabaei, T. J. Meyer and J. M. Papanikolas, ACS Appl. Mater. Interfaces, 2017, 9, 26786–26796 CAS.
- A. Morandeira, J. Fortage, T. Edvinsson, L. L. Pleux, E. Blart, G. Boschloo, A. Hagfeldt, L. Hammarström and F. Odobel, J. Phys. Chem. C, 2008, 112, 1721–1728 CAS.
- Y. Farré, M. Raissi, A. Fihey, Y. Pellegrin, E. Blart, D. Jacquemin and F. Odobel, ChemSusChem, 2017, 10, 2618–2625 CrossRef PubMed.
- I. R. Perera, T. Daeneke, S. Makuta, Z. Yu, Y. Tachibana, A. Mishra, P. Bäuerle, C. A. Ohlin, U. Bach and L. Spiccia, Angew. Chem., Int. Ed., 2015, 54, 3758–3762 CrossRef CAS PubMed.
- K. A. Click, D. R. Beauchamp, B. R. Garrett, Z. Huang, C. M. Hadad and Y. Wu, Phys. Chem. Chem. Phys., 2014, 16, 26103–26111 RSC.
- Y. Yu, K. A. Click, S. M. Polen, M. He, C. M. Hadad and Y. Wu, J. Phys. Chem. C, 2017, 121, 20720–20728 CAS.
- P. Qin, H. Zhu, T. Edvinsson, G. Boschloo, A. Hagfeldt and L. Sun, J. Am. Chem. Soc., 2008, 130, 8570–8571 CrossRef CAS PubMed.
- L. Li, E. A. Gibson, P. Qin, G. Boschloo, M. Gorlov, A. Hagfeldt and L. Sun, Adv. Mater., 2010, 22, 1759–1762 CrossRef CAS PubMed.
- C. J. Wood, G. H. Summers and E. A. Gibson, Chem. Commun., 2015, 51, 3915–3918 RSC.
- Q.-Q. Zhang, K.-J. Jiang, J.-H. Huang, C.-W. Zhao, L.-P. Zhang, X.-P. Cui, M.-J. Su, L.-M. Yang, Y.-L. Song and X.-Q. Zhou, J. Mater. Chem. A, 2015, 3, 7695–7698 CAS.
- P. Qin, J. Wiberg, E. A. Gibson, M. Linder, L. Li, T. Brinck, A. Hagfeldt, B. Albinsson and L. Sun, J. Phys. Chem. C, 2010, 114, 4738–4748 CAS.
- F. A. Black, C. A. Clark, G. H. Summers, I. P. Clark, M. Towrie, T. Penfold, M. W. George and E. A. Gibson, Phys. Chem. Chem. Phys., 2017, 19, 7877–7885 RSC.
- F. A. Black, C. J. Wood, S. Ngwerume, G. H. Summers, I. P. Clark, M. Towrie, J. E. Camp and E. A. Gibson, Faraday Discuss., 2017, 198, 449–461 RSC.
- L. D'Amario, R. Jiang, U. B. Cappel, E. A. Gibson, G. Boschloo, H. Rensmo, L. Sun, L. Hammarström and H. Tian, ACS Appl. Mater. Interfaces, 2017, 9, 33470–33477 Search PubMed.
- D. Joly, L. Pellejà, S. Narbey, F. Oswald, T. Meyer, Y. Kervella, P. Maldivi, J. N. Clifford, E. Palomares and R. Demadrille, Energy Environ. Sci., 2015, 8, 2010–2018 CAS.
- D. P. Hagberg, X. Jiang, E. Gabrielsson, M. Linder, T. Marinado, T. Brinck, A. Hagfeldt and L. Sun, J. Mater. Chem., 2009, 19, 7232–7238 RSC.
- C.-H. Chang, Y.-C. Chen, C.-Y. Hsu, H.-H. Chou and J. T. Lin, Org. Lett., 2012, 14, 4726–4729 CrossRef CAS PubMed.
- F. Wu, L. Zhu, S. Zhao, Q. Song and C. Yang, Dyes Pigm., 2016, 124, 93–100 CrossRef CAS.
- M. He, Z. Ji, Z. Huang and Y. Wu, J. Phys. Chem. C, 2014, 118, 16518–16525 CAS.
- Y.-S. Yen, W.-T. Chen, C.-Y. Hsu, H.-H. Chou, J. T. Lin and M.-C. P. Yeh, Org. Lett., 2011, 13, 4930–4933 CrossRef CAS PubMed.
- M. Weidelener, A. Mishra, A. Nattestad, S. Powar, A. J. Mozer, E. Mena-Osteritz, Y.-B. Cheng, U. Bach and P. Bäuerle, J. Mater. Chem., 2012, 22, 7366–7379 RSC.
- G.-F. Zhang, H. Wang, M. P. Aldred, T. Chen, Z.-Q. Chen, X. Meng and M.-Q. Zhu, Chem. Mater., 2014, 26, 4433–4446 CrossRef CAS.
- M. Weidelener, S. Powar, H. Kast, Z. Yu, P. P. Boix, C. Li, K. Müllen, T. Geiger, S. Kuster, F. Nüesch, U. Bach, A. Mishra and P. Bäuerle, Chem.–Asian J., 2014, 9, 3251–3263 CrossRef CAS PubMed.
- M. M. Oliva, J. Casado, M. M. M. Raposo, A. M. C. Fonseca, H. Hartmann, V. Hernández and J. T. López Navarrete, J. Org. Chem., 2006, 71, 7509–7520 CrossRef PubMed.
- R. Brisse, R. Faddoul, T. Bourgeteau, D. Tondelier, J. Leroy, S. Campidelli, T. Berthelot, B. Geffroy and B. Jousselme, ACS Appl. Mater. Interfaces, 2017, 9, 2369–2377 CAS.
- F. Brunner, N. Marinakis, C. Wobill, M. Willgert, C. D. Ertl, T. Kosmalski, M. Neuburger, B. Bozic-Weber, T. Glatzel, E. C. Constable and C. E. Housecroft, J. Mater. Chem. C, 2016, 4, 9823–9833 RSC.
- G. H. Summers, J.-F. Lefebvre, F. A. Black, E. S. Davies, E. A. Gibson, T. Pullerits, C. J. Wood and K. Zidek, Phys. Chem. Chem. Phys., 2015, 18, 1059–1070 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00474e |
| This journal is © The Royal Society of Chemistry 2018 |