Panchromatic absorption of dye sensitized solar cells by co-Sensitization of triple organic dyes
Ashraful
Islam
 *a,
Towhid H.
Chowdhury
*a,
Towhid H.
Chowdhury
 ab,
Chuanjiang
Qin
a,
Liyuan
Han
a,
Jae-Joon
Lee
c,
Idriss M.
Bedja
d,
Md
Akhtaruzzaman
*b,
Kamaruzzaman
Sopian
b,
Antoine
Mirloup
e and
Nicolas
Leclerc
f
ab,
Chuanjiang
Qin
a,
Liyuan
Han
a,
Jae-Joon
Lee
c,
Idriss M.
Bedja
d,
Md
Akhtaruzzaman
*b,
Kamaruzzaman
Sopian
b,
Antoine
Mirloup
e and
Nicolas
Leclerc
f
aPhotovoltaic Materials Group, Center for Green Research on Energy and Environmental Materials, National Institute for Materials Science, 1-2-1 Sengen, Tsukuba, Ibaraki 305-0047, Japan. E-mail: ISLAM.Ashraful@nims.go.jp
bSolar Energy Research Institute (SERI), Universiti Kebangsaan Malaysia (UKM), Bangi 43600, Selangor Darul Ehsan, Malaysia
cDepartment of Energy & Materials Engineering, Research Center for Photoenergy Harvesting and Conversion Technology, Dongguk University, Seoul 04620, Republic of Korea
dCornea Research Center, Optometry Department, College of Applied Medical Sciences, King Saud University, Riyadh 11433, Saudi Arabia
eLaboratoire de Chimie Moléculaire, et Spectroscopies Avancées (ICPEES-LCOSA), UMR 7515 au CNRS, Ecole Européenne de Chimie, Polymères et Matériaux, 25 rue Becquerel, 67087, Strasbourg Cedex 02, France
fInstitut de Chimie et Procédés pour I'Energie, I'Environnement et la Santé (ICPEES), Université de Strasbourg, Ecole Européenne de Chimie, Polymères et Matériaux, 25 rue Becquerel, 67087 Strasbourg, France
First published on 18th October 2017
Abstract
Dye sensitized solar cells (DSSCs) were co-sensitized with three custom molecularly engineered organic dyes containing butyloxyl chain induced dye (Y1), boron dipyrromethene (bodipy) dye (TP2A), and squaraine (SQ) ring configured dye (HSQ4). The individual power conversion efficiencies of the DSSCs sensitized with Y1, TP2A and HSQ4 sensitizers were 3.44%, 4.26% and 5.78%, respectively. Co-sensitized TP2A + HSQ4 dyes at a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio showed an efficiency of 7.02%. Further addition of Y1 dye with optimal TP2A + HSQ4 increased the VOC from 0.580 V to 0.605 V. The co-sensitized Y1 + TP2A + HSQ4 based DSSCs showed a new optimal efficiency (η) of 7.48%. This is the highest efficiency recorded for DSSCs based on co-sensitization of triple organic dyes. Intensity modulated photovoltage spectroscopy further confirms the longer lifetime of co-sensitized Y1 + TP2A + HSQ4 compared to that of each individual TP2A and HSQ4 and co-sensitized TP2A + HSQ4 dye.
1 molar ratio showed an efficiency of 7.02%. Further addition of Y1 dye with optimal TP2A + HSQ4 increased the VOC from 0.580 V to 0.605 V. The co-sensitized Y1 + TP2A + HSQ4 based DSSCs showed a new optimal efficiency (η) of 7.48%. This is the highest efficiency recorded for DSSCs based on co-sensitization of triple organic dyes. Intensity modulated photovoltage spectroscopy further confirms the longer lifetime of co-sensitized Y1 + TP2A + HSQ4 compared to that of each individual TP2A and HSQ4 and co-sensitized TP2A + HSQ4 dye.
Introduction
Dye sensitized solar cells (DSSCs) have attracted the attention of materials scientists due to their extraordinarily easy material synthesis process, light weight and flexible physical structure. Significant research studies have been conducted for the progress of DSSCs but they still lack in various aspects. Generally, DSSCs are comprised of an electrode, a dye sensitizer, electrolytes, a counter electrode and a transparent conducting substrate, whereas the dye dictates the performance.1–3 The main function of an efficient dye is to capture the maximum incident light over the visible spectrum and inject the photogenerated electron into the semiconductor oxide with a matched energy level. In this aspect, metal based ruthenium N719 dye has shown impressive absorption in the visible spectrum but it lacks absorption in the infrared (IR) region. Moreover, designing DSSCs with metal based dyes has been quite unattractive due to their scarcity.4 In contrast, metal free organic dyes via addition of different light absorbing groups within the organic framework can easily tune the absorption over a broad spectral range and achieve high molar extinction coefficients, which leads to the fabrication of highly efficient DSSCs.5–7 However, achieving panchromatic absorption over the whole visible spectrum by a single organic dye is difficult, as there have been only a few dyes showing this rare potential.8–13 Co-sensitization of multiple organic dyes which exhibit the maximum absorption in sensitive smaller parts of the visible region of 300–850 nm is much anticipated for obtaining high efficiency DSSCs.14–21 Nonetheless, careful molecular engineering is necessary to obtain such organic dyes of different absorption wavelength ranges and tailor them together to obtain panchromatic absorption. In our pursuit to achieve a panchromatic absorption, we have successfully designed dyes with different absorption wavelength ranges from the ultraviolet to the near infrared region.22–25 Although these dyes showed intense absorption in specific areas of the visible spectrum, they failed to provide panchromatic absorption over the whole visible spectrum. Hence, we have used a co-sensitization technique and boosted the performance of DSSCs via panchromatic engineering with metal dyes.19,26 Recently, we have employed the co-sensitization technique with all organic dyes to achieve panchromatic absorption over the whole visible spectrum and reported high performance DSSCs.27 However, tailoring three organic dyes together to achieve panchromatic absorption and produce highly efficient DSSCs has remained a challenge.Previously we have reported a small donor–π–acceptor organic dye Y1 which showed intense absorption maxima between 350 nm and 400 nm.28 The Y1 molecule was designed with butyloxyl-substituted phenyl as an electron donating unit, thiophene as a π-spacer and a cyanoacetic acid group as an acceptor. Y1 showed a strong absorption around 400 nm and successfully overcame the short wavelength dip caused by the triiodide in the incident photon to current conversion efficiency (IPCE) spectrum and successfully enhanced the IPCE of black dye in the UV region in a co-sensitized DSSC. Boron dipyrromethene (bodipy) dye TP2A was successfully investigated and a peak absorption maximum of 581 nm was reported by us.29 Upon deposition on a transparent TiO2 electrode, TP2A dye showed a wide absorption spectrum in the 500–650 nm region and showed a high open circuit voltage (VOC) of 0.526 V in a DSSC. To absorb light on the other end of the visible spectrum, we have also designed and verified near infrared (NIR) absorbing squaraine dye HSQ4 which has a broad absorption spectrum in the 620–750 nm region.23 HSQ4 dye showed a maximum IPCE value of 80% at 720 nm in a DSSC. These dyes with staggering potential of absorbing light at different wavelengths over the whole visible spectrum clearly indicate that, if co-sensitized together, they may achieve a minimum of panchromatic absorption in the 350–800 nm region.
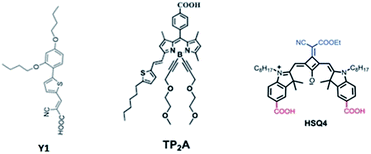
In this study, we adopted the co-sensitization technique of the aforementioned Y1, TP2A and HSQ4 organic dyes containing the molecular structures displayed in Fig. 1 for the first time. The significant panchromatic absorption occupancy in the 320–820 nm region, leading to an IPCE over 70% in the region, is yielded with a short current density (JSC) of 17.57 mA cm−2, a VOC of 0.605 V and a fill factor (FF) of 0.703 in a DSSC with an optimized molar ratio in a triple dye co-sensitized process. We report here an efficiency (η) of 7.48% for Y1 + TP2A + HSQ4 in a DSSC.
Results and discussion
Photophysical properties
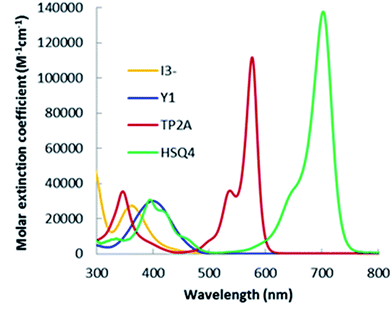
The absorption spectra of triiodide (I3−), Y1, TP2A and HSQ4 dyes in ethanol are presented in Fig. 2. The absorption spectrum of I3− shows a small molar extinction coefficient of 2.73 × 104 M−1 cm−1 with a dip at 320 nm. This dip in the UV-vis region greatly affects the IPCE spectrum in DSSC operation.30 To overcome this defect, we have successfully designed and synthesized dyes with greater absorption in the UV region. Our synthesized D–π–A structured molecule Y1 shows strong absorption between 350 nm and 450 nm with peak absorption at 390 nm. The bodipy molecule TP2A absorption is between 320 nm and 600 nm with two peaks at 350 nm and 581 nm, containing a wide gap between these two bands covering the 370–550 nm region. In our earlier work,29 we have reported the functionality of a vinyl-thienyl side arm which contributes to the absorption peak of TP2A in the lower wavelength region (350 nm). It's evident that this peak of 350 nm can surpass the dip created by the triiodide in the region successfully. The other peak of 581 nm of the TP2A corresponds to the lowest energy transition of the bodipy core. Our selected NIR dye, HSQ4, shows absorption in the 350–700 nm region with absorption maxima peaking at 390 nm and 703 nm. Interestingly, the gap between the two absorption bands of HSQ4 is covered by the second absorption band of TP2A dye, indicating an ideal match for co-sensitized DSSCs with the potential of light harvesting over the whole region.The highest molar extinction coefficients of the longer wavelength peaks of the three co-sensitizers Y1, TP2A and HSQ4 were recorded to be 3.0 × 104 M−1 cm−1 (at 390 nm), 1.15 × 105 M−1 cm−1 (at 581 nm) and 1.38 × 105 M−1 cm−1 (at 703 nm), respectively. It's evident that, with a high molar extinction coefficient of 3.8 × 10 4 M−1 cm−1 for its shorter wavelength peak at 350 nm, the TP2A may successfully overcome the loss of light absorption caused by triiodide in the region. The shorter wavelength peaks for Y1 and HSQ4 are also higher than that of triiodide in their overlying region (380–480 nm). The onset wavelength of HSQ4 at 730 nm obviously indicates strong absorption from the red to the NIR region.
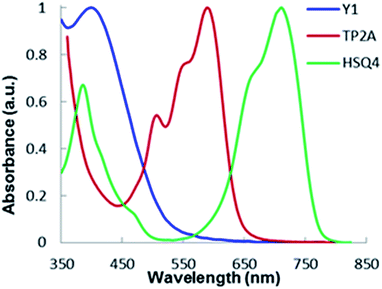
The absorption spectra of the co-sensitizers Y1, TP2A and HSQ4 absorbed onto a transparent TiO2 surface are shown in Fig. 3. Upon anchoring the dyes on the TiO2 surface, Y1 dye shows an absorption peak at 398 nm shifting 8 nm from that of ethanol solution and the energy absorption onset is red-shifted by 50 nm to absorb light up to 550 nm. The red-shifting occurs due to the strong binding between the Y1 dye and TiO2 surface. The TP2A dye also shifted 9 nm and portrayed a strong absorption peak at 590 nm with an increased absorption onset by a red shift of 50 nm. On the other hand, HSQ4 dye shows two absorption peaks at 400 nm and 740 nm, when anchored on the TiO2 surface. The highest absorption onset for HSQ4 dye is red shifted by 70 nm offering us the possibility to harvest light beyond the 800 nm region. These spectral results represent the panchromatic absorption ability in adsorbed films of Y1 + TP2A + HSQ4, which inspired their use in co-sensitized DSSCs as described in the following section.
Photovoltaic properties
We fabricated co-sensitized DSSCs with TP2A + HSQ4 and Y1 + TP2A + HSQ4 in ethanol solution. For TP2A and HSQ4 based DSSCs, we investigated the molar ratios of bodipy dye to squaraine configured dye by varying at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. Y1 + TP2A + HSQ4 was co-sensitized at a 1
1, respectively. Y1 + TP2A + HSQ4 was co-sensitized at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
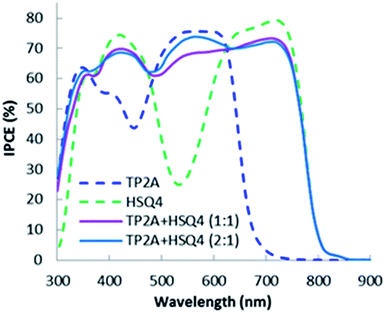
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. The co-sensitized DSSCs were investigated under standard AM 1.5 irradiation (100 mW cm−2). Fig. 4 shows IPCE spectra as functions of the wavelength of bodipy dye TP2A, squaraine dye HSQ4 and co-sensitized TP2A + HSQ4 dyes. TP2A produces a broad IPCE spectrum within the 300–670 nm region with two peaks of 63% at 345 nm (330–365 nm) and 75% at 568 nm (535–620 nm). A small dip in the IPCE spectrum at 380–470 nm observed for TP2A dye indicates the lowest absorption in the region. HSQ4 dye shows maximum absorption peaks of 80% at 750 nm and 75% at 420 nm. The two IPCE peaks are observed in 360–470 nm and 680–740 nm regions respectively with a huge dip in the IPCE spectrum below 30% at 535 nm, whereas TP2A dye shows steady absorption within the same region. The onset wavelength of HSQ4 was recorded up to 820 nm in the NIR region. As a result, HSQ4 DSSCs have a higher short circuit current (JSC = 15.61 mA cm−2) and output voltage (VOC = 0.544 V) than TP2A based DSSCs (JSC = 11.40 mA cm−2 and VOC = 0.526 V) (Table 2). This leads to an efficiency of 5.78% for HSQ4 and 4.26% for TP2A based DSSCs. But the HSQ4 based DSSCs showed a lower fill factor (FF = 0.682) than TP2A based DSSCs (FF = 0.710).
1 molar ratio. The co-sensitized DSSCs were investigated under standard AM 1.5 irradiation (100 mW cm−2). Fig. 4 shows IPCE spectra as functions of the wavelength of bodipy dye TP2A, squaraine dye HSQ4 and co-sensitized TP2A + HSQ4 dyes. TP2A produces a broad IPCE spectrum within the 300–670 nm region with two peaks of 63% at 345 nm (330–365 nm) and 75% at 568 nm (535–620 nm). A small dip in the IPCE spectrum at 380–470 nm observed for TP2A dye indicates the lowest absorption in the region. HSQ4 dye shows maximum absorption peaks of 80% at 750 nm and 75% at 420 nm. The two IPCE peaks are observed in 360–470 nm and 680–740 nm regions respectively with a huge dip in the IPCE spectrum below 30% at 535 nm, whereas TP2A dye shows steady absorption within the same region. The onset wavelength of HSQ4 was recorded up to 820 nm in the NIR region. As a result, HSQ4 DSSCs have a higher short circuit current (JSC = 15.61 mA cm−2) and output voltage (VOC = 0.544 V) than TP2A based DSSCs (JSC = 11.40 mA cm−2 and VOC = 0.526 V) (Table 2). This leads to an efficiency of 5.78% for HSQ4 and 4.26% for TP2A based DSSCs. But the HSQ4 based DSSCs showed a lower fill factor (FF = 0.682) than TP2A based DSSCs (FF = 0.710).
The co-sensitized TP2A + HSQ4 based DSSCs showed a broad IPCE spectrum from visible 320 nm to NIR 820 nm. The co-sensitized DSSCs successfully overcame the dips of each individual dye and showed an average IPCE of >60%. For a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of TP2A and HSQ4 dyes, the IPCE spectra showed a steady IPCE of 70% and above in the 540–800 nm region. The strong absorption in this region (see Fig. 3) contributes to a JSC of 16.77 mA cm−2 and a VOC of 0.570 V and η = 6.78% (Table 2). The bodipy and SQ based dyes at a 2
1 ratio of TP2A and HSQ4 dyes, the IPCE spectra showed a steady IPCE of 70% and above in the 540–800 nm region. The strong absorption in this region (see Fig. 3) contributes to a JSC of 16.77 mA cm−2 and a VOC of 0.570 V and η = 6.78% (Table 2). The bodipy and SQ based dyes at a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio shows a higher IPCE of at least 70% and above in the 490–640 nm region compared to those at a 1
1 molar ratio shows a higher IPCE of at least 70% and above in the 490–640 nm region compared to those at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
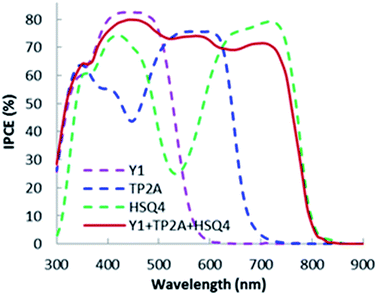
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The increased absorption in the region leads to an improved JSC of 17.06 mA cm−2. The improved short circuit current contributes to an impressive efficiency of 7.02%. The photovoltaic property data such as JSC, VOC, FF and power conversion efficiency (η) of the fabricated DSSCs are presented in Table 2. Fig. 5 shows the IPCE comparison of Y1, TP2A, HSQ4 and Y1 dye co-sensitized with the best performing TP2A + HSQ4 (at a 2
1 ratio. The increased absorption in the region leads to an improved JSC of 17.06 mA cm−2. The improved short circuit current contributes to an impressive efficiency of 7.02%. The photovoltaic property data such as JSC, VOC, FF and power conversion efficiency (η) of the fabricated DSSCs are presented in Table 2. Fig. 5 shows the IPCE comparison of Y1, TP2A, HSQ4 and Y1 dye co-sensitized with the best performing TP2A + HSQ4 (at a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio). The Y1 dye has an average IPCE >65% over the whole 315–500 nm region, with over 80% in the 400–490 nm region.
1 ratio). The Y1 dye has an average IPCE >65% over the whole 315–500 nm region, with over 80% in the 400–490 nm region.
The limited absorption range of Y1 in the UV region results in a low efficiency (η = 3.44%) DSSC (Table 2). The Y1 based DSSC shows a low JSC of 6.56 mA cm−2 with a VOC of 0.698 V. But Y1 based DSSCs yield the highest voltage (0.698 V), which is higher than that of each individual and co-sensitized DSSC in this experiment. However, the IPCE value of TP2A and HSQ4 is much lower in the 315–500 nm region. From the absorption spectra it is eminent that the dips of TP2A and HSQ4 in the UV region can be successfully overcome upon co-sensitization of Y1 with TP2A and HSQ4. The limited absorption range of Y1 in the UV region has resulted in a low DSSC efficiency (η = 3.44%) (Table 2). The Y1 based DSSC shows a low JSC of 6.56 mA cm−2. But the Y1 based DSSC yields a high voltage (0.698 V), which is higher than that of each individual and co-sensitized DSSC in this experiment. However, the IPCE value of TP2A and HSQ4 is much lower in the 315–500 nm region than that of Y1. From the film absorption spectra (Fig. 3), it is eminent that the dips of TP2A and HSQ4 in the UV region can be successfully overcome upon co-sensitization of Y1 with TP2A and HSQ4. Therefore, we co-sensitized Y1 with TP2A + HSQ4 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and achieved panchromatic absorption over the 300–820 nm region. The highest onset wavelength for the Y1 + TP2A + HSQ4 based DSSC was recorded to be as high as 820 nm. The addition of Y1 dye to the TP2A + HSQ4 successfully increases absorption in the UV region (315–380 nm) which leads to an IPCE of >60% in the region. A steady IPCE band over 70% in the 380–820 nm region is also observed for this co-sensitized triple organic dye system. The broad absorption over the visible region contributes to a higher JSC of 17.57 mA cm−2, VOC of 0.605 V and η of 7.48% for the co-sensitized Y1 + TP2A + HSQ4 DSSC, which is the highest efficiency of triple co-sensitized DSSCs as seen in Table 1. This indicates a better injection efficiency of a triple co-sensitized dye system than each of the individual dyes and co-sensitized TP2A + HSQ4 dye systems.
1) and achieved panchromatic absorption over the 300–820 nm region. The highest onset wavelength for the Y1 + TP2A + HSQ4 based DSSC was recorded to be as high as 820 nm. The addition of Y1 dye to the TP2A + HSQ4 successfully increases absorption in the UV region (315–380 nm) which leads to an IPCE of >60% in the region. A steady IPCE band over 70% in the 380–820 nm region is also observed for this co-sensitized triple organic dye system. The broad absorption over the visible region contributes to a higher JSC of 17.57 mA cm−2, VOC of 0.605 V and η of 7.48% for the co-sensitized Y1 + TP2A + HSQ4 DSSC, which is the highest efficiency of triple co-sensitized DSSCs as seen in Table 1. This indicates a better injection efficiency of a triple co-sensitized dye system than each of the individual dyes and co-sensitized TP2A + HSQ4 dye systems.
| Dye | Solution 1st peak ext. coef. (λmax) | Solution 2nd peak ext. coef. (λmax) | Film 1st peak λmax | Film 2nd peak λmax | Film absorption onset |
|---|---|---|---|---|---|
| Y1 | 3.0 × 104 (390) | — | 398 | — | 550 |
| TP2A | 3.8 × 104 (350) | 1.15 × 105 (581) | 359 | — | 660 |
| HSQ4 | 3.08 × 104 (395) | 1.38 × 105 (703) | 400 | 740 | 810 |
| I3− | 2.73 × 104 (360) | — | — | — | — |
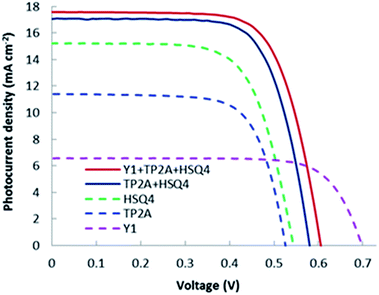
The current–voltage properties (I–V curves) of Y1, TP2A, and HSQ4, and co-sensitized TP2A + HSQ4 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and Y1 + TP2A + HSQ4 (1
1) and Y1 + TP2A + HSQ4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) dye systems under standard AM 1.5 illumination are shown in Fig. 6 and their corresponding JSC, VOC, FF and η are presented in Table 2. DSSCs based on TP2A dye show a JSC of 11.40 mA cm−2 which increased to 16.77 mA cm−2 when co-sensitized with HSQ4 in a molar ratio of 1
1) dye systems under standard AM 1.5 illumination are shown in Fig. 6 and their corresponding JSC, VOC, FF and η are presented in Table 2. DSSCs based on TP2A dye show a JSC of 11.40 mA cm−2 which increased to 16.77 mA cm−2 when co-sensitized with HSQ4 in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The best cell efficiency was observed for TP2A + HSQ4 based DSSCs when the molar ratio was varied from 1
1. The best cell efficiency was observed for TP2A + HSQ4 based DSSCs when the molar ratio was varied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In the latter case, JSC increased up to 17.06 mA cm−2, while the VOC and FF remained almost the same. Upon co-sensitization of Y1 with optimal TP2A + HSQ4, an increased VOC from 0.580 to 0.605 V has led to a power conversion efficiency of 7.48%. The increased VOC of the co-sensitized Y1 + TP2A + HSQ4 based DSSC seems to have more contribution to the improved efficiency than the increased JSC of 17.57 mA cm−2 for DSSCs based on co-sensitized TP2A + HSQ4 dye. The DSSC consisting of co-sensitized Y1 + TP2A + HSQ4 dyes showed a VOC of 0.605 V which is 0.025 V higher than that of the best TP2A + HSQ4 based DSSC. The beneficial role of Y1 contributes to the suppression of the charge recombination of electrons in the TiO2 film with I3− and have a positive impact on increasing the VOC. This intrigued the necessity to explore the electron lifetime related to charge recombination.
1. In the latter case, JSC increased up to 17.06 mA cm−2, while the VOC and FF remained almost the same. Upon co-sensitization of Y1 with optimal TP2A + HSQ4, an increased VOC from 0.580 to 0.605 V has led to a power conversion efficiency of 7.48%. The increased VOC of the co-sensitized Y1 + TP2A + HSQ4 based DSSC seems to have more contribution to the improved efficiency than the increased JSC of 17.57 mA cm−2 for DSSCs based on co-sensitized TP2A + HSQ4 dye. The DSSC consisting of co-sensitized Y1 + TP2A + HSQ4 dyes showed a VOC of 0.605 V which is 0.025 V higher than that of the best TP2A + HSQ4 based DSSC. The beneficial role of Y1 contributes to the suppression of the charge recombination of electrons in the TiO2 film with I3− and have a positive impact on increasing the VOC. This intrigued the necessity to explore the electron lifetime related to charge recombination.
| Dyes | Dye ratio | J SC (mA cm−2) | V OC (V) | FF | η (%) |
|---|---|---|---|---|---|
| Y1 | 6.56 | 0.698 | 0.750 | 3.44 | |
| TP2A | 11.40 | 0.526 | 0.710 | 4.26 | |
| HSQ4 | 15.61 | 0.544 | 0.682 | 5.78 | |
| TP2A + HSQ4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16.77 | 0.570 | 0.709 | 6.78 |
| TP2A + HSQ4 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
17.06 | 0.580 | 0.709 | 7.02 |
| Y1 + TP2A + HSQ4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
17.57 | 0.605 | 0.703 | 7.48 |
Intensity-modulated photovoltage spectrometry characterization
The electron lifetime (τ) in the DSSCs is a central quantity to determine the dynamics of charge recombination which is related to the VOC of the cell. The VOC dependence on charge recombination reactions rather than the molecular structures of the dyes has been well explored. Our earlier work shows that the conduction band edge of TiO2 remains unchanged in Y1, TP2A or HSQ4 sensitized cells,27–29 and thus the quasi-Fermi level of TiO2 is only influenced by the electron density in TiO2, which itself is affected by the electron leakage (recombination with the electrolyte (I3−)).To investigate the reason for the differences between the VOC values, we measured the electron lifetime (τ, which reflects the degree of electron recombination with the electrolyte (I3−)) of the DSSCs sensitized with Y1, TP2A, HSQ4, TP2A + HSQ4 and Y1 + TP2A + HSQ4 by means of intensity-modulated photovoltage spectroscopy. Fig. 7 shows the plot of the electron lifetimes (τ) of the DSSCs as a function of VOC using Y1, TP2A, and HSQ4 and co-sensitized optimal ratios of TP2A + HSQ4 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and Y1 + TP2A + HSQ4 (1
1) and Y1 + TP2A + HSQ4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) dyes. It is clearly obvious that the lifetime increases as TP2A < HSQ4 < TP2A + HSQ4 < Y1 + TP2A + HSQ4 < Y1, which is in accord with the order of increasing VOC observed in the DSSCs. The longer lifetime of Y1 + TP2A + HSQ4 associated with TP2A + HSQ4 suggests that the UV dye Y1 not only overcame the I3− light absorption but sufficiently suppressed the recombination of electrons in the TiO2 with the I3−. The longer lifetime of Y1 + TP2A + HSQ4 compared to those of TP2A + HSQ4, TP2A and HSQ4 individually suggests the successful regeneration of Y1 dye on the TiO2 by the redox electrolyte which suppressed strongly the TiO2 injected electron recombination with the electrolyte (I3−).
1) dyes. It is clearly obvious that the lifetime increases as TP2A < HSQ4 < TP2A + HSQ4 < Y1 + TP2A + HSQ4 < Y1, which is in accord with the order of increasing VOC observed in the DSSCs. The longer lifetime of Y1 + TP2A + HSQ4 associated with TP2A + HSQ4 suggests that the UV dye Y1 not only overcame the I3− light absorption but sufficiently suppressed the recombination of electrons in the TiO2 with the I3−. The longer lifetime of Y1 + TP2A + HSQ4 compared to those of TP2A + HSQ4, TP2A and HSQ4 individually suggests the successful regeneration of Y1 dye on the TiO2 by the redox electrolyte which suppressed strongly the TiO2 injected electron recombination with the electrolyte (I3−).
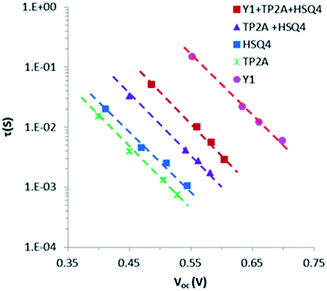 | ||
| Fig. 7 Electron lifetime (τ) as a function of open circuit voltage for DSSCs sensitized with Y1, TP2A, HSQ4, TP2A + HSQ4 and Y1 + TP2A + HSQ4. | ||
Conclusions
We have fabricated DSSCs by co-sensitization of organic butyloxyl chain induced Y1 dye, bodipy dye TP2A, squaraine based dye HSQ4 and achieved panchromatic absorption in the 300–820 nm range. The panchromatic absorption range in this work is the highest recorded value for triple dye based DSSCs till date. The Y1 + TP2A + HSQ4 dye based DSSC shows a JSC of 17.57 mA cm−2 and a VOC of 0.605 V and η = 7.48%. This performance is higher than that of individual dye and TP2A + HSQ4 based DSSCs. The IPCE spectrum of the Y1 + TP2A + HSQ4 dye based DSSC indicates a better injection efficiency than each of the individual dyes and TP2A + HSQ4 dyes. The addition of butyloxyl chain induced Y1 dye to TP2A + HSQ4 dye significantly improves the IPCE spectrum in the 300–400 nm region which leads to an enhanced VOC of the Y1 + TP2A + HSQ4 dye based DSSC. The VOC enhancement is attributed to the combined and complementary light harvesting ability of Y1, TP2A and HSQ4 dyes. These triple dye co-sensitized solar cells showed a panchromatic response with an IPCE >70% over the entire visible spectrum extending to the NIR region, suggesting their usage in indoor applications.Experimental
Materials and methods
All chemicals and reagents were purchased from Sigma-Aldrich and Alfa Aesar and used as received without further purification. The synthesis and characterization of Y1, TP2A and HSQ4 have been reported previously.23,28,29 UV-vis-NIR spectra were recorded in a 1 cm path length quartz cell in ethanol solution or on a TiO2 film (5 μm thickness) with a Shimadzu UV-vis to near IR UV-3600 spectrometer. The photoanode was prepared as reported previously.31 A fluorine-doped tin oxide (FTO) conducting glass substrate with a resistance of 8–10 Ω per sq and an optical transmission of greater than 80% in the visible range was used. A screen-printed double layer TiO2 film of (8 + 5) μm in thickness (0.25 cm2 cell area) with an 8 μm transparent layer of TiO2 particles (approximately 20 nm in diameter) and a 5 μm scattering layer of TiO2 particles (approximately 400 nm in diameter) was prepared. The films were sintered at 500 °C for 1 h. The films were further treated with 0.1 M HCl solutions before use. The thickness of the films was measured with a Surfcom 1400A surface profiler (Tokyo Seimitsu Co. Ltd.). A 0.2 mM solution of Y1, TP2A or HSQ4 and deoxycholic acid (20 mM) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) acetonitrile/tert-butyl alcohol was used to coat the TiO2 film for a single-dye-based DSSC. Mixtures of TP2A and HSQ4 with 1
1 (v/v) acetonitrile/tert-butyl alcohol was used to coat the TiO2 film for a single-dye-based DSSC. Mixtures of TP2A and HSQ4 with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (0.2 mM
1 (0.2 mM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 mM) and 2
0.2 mM) and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (0.26 mM
1 (0.26 mM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.13 mM) molar ratios and deoxycholic acid (20 mM) in 1
0.13 mM) molar ratios and deoxycholic acid (20 mM) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetonitrile and tert-butyl alcohol were used as co-sensitization dye solutions. For the triple dye co-sensitization system, a mixture of Y1, TP2A and HSQ4 with a 1
1 acetonitrile and tert-butyl alcohol were used as co-sensitization dye solutions. For the triple dye co-sensitization system, a mixture of Y1, TP2A and HSQ4 with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (0.13 mM
1 (0.13 mM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.26 mM
0.26 mM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.13 mM) molar ratio and deoxycholic acid (20 mM) in 1
0.13 mM) molar ratio and deoxycholic acid (20 mM) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetonitrile and tert-butyl alcohol was used. The TiO2 films were immersed in the dye solutions and then maintained at 25 °C for 15 h. To assemble each cell, each dye-coated TiO2 film and a platinum-coated conducting glass were separated by a 40 μm thick Surlyn spacer and sealed by heating the polymer frame at 100 °C. An electrolyte consisting of a mixture of 0.6 M dimethylpropyl-imidazolium iodide, 0.05 M I2, 0.1 M LiI, and 0.4 M tert-butylpyridine in acetonitrile was used in each cell.
1 acetonitrile and tert-butyl alcohol was used. The TiO2 films were immersed in the dye solutions and then maintained at 25 °C for 15 h. To assemble each cell, each dye-coated TiO2 film and a platinum-coated conducting glass were separated by a 40 μm thick Surlyn spacer and sealed by heating the polymer frame at 100 °C. An electrolyte consisting of a mixture of 0.6 M dimethylpropyl-imidazolium iodide, 0.05 M I2, 0.1 M LiI, and 0.4 M tert-butylpyridine in acetonitrile was used in each cell.
Photovoltaic performance measurements
The current–voltage characteristics were measured using a black metal mask with an area of 0.25 cm2 under AM 1.5 sunlight (100 mW cm−2, WXS-155S-10: Wacom Denso Co. Japan). The IPCE spectra were measured with a monochromatic incident light of 1 × 1016 photons cm−2 in direct current mode (CEP-2000BX, Bunko-Keiki).IMVS measurements
Intensity-modulated photovoltage spectroscopy (IMVS) was performed with a potentiostat (Solartron 1287) equipped with a frequency response analyzer (Solartron 1255B) under open-circuit conditions, based on monochromatic illumination (420 nm) controlled by a Labview system, to obtain the photovoltaic response induced by the modulated light. The modulated light was driven with a 10% AC perturbation current superimposed on a DC current in a frequency range from 0.1 to 106 Hz.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partly supported by the JSPS KAKENHI grant No. 26288113. MA and KS acknowledge the support of the Science Fund Grant with code 03-01-02 SF1149 of the Ministry of Science, Technology, and Innovation (MOSTI), Malaysia. MA and KS also acknowledge the financial support from the Solar Energy Research Institute (SERI) of Universiti Kebangsaan Malaysia (UKM) through the Dana Impak Perdana (DIP) research grant No. DIP-2015-027. This work was partly supported by the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2016M1A2A2940912). I. Bedja extends his appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No. (RG-1438-041).References
- A. Mishra, M. K. Fischer and P. Bäuerle, Angew. Chem., Int. Ed., 2009, 48, 2474–2499 CrossRef CAS PubMed
.
- M. Akhtaruzzaman, A. Islam, A. El-Shafei, N. Asao, T. Jin, L. Han, K. A. Alamry, S. A. Kosa, A. M. Asiri and Y. Yamamoto, Tetrahedron, 2013, 69, 3444–3450 CrossRef CAS
.
- M. Akhtaruzzaman, H. E. Mahmud, A. Islam, A. E. Shafei, M. R. Karim, K. Sopian, L. Han and Y. Yamamoto, Mater. Chem. Phys., 2013, 142, 82–86 CrossRef CAS
.
- M. Grätzel, J. Photochem. Photobiol., A, 2004, 168, 235 CrossRef
.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed
.
- Y. Wu and W. Zhu, Chem. Soc. Rev., 2013, 42, 2039–2058 RSC
.
- S. Zhang, X. Yang, Y. Numata and L. Han, Energy Environ. Sci., 2013, 6, 1443–1464 CAS
.
- Y. Chiba, A. Islam, Y. Watanabe, R. Komiya, N. Koide and L. Han, Jpn. J. Appl. Phys., 2006, 45, L638 CrossRef CAS
.
- H. Tian, X. Yang, R. Chen, A. Hagfeldt and L. Sun, Energy Environ. Sci., 2009, 2, 674–677 CAS
.
- J.-H. Yum, E. Baranoff, S. Wenger, M. K. Nazeeruddin and M. Gratzel, Energy Environ. Sci., 2011, 4, 842–857 CAS
.
- N. Koumura, Z.-S. Wang, S. Mori, M. Miyashita, E. Suzuki and K. Hara, J. Am. Chem. Soc., 2006, 128, 14256–14257 CrossRef CAS PubMed
.
- C. Qin, A. Islam and L. Han, Dyes Pigm., 2012, 94, 553–560 CrossRef CAS
.
- S. Qu, C. Qin, A. Islam, J. Hua, H. Chen, H. Tian and L. Han, Chem.–Asian J., 2012, 7, 2895–2903 CrossRef CAS PubMed
.
- A. Ehret, L. Stuhl and M. Spitler, J. Phys. Chem. B, 2001, 105, 9960–9965 CrossRef CAS
.
- R. Y. Ogura, S. Nakane, M. Morooka, M. Orihashi, Y. Suzuki and K. Noda, Appl. Phys. Lett., 2009, 94, 073308 CrossRef
.
- A. Islam, T. Swetha, M. R. Karim, M. Akhtaruzzaman, L. Han and S. P. Singh, Phys. Status Solidi A, 2015, 212, 651–656 CrossRef CAS
.
- C.-M. Lan, H.-P. Wu, T.-Y. Pan, C.-W. Chang, W.-S. Chao, C.-T. Chen, C.-L. Wang, C.-Y. Lin and E. W.-G. Diau, Energy Environ. Sci., 2012, 5, 6460–6464 CAS
.
- J. Chang, C.-P. Lee, D. Kumar, P.-W. Chen, L.-Y. Lin, K. J. Thomas and K.-C. Ho, J. Power Sources, 2013, 240, 779–785 CrossRef CAS
.
- S. Zhang, A. Islam, X. Yang, C. Qin, K. Zhang, Y. Numata, H. Chen and L. Han, J. Mater. Chem. A, 2013, 1, 4812–4819 CAS
.
- Y. Xie, Y. Tang, W. Wu, Y. Wang, J. Liu, X. Li, H. Tian and W.-H. Zhu, J. Am. Chem. Soc., 2015, 137, 14055–14058 CrossRef CAS PubMed
.
- K. Pei, Y. Wu, H. Li, Z. Geng, H. Tian and W.-H. Zhu, ACS Appl. Mater. Interfaces, 2015, 7, 5296–5304 CAS
.
- G. Ferrara, T. Jin, M. Akhtaruzzaman, A. Islam, L. Han, H. Jiang and Y. Yamamoto, Tetrahedron Lett., 2012, 53, 1946–1950 CrossRef CAS
.
- C. Qin, Y. Numata, S. Zhang, X. Yang, A. Islam, K. Zhang, H. Chen and L. Han, Adv. Funct. Mater., 2014, 24, 3059–3066 CrossRef CAS
.
- M. Akhtaruzzaman, A. Islam, F. Yang, N. Asao, E. Kwon, S. P. Singh, L. Han and Y. Yamamoto, Chem. Commun., 2011, 47, 12400–12402 RSC
.
- H. Jiang, K. Oniwa, A. Islam, J. Zhao, L. Han, Y.-J. Sun, M. Bao, N. Asao, Y. Yamamoto and T. Jin, Tetrahedron, 2015, 71, 6534–6540 CrossRef CAS
.
- S. P. Singh, M. Chandrasekharam, K. S. V. Gupta, A. Islam, L. Han and G. D. Sharma, Org. Electron., 2013, 14, 1237–1241 CrossRef CAS
.
- A. Islam, M. Akhtaruzzaman, T. H. Chowdhury, C. Qin, L. Han, I. M. Bedja, R. Stalder, K. S. Schanze and J. R. Reynolds, ACS Appl. Mater. Interfaces, 2016, 8(7), 4616–4623 CAS
.
- L. Han, A. Islam, H. Chen, C. Malapaka, B. Chiranjeevi, S. Zhang, X. Yang and M. Yanagida, Energy Environ. Sci., 2012, 5, 6057–6060 CAS
.
- C. Qin, A. Mirloup, N. Leclerc, A. Islam, A. El-Shafei, L. Han and R. Ziessel, Adv. Energy Mater., 2014, 4, 1400085 CrossRef
.
- M. Grätzel, Inorg. Chem., 2005, 44, 6841–6851 CrossRef PubMed
.
- P. Wang, S. M. Zakeeruddin, P. Comte, R. Charvet, R. Humphry-Baker and M. Grätzel, J. Phys. Chem. B, 2003, 107, 14336–14341 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2018 |