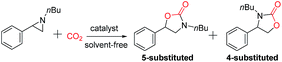Imidazolium-based ionic liquid decorated zinc porphyrin catalyst for converting CO2 into five-membered heterocyclic molecules†
Yaju
Chen
 a,
Rongchang
Luo
a,
Rongchang
Luo
 *a,
Zhi
Yang
a,
Xiantai
Zhou
b and
Hongbing
Ji
*a,
Zhi
Yang
a,
Xiantai
Zhou
b and
Hongbing
Ji
 *a
*a
aFine Chemical Industry Research Institute, Key Laboratory of Low-Carbon Chemistry & Energy Conservation of Guangdong Province, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275, China. E-mail: luorongc@mail.sysu.edu.cn; jihb@mail.sysu.edu.cn
bSchool of Chemical Engineering and Technology, Sun Yat-Sen University, Zhuhai, Guangdong 519082, China
First published on 31st October 2017
Abstract
An imidazolium-based ionic liquid (IL) functionalized zinc porphyrin catalyst was successfully synthesized for the first time, and was employed as an efficient catalyst for CO2 transformation including both the chemoselective synthesis of cyclic carbonates from various epoxides and the regioselective synthesis of 5-aryl-2-oxazolidinones from N-substituted aziridines. Detailed studies demonstrated that the intramolecular cooperative effect between the coordinated zinc center (electrophile) and flexible bromine anions (nucleophile) could mediate these reactions effectively under solvent-free and mild conditions. More importantly, the IL-based catalyst could be readily recovered by solvent precipitation and reused with retention of high activity and selectivity more than ten times based on the concept of “one-phase catalytic coupling with two-phase separation”.
Introduction
As an abundant, eco-friendly, cheap and renewable C1 building block, carbon dioxide (CO2) has tremendously attracted continuous and increasing attention in recent years.1–3 Chemical conversion of captured CO2 into high-value products is a promising and important strategy to reutilize carbon resources, which is in line with the development tendency of human society over the course of this century.4,5 To date, more than 20 reactions involving CO2 as a raw material have been intensively developed over the past few decades.6 Among these, the synthesis of five-membered heterocyclic compounds from the cycloaddition reaction of CO2 and three-membered heterocyclic molecules (including terminal epoxides and N-substituted aziridines) is generally regarded as the 100% atom-economic and cost-effective method.7–9 In addition, the resulting cyclic products have been widely used as important intermediates in the synthesis of a variety of pharmaceutical compounds (such as antibiotics linezolid and tedizolid)10,11 and fine chemicals (such as electrolytes and polar aprotic solvents).12,13So far, various catalysts for this transformation have been intensively reported such as N-heterocyclic carbenes,14 ionic liquids (ILs),15,16 organic bases,17,18 alkali metal halides,19,20 metal oxides,21 metallosalen complexes,22–28 metalloporphyrins,29–36etc. Through careful analysis of the above catalytic systems, metalloporphyrins and metallosalen complexes have been well documented as highly active catalysts in comparison with traditional organocatalysts because of their designable framework and the unique conjugation properties. Based on the general character of dual activation mode in the metal-catalyzed CO2-cycloaddition reactions, either a nucleophile group (quaternary onium salt) or organic base was required for the ring opening of the epoxide and/or aziridine. However, on account of the introduction of additional co-catalysts, some obvious drawbacks such as the intricate recycling problem and the unavoidable contamination of the products in binary catalytic systems have greatly limited their industrial application. In this regard, vigorous efforts have been made to develop bifunctional single-component catalysts, which were in combination with metal active sites and nucleophile groups. For instance, in 2014, our group developed a series of novel IL-functionalized salen aluminum complexes with built-in “CO2 capture” capability and used them as highly efficient catalysts for the conversion of CO2 into cyclic carbonates.23 A more recent study reported by Jing and co-workers identified a new type of zinc porphyrin bearing quaternary ammonium halogenides, which showed high catalytic activity in this coupling reaction.37 However, in these catalytic systems, high temperature (≥100 °C) was still required to raise the catalytic performance. Thus, there is still much room for the improvement of currently available catalysts for this transformation. Recently, ILs have attracted great attention because of their applications, specifically in CO2 capture and conversion. This is mainly because their significant CO2 solubility and CO2-philic ability derive from either varying the type of cations and anions or introducing various functional groups.38 Accordingly, together with the superiority of task-specific ILs and the high activity of porphyrin complexes, our design is to strive for developing bifunctional catalysts combining the advantages of ILs and metalloporphyrins to facilitate the CO2-involved coupling reaction at relatively lower temperatures and pressures.
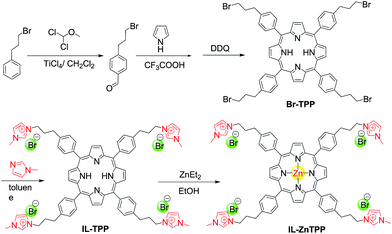
Herein, we reported a new bifunctional catalyst (denoted as IL-ZnTPP) synthesized by grafting quaternary imidazolium-based IL moieties onto the para position of each phenyl ring in the zinc porphyrin framework through the highly stable covalent linkage (Scheme 1). As a result of a cooperative effect between the Lewis acidic metal center (Zn) and the flexible nucleophile (Br−) in one molecule, this homogeneous IL-based catalyst possessed excellent catalytic performance for producing five-membered cyclic products from CO2 and epoxides and/or aziridines at a low catalyst loading under solvent- and additive-free conditions. It is worth noting that this new catalyst also exhibited broad substrate scope for the transformation. Moreover, considering the special solubility of IL-based catalysts and the concept of “one-phase catalysis and biphase catalyst separation”, catalyst IL-ZnTPP can be conveniently reused more than ten times with the maintenance of good catalytic activity and selectivity, showing its superior stability. Therefore, our new-prepared bifunctional catalyst might provide a promising candidate for the catalytic synthesis of five-membered heterocyclic products from CO2 under mild conditions, simultaneously resolving the inevitable problem of difficulty in recycling the homogeneous catalyst.
 | ||
| Scheme 1 Structure of IL-functionalized zinc porphyrin (IL-ZnTPP) and its application in the CO2 cycloaddition reaction. | ||
Results and discussion
Catalyst synthesis and characterization
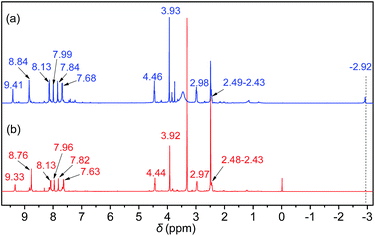
Initially, the 1H NMR spectra of the porphyrin ligand IL-TPP and catalyst IL-ZnTPP are depicted in Fig. 1. The proton signals at 8.84, 8.13, 7.68 and −2.92 ppm are assigned to the hydrogen atom of the porphyrin macroring,39 and the peaks at 4.46, 2.98 and 2.49–2.43 ppm are ascribed to the bromopropyl groups in the spectrum of IL-TPP (Fig. 1a). It can also be seen that the obtained IL-TPP shows additional signals at about 9.41, 7.99, 7.84 and 3.93 ppm after being functionalized with imidazolium-based ILs, which are attributable to those of imidazolium rings and methyl groups. After metalation of zinc salts, the peaks in the spectrum of IL-ZnTPP are consistent with those of IL-TPP (Fig. 1b). Nevertheless, the peak at −2.92 ppm assigned to the pyrrolic H (–NH–) in the porphyrin ring almost cannot be observed, indicating that the IL-ZnTPP has a very high degree of metallization with the zinc cation.39Afterwards, FT-IR, XPS and UV-vis analyses were used to further validate the structure and composition of the catalysts. In the FT-IR spectra, compared to the neat Zn-TPP, the IL-functionalized sample exhibits additional characteristic peaks at around 1160 and 619 cm−1, which are assigned to the stretching vibration of the imidazole units (Fig. 2A). These new stretching vibrations further confirmed the successful grafting of the imidazolium-based ILs onto the porphyrin framework. Meanwhile, Fig. 2C and D show the XPS spectra of the Zn-TPP and IL-ZnTPP. The N 1s spectrum of the IL-ZnTPP exhibits two distinct peaks at 398.2 and 401.7 eV, which are related to the contribution of two kinds of nitrogen bonding pairs, that is, the Zn–N bonds in the porphyrin ring and the C–N bonds in the imidazolium ring, respectively.40,41 Additionally, the absence of the value of N 1s at around 399.8 eV assigned to the pyrrolic N (–NH–) suggests that the porphyrin ring is fully metalated, which strongly supports the conclusion made by 1H NMR analysis. The Zn 2p spectrum of IL-ZnTPP shows the values of Zn 2p1/2 and Zn 2p3/2 at 1045.6 and 1022.5 eV, respectively, which is well consistent with that of the ZnTPP (1045.9 and 1022.8 eV), indicating that both the ZnTPP and IL-ZnPP had an identical metal active center and chemical environment. Therefore, these observations provide indirect proof for the successful immobilization of the imidazolium-based ILs into the zinc porphyrin framework. Furthermore, UV-vis spectroscopy was also used to investigate the Q and Soret bands of the porphyrin framework in samples. As shown in Fig. S1,†IL-TPP has absorption peaks at 422, 519, 556, 593 and 649 nm, which is consistent with tetraphenylporphyrin (429, 520, 554, 595, and 653 nm),42 whereas the sample gives new absorption peaks at 439, 570 and 613 nm after the metalation with the zinc cation. Comparing each other, a red shift of the Soret band from 422 to 439 nm can be clearly observed, demonstrating the full metalation in the porphyrin rings.43 Additionally, the decomposition profiles of the samples were monitored by thermogravimetry and differential thermogravimetric (TG-DTG) analysis under an air atmosphere. As shown in Fig. 2B, the bifunctional catalyst IL-ZnTPP can endure about 250 °C with little loss of its weight, indicating its high thermal stability.
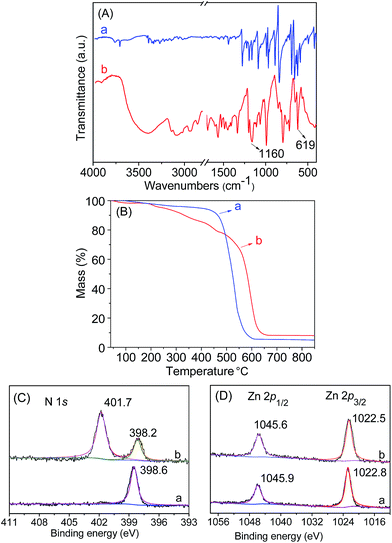 | ||
| Fig. 2 (A) FT-IR spectra of (a) Zn-TPP and (b) IL-ZnTPP. (B) TG curves of (a) Zn-TPP and (b) IL-ZnTPP. (C) N 1s and (D) Zn 2p XPS spectra of (a) Zn-TPP and (b) IL-ZnTPP. | ||
CO2/epoxide cycloaddition reaction
Cyclic carbonates, as an important class of chemicals, have been broadly used as electrolytes in lithium-ion batteries, environmentally friendly polar aprotic solvents, raw materials for plastics, and intermediates in the production of fine chemicals. Thereinto, the coupling of epoxides and CO2 has been generally regarded as one of most promising strategies for the synthesis of cyclic carbonates. Moreover, the successful application of the IL-functionalized 2,2′-bipyridine zinc(II) complex (IL-BPZ)22 in our previous work encouraged us to exploit the catalytic potential of our bifunctional catalyst IL-ZnTPP. As a result, this cycloaddition reaction under solvent-free conditions was firstly chosen as a model reaction. The original conditions for this reaction were systematically researched by using styrene oxide (SO) as a representative substrate at 60 °C and 2.0 MPa (initial CO2 pressure), and the related results are listed in Table 1.| Entry | Catalyst | P/MPa | T/°C | t/h | Conv.b/% | Yieldb/% |
|---|---|---|---|---|---|---|
| a Reaction conditions: 10 mL stainless-steel autoclave, SO (1.0 mmol), catalyst (0.1 mol%). b Determined by GC with biphenyl as an internal standard. c Catalyst (0.02 mol%). d Catalyst (0.2 mol%). | ||||||
| 1 | — | 2 | 60 | 72 | 7 | 5 |
| 2 | IL-TPP | 2 | 60 | 30 | 63 | 61 |
| 3 | ZnTPP | 2 | 60 | 30 | 24 | 20 |
| 4 | IL-ZnTPP | 2 | 60 | 30 | 97 | 96 |
| 5c | IL-ZnTPP | 2 | 60 | 30 | 13 | 10 |
| 6d | IL-ZnTPP | 2 | 60 | 30 | 99 | 94 |
| 7 | IL-ZnTPP | 2 | 35 | 48 | 34 | 33 |
| 8 | IL-ZnTPP | 2 | 35 | 72 | 72 | 70 |
| 9 | IL-ZnTPP | 1 | 60 | 30 | 87 | 85 |
| 10 | IL-ZnTPP | 0.5 | 60 | 30 | 67 | 64 |
Firstly, the control experiment gave a negligible reaction product in the absence of a catalyst in spite of prolonging the reaction time to 72 h at 60 °C and 2.0 MPa (Table 1, entry 1). Next, we observed that the neat Zn-TPP could only give 24% conversion, and the metal-free IL-TPP could give a better conversion (63%), indicating that both an electrophilic metal center and a nucleophilic group play important roles in the catalytic cycle (Table 1, entries 2 and 3). As expected, the bifunctional catalyst IL-ZnTPP could smoothly catalyze the reaction to afford the corresponding product with a high yield of 96% without any additives at only a catalyst loading of 0.1 mol% under identical conditions, which was due to the dual cooperative activation of epoxide by the coordinated zinc active sites and the flexible quaternary imidazolium-based ILs at the para position of each phenyl ring (Table 1, entry 4). Additionally, the influences of different parameters including the catalyst loading, CO2 pressure and reaction temperature on the yield of this reaction were also investigated in detail. It can be seen that the catalytic activity was found to increase remarkably in the presence of a higher amount of IL-ZnTPP (Table 1, entries 4 and 5). However, when the catalyst amount was further increased to 0.2 mol% (Table 1, entry 6), the conversion of SO did not increase observably. Under the rational analysis of the results, the optimum amount of IL-ZnTPP was chosen as 0.1 mol% for the following parameter studies. From the results in entries 4, 7 and 8, it was demonstrated that the reaction temperature had a positive effect on the catalytic reaction. As mentioned above, a high yield of 96% was obtained at 60 °C. However, when the temperature was decreased to 35 °C, the yield of SC dropped dramatically, even when prolonging the reaction time to 72 h (Table 1, entries 7 and 8). Even so, this represents an encouraging result (conversion: 72%) for this reaction at such a mild temperature (35 °C). Thereafter, the CO2 pressure was also investigated in the range of 0.5 and 2.0 MPa at 60 °C. Styrene cyclic carbonate (SC) was obtained in excellent yields at the CO2 pressure of 2.0 MPa. At a relatively lower pressure (1.0 and 0.5 MPa), the conversion of SO decreased obviously to a certain extent (Table 1, entries 9 and 10). In order to ensure the higher efficiency for CO2 conversion, a CO2 pressure of 2.0 MPa was selected for further investigations.
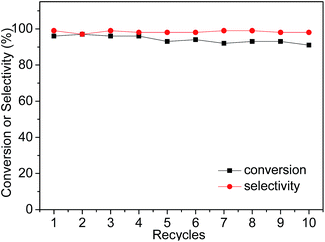
Subsequently, owing to the insolubility of the imidazolium ILs in non-polar solvents (e.g. hexane and diethyl ether), the IL-ZnTPP could be easily precipitated from the reaction mixture by adding sufficient diethyl ether. The supernatant with the product was separated from the catalyst by simple filtration or centrifugation, and the reusability of the IL-ZnTPP was evaluated in the cycloaddition reaction of CO2 with SO at 60 °C and 2.0 MPa. As shown in Fig. 3, IL-ZnTPP could be reused up to ten times with no significant loss in conversion and selectivity. Slightly decreased yields of products for each cycle were observed, mainly as a result of the catalyst loss in the recovery process. In order to confirm the stability of our catalyst in this reaction system, leaching tests to the reaction medium were carried out by directly determining the zinc species in the supernatant through ICP-OES elemental analysis, and almost no zinc ion was detected. Moreover, the 1H NMR and FTIR spectra of the reused IL-ZnTPP were confirmed, and the structure of the catalyst showed no obvious change (Fig. S3 and S4 in the ESI†). These encouraging results might demonstrate that the IL-functionalized zinc porphyrin could be considered as a promising catalyst for efficient and repetitive conversion of CO2 and epoxides into cyclic carbonates under solvent- and additive-free conditions.
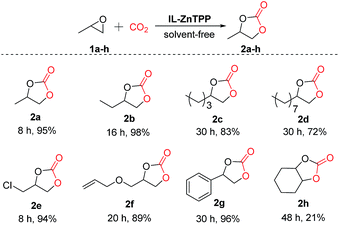
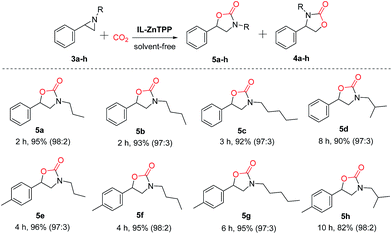
Moreover, in order to study the scope of the cycloaddition reaction, this bifunctional catalytic system was extended to a number of other representative epoxides such as propylene oxide, 1,2-epoxybutane, 1,2-epoxyhexane, 1,2-epoxydodecane, epichlorohydrin, allyl glycidyl ether and cyclohexene oxide, and the results are illustrated in Scheme 2. To our delight, most of the terminal epoxides (1a–g) can be successfully converted to the desired cyclic carbonates in good-to-excellent yields (72–98%) and high selectivities (>97%) at 60 °C and 2.0 MPa CO2 pressure in the absence of any additives. However, when the internal epoxide (1h), a very challenging substrate for this reaction, was employed, the yield of the corresponding product was only 21% even at a longer reaction time of 48 h under similar conditions, indicating that the reactivity of the substrate is strongly influenced by the steric hindrance effect.23 As a consequence, this new bifunctional catalyst exhibited broad substrate scope for the synthesis of various organic carbonates.
CO2/aziridine cycloaddition reaction
Encouraged by the successful results of the synthesis of cyclic carbonates, we further explored the application of the bifunctional catalyst IL-ZnTPP for the cycloaddition reaction of CO2 and various N-substituted aziridines into oxazolidinones, which are considered as another class of important compounds in synthetic and medicinal chemistry. Then 1-nbutyl-2-phenylaziridine was chosen as a model substrate to investigate the catalytic performance of various catalysts under solvent-free conditions. According to the literature, the cycloaddition of CO2 with N-substituted aziridines often gives two kinds of products (5-substituted and 4-substituted oxazolidinones), compared with the sole product rooting in CO2/epoxides coupling. Indeed, both 5-substituted and 4-substituted oxazolidinones were formed in this catalytic system, and the former was obtained as the major product (Table 2). As expected, due to the dual cooperative activation of aziridine, IL-ZnTPP was found to be very effective in catalyzing the coupling of CO2 with N-substituted aziridines without any additives. Concretely, as shown in entry 1, IL-ZnTPP afforded the coupling reaction with a 93% yield and up to 97% regioselectivity for the 5-aryl-2-oxazolidinone as the desired product within 2 h at only a catalyst loading of 0.1 mol%, 90 °C and 2.0 MPa CO2 pressure. As a control experiment, only 18% conversion was observed in the absence of a catalyst at 90 °C and an initial CO2 pressure of 2.0 MPa (Table 2, entry 2). Then, this reaction catalyzed by [BMIm]Br alone gave a 30% yield (Table 2, entry 3). Moreover, without [BMIm]Br, the neat ZnTPP could give a 41% yield of desired product (Table 2, entry 4). However, we observed that the binary ZnTPP/[BMIm]Br catalyst promoted this reaction with a 58% yield under the same conditions (Table 2, entry 5), indicating that both the zinc component and [BMIm]Br are beneficial for the ring opening of 1-nbutyl-2-phenylaziridine. Entries 6–8 in Table 2 show the catalytic performance of IL-ZnTPP at different temperatures, and the conversion of the substrate was increased from 48% to 80% with the increase of temperature from 40 °C to 90 °C. Notably, the regioselectivity of the product is almost unaffected by the variation of temperature which indicates that IL-ZnTPP exhibits good regioselective ring opening of the substrate. In addition, when the pressure was decreased to 0.5 MPa, this bifunctional catalyst showed a lower activity, and only 50% of 1-nbutyl-2-phenylaziridine was converted within 2 h (Table 2, entry 9). Above all, these results demonstrated that the excellent performance of the bifunctional catalyst reflected the intramolecular cooperative effect between zinc species and bromine anions, and the reaction temperature and the pressure of CO2 affected strongly this model reaction.| Entry | Catalyst | T/°C | P/MPa | Conv.b/% | Yieldb/% | Sel. (Regio)c/% |
|---|---|---|---|---|---|---|
| a Reaction conditions: 10 mL stainless-steel autoclave, 1-nbutyl-2-phenylaziridine (1.0 mmol), catalyst (0.1 mol%), 2 h. b Determined by GC by using naphthalene as an internal standard. c Molar ratio of 5-substituted oxazolininone to 4-substituted oxazolidinone, as determined by GC. d [BMIm]Br (0.4 mol%). e [BMIm]Br (0.4 mol%), ZnTPP (0.1 mol%). | ||||||
| 1 | IL-ZnTPP | 90 | 2 | 99 | 93 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 2 | — | 90 | 2 | 18 | 13 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 3d | [BMIm]Br | 90 | 2 | 37 | 30 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 4 | ZnTPP | 90 | 2 | 46 | 41 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 5e | ZnTPP/[BMIm]Br | 90 | 2 | 64 | 58 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 6 | IL-ZnTPP | 50 | 1 | 48 | 45 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 7 | IL-ZnTPP | 70 | 1 | 64 | 60 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 8 | IL-ZnTPP | 90 | 1 | 80 | 75 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 9 | IL-ZnTPP | 90 | 0.5 | 54 | 50 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Furthermore, IL-ZnTPP was also employed as a bifunctional catalyst for the coupling of CO2 with various aziridines, including 1-propyl-2-phenylaziridine, 1-nbutyl-2-phenylaziridine, 1-amyl-2-phenylaziridine, 1-isobutyl-2-phenylaziridine, etc. As summarized in Scheme 3, this catalyst exhibited excellent catalytic performance for these substrates, giving >92% yields and >97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 regioselectivities of the corresponding oxazolidinones at 90 °C and 2.0 MPa. Also, it can be observed clearly that the catalytic activity of the catalyst IL-ZnTPP decreased in tandem with the increment of the length of the alkyl chain on the nitrogen atom of aziridine (5a > 5b > 5c and 5e > 5f > 5g). Compared with the above substrates bearing a linear alkyl group, the substrate with a branched alkyl group (e.g. isobutyl) at the nitrogen atom showed a slower reaction rate with excellent regioselectivity (5a > 5d and 5e > 5h), possibly owing to the steric effect.44 Even so, the yields of desired oxazolidinones (5d and 5h) could be substantially enhanced by prolonging the reaction time. Additionally, it was found that the activity of the aziridines with an electron-donating group (methyl) in the phenyl ring was relatively lower than that of the methyl-free aziridines (5a > 5e, 5b > 5f, 5c > 5g and 5d > 5h). As a result, our bifunctional catalyst showed good efficiency and excellent regioselectivity for the synthesis of various oxazolidinones from CO2 under solvent-free conditions, and the activity was greatly influenced by the steric effect and the electronic effect resulting from the nature of the substrate itself.45
3 regioselectivities of the corresponding oxazolidinones at 90 °C and 2.0 MPa. Also, it can be observed clearly that the catalytic activity of the catalyst IL-ZnTPP decreased in tandem with the increment of the length of the alkyl chain on the nitrogen atom of aziridine (5a > 5b > 5c and 5e > 5f > 5g). Compared with the above substrates bearing a linear alkyl group, the substrate with a branched alkyl group (e.g. isobutyl) at the nitrogen atom showed a slower reaction rate with excellent regioselectivity (5a > 5d and 5e > 5h), possibly owing to the steric effect.44 Even so, the yields of desired oxazolidinones (5d and 5h) could be substantially enhanced by prolonging the reaction time. Additionally, it was found that the activity of the aziridines with an electron-donating group (methyl) in the phenyl ring was relatively lower than that of the methyl-free aziridines (5a > 5e, 5b > 5f, 5c > 5g and 5d > 5h). As a result, our bifunctional catalyst showed good efficiency and excellent regioselectivity for the synthesis of various oxazolidinones from CO2 under solvent-free conditions, and the activity was greatly influenced by the steric effect and the electronic effect resulting from the nature of the substrate itself.45
Reaction mechanism
On the basis of our observations and the molecular structure of bifunctional catalyst IL-ZnTPP, a tentative Lewis acid–base cooperative activation mechanism was proposed for the catalytic conversion of CO2 to five-membered heterocyclic products (Scheme 4). Firstly, the process of the synthesis of cyclic carbonate from CO2 with epoxides is shown in Scheme 4A, which is in accordance with literature reports.22,23,37,45 The cycle is initiated by binding the epoxide with the coordinated Lewis acidic zinc site of IL-ZnTPP thereby activating the epoxy ring. At the same time, an intramolecular nucleophilic attack by the freely moving bromine anion on the less-hindered side of the activated epoxide leads to the ring opening of epoxide, affording an intermediate of metal-bound alkoxide, which is the rate-determining step. Nevertheless, when the styrene oxide was used as the substrate, DFT analyses suggest that the nucleophile favors the attack at the benzylic position.46 Subsequently, CO2 coordinated to the same zinc center and allowed intermolecular transfer of the alkoxide to coordinated CO2, resulting in a metal carbonate. At last, the metal carbonate is converted to the corresponding cyclic carbonate with regeneration of the catalyst through an intramolecular SN2 cyclization reaction.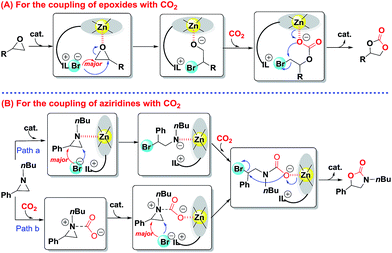 | ||
| Scheme 4 Proposed mechanism for the catalytic conversion of CO2 to five-membered heterocyclic products over IL-ZnTPP. | ||
Next, Scheme 4B shows the cycloaddition reaction of CO2 with aziridines. According to some previous studies,34,47–49 the proposed mechanism of this transformation is similar to the above coupling of CO2 and epoxides over the Lewis acid–base bifunctional catalyst, which is illustrated in Scheme 4B, path a. However, considering the strong nucleophilicity of the nitrogen atom of aziridine, the coupling reaction occurs through another path (Scheme 4B, path b).34 Initially, the nitrogen atom of aziridine acts as a Lewis base site to activate free CO2 and a new CO2-aziridine intermediate is formed. Subsequently, this intermediate binds with the Lewis acidic zinc site of IL-ZnTPP through the oxygen atom of CO2 followed by an intramolecular nucleophilic substitution reaction mainly at the less-hindered side of the activated aziridine, affording a ring-opened intermediate. Finally, the reaction is completed though a ring closing step that gives 5-substituted oxazolidinone as the major product and regenerates the catalyst. It is worthwhile to note that extremely flexible imidazolium-based ILs at the para position of a zinc porphyrin framework are accessible to the activated epoxides or aziridines in the above two cycloaddition reactions, which could accelerate the rate-determining step, thereby promoting the kinetic behavior.
Experimental
Materials and methods
Dimethyl sulfide and 4-chlorostyrene oxide were purchased from Alfa Aesar Chemical Reagent Co. Ltd. 1-Bromo-3-phenylpropane, 1,1-dichlorodimethyl ether, titanium tetrachloride (1.0 mol L−1 in methylene chloride), 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), boron trifluoride, and diethylzinc (1.0 mol L−1 in hexane) were available from Energy Chemical. Olefins, 1-methylimidazole, diethyl aluminum chloride (Et2AlCl, 0.9 M solution in toluene) and epoxides were obtained from J&K Scientific Ltd. Various N-substituted aziridines50 and tetraphenylporphyrin zinc (ZnTPP)51 were synthesized according to the procedure in the literature. Other chemicals and solvents were purchased from commercial vendors and used as received unless indicated otherwise.1H and 13C NMR data were collected on a Bruker Varian INOVA500NB spectrometer using TMS as an internal standard. Elemental analyses of C, H, and N were performed on a Vario EL cube instrument. FT-IR spectra of the samples were obtained under ambient conditions at a resolution of 4 cm−1 in the wave number range of 4000–400 cm−1 by using an EQUINOX 55 spectrometer. The ultra-violet-visible light (UV-vis) spectra were recorded on a Shimadzu UV-2450 spectrophotometer. Thermogravimetry and differential thermogravimetric (TG-DTG) was carried out in a NETZSCH TG 209 F3 Tarsus instrument by heating samples from 40 °C to 850 °C at a heating rate of 10 °C min−1 under an air atmosphere. X-ray photoelectron spectroscopy (XPS) analysis was carried out on an ESCALAB 250 spectrometer. Matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy (MALDI-TOF/MS) was performed on a Bruker ultrafleXtreme MALDI TOF mass spectrometer. Inductively coupled plasma optical emission spectroscopy (ICP-OES) was performed on an OPTIMA 8000DV (PerkinElmer). Gas chromatographic (GC) analysis was performed on a GC2010 gas chromatograph (Shimadzu) equipped with a flame ionization detector and a capillary column (Rtx-5, 30 m × 0.32 mm × 0.25 μm).
Synthesis of catalyst
The synthetic process is shown as follows:Typical procedures for the cycloaddition of CO2 with epoxides or aziridines
All the cycloaddition reactions were conducted in a 10 mL stainless steel autoclave. Typically, the epoxide or aziridine and catalyst were quickly added into the autoclave. After sealing and purging with CO2 3 times, the autoclave was pressurized with CO2 to the required pressure, followed by stirring at the desired temperature. After the appropriate time, the autoclave was cooled to 0 °C and the excess of CO2 was released slowly. Subsequently, the reaction mixture was extracted with ethyl acetate (3 × 2 mL), and the product yield and selectivity were determined by GC analysis through the internal standard method with biphenyl as the standard. The purity and structure of the products were also confirmed by FT-IR, 1H NMR, 13C NMR spectra and GC-MS analysis. The recycled catalyst was precipitated by adding a certain amount of diethyl ether at the end of the cycloaddition reaction. After being centrifuged, washed and dried, the recycled catalyst was used for the next run.Conclusions
In summary, we developed a new bifunctional catalyst by the covalent confinement of multiple flexible imidazolium-based ILs at the para position of each phenyl ring in the zinc porphyrin framework. It was demonstrated that this resultant catalyst exhibited good catalytic activity and high selectivity for the coupling reaction of CO2 with three-membered heterocyclic compounds (epoxides or aziridines) without any additives under solvent-free conditions, due to the intramolecular cooperative effect between the electrophilic zinc center and the nucleophilic bromine anions. Notably, the catalytic system displayed broad substrate scope for the synthesis of the corresponding five-membered cyclic products in good-to-excellent yields. Moreover, this catalyst could be readily recovered and it maintained its catalytic activity and selectivity even after reusing ten times. As a consequence, the bifunctional catalyst provides a promising candidate for the catalyzed fixation of CO2 into value-added chemicals.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported by the National Natural Science Foundation of China NSFC (No. 21676306 and 21425627), the National Key Research and Development Program of China (2016YFA0602900), the Natural Science Foundation of Guangdong Province (2016A030310211), and the Characteristic Innovation Project (Natural Science) of Guangdong Colleges (2016KTSCX004).Notes and references
- A. Goeppert, M. Czaun, J.-P. Jones, G. K. Surya Prakash and G. A. Olah, Chem. Soc. Rev., 2014, 43, 7995 RSC.
- Q. Liu, L. Wu, R. Jackstell and M. Beller, Nat. Commun., 2015, 6, 5933 CrossRef PubMed.
- J. Klankermayer, S. Wesselbaum, K. Beydoun and W. Leitner, Angew. Chem., Int. Ed., 2016, 55, 7296 CrossRef CAS PubMed.
- A. Banerjee, G. R. Dick, T. Yoshino and M. W. Kanan, Nature, 2016, 531, 215 CrossRef CAS PubMed.
- M. North, R. Pasquale and C. Young, Green Chem., 2010, 12, 1514 RSC.
- H. Zhou, G.-X. Wang, W.-Z. Zhang and X.-B. Lu, ACS Catal., 2015, 5, 6773 CrossRef CAS.
- C. Qi, J. Ye, W. Zeng and H. Jiang, Adv. Synth. Catal., 2010, 352, 1925 CrossRef CAS.
- T. Sakakura and K. Kohno, Chem. Commun., 2009, 11, 1312 RSC.
- F. D. Bobbink and P. J. Dyson, J. Catal., 2016, 343, 52 CrossRef CAS.
- S. M. Kelly, C. Han, L. Tung and F. Gosselin, Org. Lett., 2017, 19, 3021 CrossRef CAS PubMed.
- M. S. Deshmukh and N. Jain, ACS Med. Chem. Lett., 2017 DOI:10.1021/acsmedchemlett.7b00263.
- S. Grama, J. Lejnieks, M. Enayati, R. B. Smail, L. Ding, G. Lligadas, M. J. Monteiro and V. Percec, Polym. Chem., 2017, 8, 5865 RSC.
- S. A. Freunberger, Y. Chen, Z. Peng, J. M. Griffin, L. J. Hardwick, F. Barde, P. Novak and P. G. Bruce, J. Am. Chem. Soc., 2011, 133, 8040 CrossRef CAS PubMed.
- L. H. Yang and H. M. Wang, ChemSusChem, 2014, 7, 962 CrossRef CAS PubMed.
- Z. Z. Yang, L. N. He, C. X. Miao and S. Chanfreau, Adv. Synth. Catal., 2010, 352, 2233 CrossRef CAS.
- Z.-Z. Yang, Y.-N. Li, Y.-Y. Wei and L.-N. He, Green Chem., 2011, 13, 2351 RSC.
- B. Wang, Z. Luo, E. H. M. Elageed, S. Wu, Y. Zhang, X. Wu, F. Xia, G. Zhang and G. Gao, ChemCatChem, 2016, 8, 830 CrossRef CAS.
- L. Wang, K. Kodama and T. Hirose, Catal. Sci. Technol., 2016, 6, 3872 CAS.
- X. B. Lu, Y. J. Zhang, K. Jin, L. M. Luo and H. Wang, J. Catal., 2004, 227, 537 CrossRef CAS.
- K. R. Roshan, A. C. Kathalikkattil, J. Tharun, D. W. Kim, Y. S. Won and D. W. Park, Dalton Trans., 2014, 43, 2023 RSC.
- S. Roy, B. Banerjee, A. Bhaumik and S. M. Islam, RSC Adv., 2016, 6, 31153 RSC.
- R. C. Luo, X. T. Zhou, W. Y. Zhang, Z. X. Liang, J. Jiang and H. B. Ji, Green Chem., 2014, 16, 4179 RSC.
- R. C. Luo, X. T. Zhou, S. Y. Chen, Y. Li, L. Zhou and H. B. Ji, Green Chem., 2014, 16, 1496 RSC.
- M. North, S. C. Z. Quek, N. E. Pridmore, A. C. Whitwood and X. Wu, ACS Catal., 2015, 5, 3398 CrossRef CAS.
- R. C. Luo, X. T. Zhou, Y. X. Fang and H. B. Ji, Carbon, 2015, 82, 1 CrossRef CAS.
- S. Duan, X. Jing, D. Li and H. Jing, J. Mol. Catal. A: Chem., 2016, 411, 34 CrossRef CAS.
- Y. A. Rulev, V. A. Larionov, A. V. Lokutova, M. A. Moskalenko, O. L. Lependina, V. I. Maleev, M. North and Y. N. Belokon, ChemSusChem, 2016, 9, 216 CrossRef CAS PubMed.
- Z. Zhu, Y. Zhang, K. Wang, X. Fu, F. Chen and H. Jing, Catal. Commun., 2016, 81, 50 CrossRef CAS.
- Y. S. Qin, H. C. Guo, X. H. Sheng, X. H. Wang and F. S. Wang, Green Chem., 2015, 17, 2853 RSC.
- X. Jiang, F. L. Gou, F. J. Chen and H. W. Jing, Green Chem., 2016, 18, 3567 RSC.
- C. Maeda, J. Shimonishi, R. Miyazaki, J. Y. Hasegawa and T. Ema, Chem.–Eur. J., 2016, 22, 6556 CrossRef CAS PubMed.
- R. K. Totten, Y. S. Kim, M. H. Weston, O. K. Farha, J. T. Hupp and S. T. Nguyen, J. Am. Chem. Soc., 2013, 135, 11720 CrossRef CAS PubMed.
- X. Jiang, F. Gou and H. Jing, J. Catal., 2014, 313, 159 CrossRef CAS.
- W. M. Ren, Y. Liu and X.-B. Lu, J. Org. Chem., 2014, 79, 9771 CrossRef CAS PubMed.
- C. E. Anderson, S. I. Vagin, W. Xia, H. Jin and B. Rieger, Macromolecules, 2012, 45, 6840 CrossRef CAS.
- C. Maeda, T. Taniguchi, K. Ogawa and T. Ema, Angew. Chem., Int. Ed., 2015, 54, 134 CrossRef CAS PubMed.
- X. Jiang, F. Gou, X. Fu and H. Jing, J. CO2 Util., 2016, 16, 264 CrossRef CAS.
- S. Zulfiqar, M. I. Sarwar and D. Mecerreyes, Polym. Chem., 2015, 6, 6435 RSC.
- Y. Fan, Y. Huang, Y. Jiang, X. Ning, X. Wang, D. Shan and X. Lu, J. Colloid Interface Sci., 2016, 462, 100 CrossRef CAS PubMed.
- Y. J. Chen, R. C. Luo, Q. H. Xu, W. Y. Zhang, X. T. Zhou and H. B. Ji, ChemCatChem, 2017, 9, 767 CrossRef CAS.
- Y. J. Chen, R. C. Luo, Q. H. Xu, J. Jiang, X. T. Zhou and H. B. Ji, ChemSusChem, 2017, 10, 2534 CrossRef CAS PubMed.
- X. M. Liu, Z. Y. Dou, L. Xu, Y. F. Zhi, Y. W. Zhang, H. Xia and Y. Mu, Chem.–Eur. J., 2016, 22, 9919 CrossRef PubMed.
- Z. F. Dai, Q. Sun, X. L. Liu, C. Q. Bian, Q. M. Wu, S. X. Pan, L. Wang, X. J. Meng, F. Deng and F.-S. Xiao, J. Catal., 2016, 338, 202 CrossRef CAS.
- S. Kumar and S. L. Jain, Ind. Eng. Chem. Res., 2013, 53, 541 CrossRef.
- R. C. Luo, Z. Yang, W. Y. Zhang, X. T. Zhou and H. B. Ji, Sci. China: Chem., 2017, 60, 979 CrossRef CAS.
- F. Castro-Gomez, G. Salassa, A. W. Kleij and C. Bo, Chem.–Eur. J., 2013, 19, 6289 CrossRef CAS PubMed.
- D. Adhikari, A. W. Miller, M.-H. Baik and S. T. Nguyen, Chem. Sci., 2015, 6, 1293 RSC.
- A. W. Miller and S. T. Nguyen, Org. Lett., 2004, 6, 2301 CrossRef CAS PubMed.
- Y. Zhao, Z. Yang, S. Luo and L. He, Catal. Today, 2013, 200, 2 CrossRef CAS.
- Y. Du, Y. Wu, A.-H. Liu and L.-N. He, J. Org. Chem., 2008, 73, 4709 CrossRef CAS PubMed.
- M. E. El-Khouly, J. B. Ryu, K.-Y. Kay, O. Ito and S. Fukuzumi, J. Phys. Chem. C, 2009, 113, 15444 CAS.
- C. B. Minkenberg, F. Li, P. van Rijn, L. Florusse, J. Boekhoven, M. C. Stuart, G. J. Koper, R. Eelkema and J. H. van Esch, Angew. Chem., Int. Ed., 2011, 50, 3421 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00464h |
| This journal is © The Royal Society of Chemistry 2018 |