Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Intramolecular hydrogen-bonding in a cobalt aqua complex and electrochemical water oxidation activity†
Juliet F.
Khosrowabadi Kotyk
,
Caitlin M.
Hanna
,
Rebecca L.
Combs
,
Joseph W.
Ziller
and
Jenny Y.
Yang
 *
*
Department of Chemistry, University of California, Irvine, USA. E-mail: j.yang@uci.edu
First published on 6th February 2018
Abstract
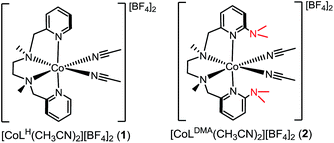
Water oxidation is catalysed in Nature by a redox cofactor embedded in a hydrogen-bonded network designed to orchestrate proton transfer throughout the challenging 4 electron reaction. In order to mimic aspects of this microenvironment, [CoLDMA(CH3CN)2][BF4]2 (2) was synthesized, where LDMA is a dipyridyldiamine ligand with two dimethylamine bases in the secondary coordination sphere. Structural characterization of the corresponding aqua complexes establish hydrogen bonding between the bound water and pendant base(s). Cyclic voltammetry of [CoLDMA(CH3CN)2][BF4]2 (2) reveals enhanced oxidative current upon titration with water and controlled potential electrolysis confirms evolution of O2. The related complex [CoLH(CH3CN)2][BF4]2 (1), which has the same primary coordination environment as 2 but lacks pendant bases, is inactive. The structural and electrochemical studies illustrate the role positioned proton relays can play in promoting redox reactivity.
Introduction
In Nature, the Oxygen Evolving Complex (OEC) in photosystem II catalyses the oxidation of water to liberate proton and electron equivalents for carbon dioxide reduction. The challenging 4-electron, 4-proton reaction is choreographed using a precisely tuned network of hydrogen-bonding interactions.1–5 Efforts to design synthetic catalysts that mimic aspects of this microenvironment have primarily utilized distal interactions to orient water for O–O bond formation. These interactions have either been incorporated into the ligand,6–11 or are assembled through the use of appropriate buffers.12Proximal interactions are also important for managing proton inventory at the reaction site. Sequential removal of protons with electrons enables successive oxidation events to occur in a smaller potential window (redox-levelling). The magnitude of this effect has been quantified in synthetic models.13,14Concerted proton and electron transfer provides an even lower energetic route for water oxidation and can be achieved through proton acceptors of correct positioning and pKb.15–17 This mechanism is believed to be important to the OEC, which performs the rapid four-electron oxidation of water within a narrow 0.3 V range.18–24 Concerted proton transfer is also cited in the synthetic catalyst [RuII(damp)(bpy)(H2O)]2+, which orients external water molecules to couple proton removal with O–O bond formation.25,26
Constructing a synthetic scaffold to facilitate concerted proton transfer presents challenges as the base must be positioned and matched to both the substrate and reaction conditions. For example, appropriately positioned bases were previously installed in a series of iron complexes. Their activity for water oxidation was investigated using the chemical oxidant Ce(IV) at very low pH. Under these conditions the bases were likely already protonated, and negligible changes in activity were observed.27 Likewise, Ru complexes modified with naphthyridine-functionalized ligands display no improvement in activity;28–30 spectrophotometric titration established that the intended base is a poor proton acceptor as it is unprotonated at pH values as low as zero.30 Two copper complexes,31,32 Cp*Ir(pyalc)Cl,33 and most recently a Ru complex34 represent the few synthetic examples where proximal proton acceptors contribute to enhance water oxidation activity.
Our interest in concerted proton transfer for small molecule redox catalysis led us to synthesize a series of N,N′-dimethyl-N,N′-bis(2-pyridylmethyl)ethane-1,2-diamine (BPMEN) ligands with pendant bases in the secondary coordination sphere.27,35 In this study, we focused our efforts on [CoLDMA(CH3CN)2]2+ (2, Chart 1), where LDMA is a BPMEN ligand modified with two dimethylamine pendant bases. In [CoLDMA(CH3CN)2]2+ (2), the location and pKb of the pendant bases are matched for concerted proton transfer upon oxidation of aqua ligands in the primary coordination sphere (vide infra). Our choice was supported by structural studies establishing intramolecular hydrogen-bonding interactions in Co(II) LDMA aqua complexes. Additionally, electrochemical studies demonstrate 2 is competent for water oxidation while [CoLH(CH3CN)2]2+ (1, Chart 1), which lacks pendant bases, is inactive, supporting the role proximal proton acceptors can play in enabling redox reactivity.
Results and discussion
Synthetic procedures
The divalent cobalt complexes [CoLH(CH3CN)2][BF4]2 (1) and [CoLDMA(CH3CN)2][BF4]2 (2) shown in Chart 1 were synthesized by adding the respective ligands to an acetonitrile solution of [Co(CH3CN)6][BF4]2,36 and were isolated as orange solids in 78–89% yield. The formulations were confirmed by ESI-MS and purity by elemental analysis (see ESI†). Complexes 1 and 2 were also characterized by electron absorption (Fig. S1†) and electron paramagnetic resonance spectroscopy (Fig. S2†).Crystallography
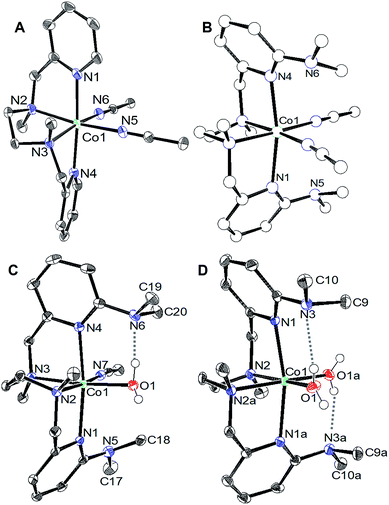
Crystals suitable for single crystal X-ray diffraction were grown by diethyl ether diffusion into acetonitrile. The solid-state structures for 1 and 2 are shown in Fig. 1a and b, respectively. The quality of the diffraction data for 2 was poor, and is only depicted to establish connectivity. As expected, the dimethylamine functional groups in [CoLDMA(CH3CN)2][BF4]2 (2) are poised over the labile coordination sites occupied by solvent.Diethyl ether diffusion into a mixed H2O/CH3CN solution of [CoLDMA(CH3CN)2][BF4]2 (2) resulted in crystallization of the mono-aqua complex [CoLDMA(CH3CN)(H2O)][BF4]2. The corresponding bis-aqua complex [CoLDMA(H2O)2][BF4]2 was isolated by diffusing diethyl ether into a 5 mL solution of [CoLDMA(CH3CN)2][BF4]2 (2) in the less coordinating solvent, CH2Cl2 with 1.1 μL H2O (2 equiv.). The solid state structures of [CoLDMA(CH3CN)(H2O)][BF4]2 and [CoLDMA(H2O)2][BF4]2 are shown in Fig. 1c and d, respectively, and selected bond angles are listed in Table 1. The hydrogen atoms on the aqua ligand(s) were located in the difference map. Although the products were isolated as crystals suitable for X-ray analysis, the complexes turned from pink to blue under reduced pressure, suggesting desolvation37 and precluded isolation of the aqua complexes for further characterization.
| [CoLDMA(CH3CN)(H2O)][BF4]2 | [CoLDMA(H2O)2][BF4]2 | ||
|---|---|---|---|
| C1–N5–C17 | 119.67(13) | C1–N3–C9 | 113.56(11) |
| C1–N5–C18 | 120.65(12) | C1–N3–C10 | 115.43(11) |
| C14–N6–C19 | 114.75(11) | N3⋯O1 | 2.8692(16) |
| C14–N6–C20 | 110.71(11) | ||
| N6⋯O1 | 2.6530(16) | ||
| N5⋯N7 | 3.1282(17) | ||
The solid-state structures reveal hydrogen-bonding interactions between the aqua ligands and the dimethylamine functionalities. In [CoLDMA(CH3CN)(H2O)][BF4]2, the distance between the dimethylamine nitrogen (N6) and aqua oxygen (O1) is 2.6530(16) Å, compared to 3.1282(17) Å between the other dimethylamine nitrogen (N5) and nitrogen on the adjacent acetonitrile ligand (N7). For [CoLDMA(H2O)2][BF4]2, the structure was generated from a two-fold C2 axis; only one of the two hydrogen bonding interactions between the aqua ligands and dimethylamines are crystallographically unique. The distance between the dimethylamine nitrogen (N3) to oxygen (O1) on the aqua ligand is 2.8692(16) Å. The distances observed between the hydrogen bond acceptor and donor are in the range of a moderate hydrogen-bonding interaction.38
The hydrogen-bonding interactions are also evident from the geometry at the dimethylamines associated with aqua ligands compared to those with no exogenous interactions (Table 1). In [CoLDMA(CH3CN)(H2O)][BF4]2, the C(pyridine)–N(dimethylamine)–CH3 angles for N5, which does not participate in hydrogen-bonding, are 119.67(13)° and 120.65(12)°, which are expected for a tertiary amine. In contrast, N6, which is poised over the aqua ligand, has more acute angles, 114.75(11)° and 110.71(11)°, indicating a contraction to accommodate the hydrogen-bonding interaction with a proton from water. The bis-aqua bound structure [CoLDMA(H2O)2][BF4]2 also demonstrates angular distortion with acute N3 angles of 113.56(11)° and 115.43(11)°. Complete information regarding the refinement of each structure, as well as tables for all bond angles and distances can be found in the ESI (Fig. S16–S19, Tables S1–S7†).
Electrochemical studies
The cyclic voltammograms for the oxidation of [CoLH(CH3CN)2][BF4]2 (1) and [CoLDMA(CH3CN)2][BF4]2 (2) are shown as the black trace in Fig. 2a and b, respectively. Complex 1 and 2 exhibit an oxidation at Epa = 0.77 V and 0.96 V versus Fe(C5H5)2+/0, respectively. Both oxidations are irreversible at scan rates up to 1600 mV s−1 (Fig. S4†). Cyclic voltammograms of 1 and 2 over a larger potential window are shown in Fig. S3.†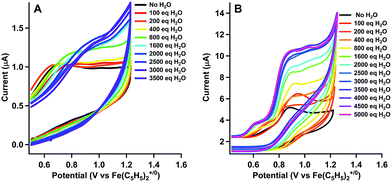 | ||
| Fig. 2 Cyclic voltammograms upon addition of water to 1 mM solutions of (a) [CoLH(CH3CN)2][BF4]2 (1) and (b) [CoLDMA(CH3CN)2][BF4]2 (2) in CH3CN at 100 mV s−1. | ||
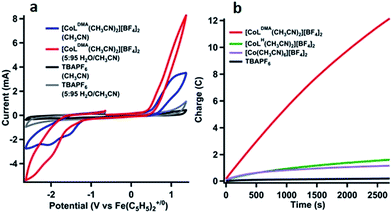
We tested activity for electrochemical water oxidation by adding aliquots of water to 1 and 2 in acetonitrile since the cobalt complexes are minimally soluble in water. The resulting cyclic voltammograms are shown as coloured traces in Fig. 2. The oxidative current for [CoLH(CH3CN)2][BF4]2 (1), which contains no pendant amines in the secondary coordination sphere, displayed minimal changes upon addition of up to 400 equivalents of water. A small current increase is observed with 400 to 3500 equivalents of water which does not appear to be due to water oxidation since negligible O2 is detected under controlled potential electrolysis at 1.07 V vs. Fe(C5H5)2+/0 (vide infra). The mild current increase may be due to catalytic hydration of acetonitrile to acetamide, a reaction that has been observed for similar Co(III) complexes.39 Addition of between 3500 up to 5000 equivalents of water resulted in loss of the oxidation event. Full cyclic voltammetry data for [CoLH(CH3CN)2][BF4]2 (1) and water titration can be seen in Fig. S5.†
In contrast, [CoLDMA(CH3CN)2][BF4]2 (2) displayed an enhanced current response with increasing water content (up to 4000 equivalents), leading to a plateau profile characteristic of electrocatalytic activity where the catalyst is operating under pseudo-first order conditions.40 A negative shift in the electrochemical potential appears with increasing concentrations of water, which may be due to displacement of one or both acetonitrile ligands with aqua ligands. The magnitude of the plateau current increased linearly with the concentration of 2, indicating the oxidation reaction is first order in 2 (Fig. S7 and S8†).
We also compared the electrochemical oxidation of 2 in the presence of D2O. Increasing equivalents of D2O leads to a similar current enhancement and plateau (see Fig. S6†). However, the current ceases to increase after addition of 800 equivalents. An accurate determination of the rate of water oxidation using cyclic voltammetry is challenging because the oxidation of 2 is not reversible under non-catalytic conditions. However the ratio of the current enhancement vs. the non-catalytic current decreases from 4 to 2.4 with H2O vs. D2O as substrate, indicating a 2-fold decrease in rate for a 4 electron process.41
The product of oxidation in the presence of water with [CoLDMA(CH3CN)2][BF4]2 (2) was identified using controlled potential electrolyses (CPE) and analysis of the headspace by gas chromatography.42Fig. 3a shows the cyclic voltammetry of 1 mM solutions of [CoLDMA(CH3CN)2][BF4]2 (2) prior to electrolysis in acetonitrile (blue trace) and in 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 CH3CN
5 CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v, red trace). The cyclic voltammograms with no compound present are shown in black (acetonitrile) and grey (95
H2O (v/v, red trace). The cyclic voltammograms with no compound present are shown in black (acetonitrile) and grey (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 CH3CN
5 CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, v/v) in Fig. 3a, demonstrating no appreciable background current in the potential window under these conditions. Cyclic voltammetry with [CoLH(CH3CN)2][BF4]2 (1) under equivalent conditions in the controlled potential electrolysis cell are shown in Fig. S9.†
H2O, v/v) in Fig. 3a, demonstrating no appreciable background current in the potential window under these conditions. Cyclic voltammetry with [CoLH(CH3CN)2][BF4]2 (1) under equivalent conditions in the controlled potential electrolysis cell are shown in Fig. S9.†
Charge passed during controlled potential electrolysis at 1.07 V vs. Fe(C5H5)2+/0 for 1 mM [CoLDMA(CH3CN)2][BF4]2 (2) in 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 CH3CN
5 CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v) is shown as the red trace in Fig. 3b. By comparison, a low current response is observed for [CoLH(CH3CN)2][BF4]2 (1) (green trace, Fig. 3b), which lacks pendant bases.
H2O (v/v) is shown as the red trace in Fig. 3b. By comparison, a low current response is observed for [CoLH(CH3CN)2][BF4]2 (1) (green trace, Fig. 3b), which lacks pendant bases.
Analysis of the O2 concentration in the headspace after electrolysis of [CoLDMA(CH3CN)2][BF4]2 (2) (Fig. S11†) leads to a faradic yield of 99% for the oxidation of water to oxygen (∼1 TON). Longer electrolyses result in a decline of the faradic yield of O2. Electronic absorption spectra taken before and after electrolysis reveal changes in solution composition (Fig. S10†). These changes, coupled with a gradual decrease in current (Fig. 3b & S10†) and faradic yield under extended electrolysis, suggests degradation of 2 under oxidative conditions. Previous studies with ligands of this type have shown long-term instability under oxidizing conditions, with evidence of demetallation and ligand decomposition.26,43,44 Acetonitrile may also be susceptible to oxidative degradation under these conditions. An infrared spectrum of the post-electrolysis solution after solvent removal exhibits a small peak at 2060 cm−1, a value similar to cobalt cyanide complexes.45 (see Fig. S11†) Another contribution to the decline in activity may be an increase in solution acidity during electrolysis that promotes decomposition by ligand deprotonation. Studies have been performed on water oxidation in buffered mixed organic-aqueous solutions.46 However we found aqueous buffers (phosphate, carbonate) were insufficiently soluble in our solution and soluble organic bases (primarily amines) were oxidized at potentials negative of 2.
The concentration-dependent oxidation activity (measured by the plateau current in the CV) indicates the active species is likely 2 (Fig. S7 and S8†). However, prior studies have shown that Co(II) ions can leach into solution from molecular species to form active heterogeneous electrocatalysts in aqueous phosphate buffer.47 To minimize this possibility, we performed several additional experiments that support homogeneous water oxidation activity by 2. An electrolysis of 1 mM solution of [Co(CH3CN)6][BF4]2 under identical conditions passed minimal current (lavender trace, Fig. 3b). For both [CoLH(CH3CN)2][BF4]2 (1) and [Co(CH3CN)6][BF4]2 (green and lavender trace, respectively, in Fig. 3b), the amount of oxygen in the headspace was below the detectable limit of our GC calibration curve, or less than 0.1%. All electrolyses were also performed with a mercury pool in the electrolysis cell to form an amalgam with potential cobalt oxide heterogeneous particles forming in situ.47,48 Additionally, the working electrode was rinsed with water and acetonitrile and reused in a fresh solution of 5% v/v solution of H2O in CH3CN to confirm no heterogeneous cobalt compounds had deposited onto the electrode surface (Fig. 3b, black trace). Due to sample size constraints we are unable to perform an XPS on the high-surface area vitreous carbon working electrode used to quantify O2 after controlled potential electrolysis. We instead performed an identical electrolysis using a smaller glassy carbon electrode. The current vs. time profile is similar to that observed with vitreous glassy carbon (Fig. S14†). No evidence of cobalt or cobalt oxide was observed in the XPS spectrum (Fig. S15†). Although it is difficult to completely rule out any contributions to water oxidation by heterogeneous species, the lack of induction period also suggests the initial species is evolving oxygen, and the decrease in activity indicates degradation into an inactive species.
The slightly higher reduction potential of [CoLDMA(CH3CN)2][BF4]2 (2) compared to [CoLH(CH3CN)2][BF4]2 (1) could potentially contribute to the difference in activity between the two complexes. However, this effect is minimal at higher concentrations of water since the onset potential for 2 decreases to roughly the same potential as 1 while maintaining an enhanced current response.
The water oxidation activity of 2vs.1 is more likely enabled by the presence of the pendant bases. It is possible the pendant bases facilitate catalysis by providing a favourable H2O binding environment through the hydrogen-bonding interactions. However, the pKb and proximity of dimethylamine proton acceptors in the secondary coordination sphere are also appropriate for supporting concerted proton transfer. The pKa's of related N-coordinated Co(II) aqua complexes are between 6 and 8.49 Upon oxidation, the pKa of metal aqua complexes typically increase by at least 6 units.16,50 We expect the dimethylamine pendant bases in LDMA to have a similar pKa to protonated dimethylaniline (5.07).51 The pendant bases are thus matched to accept a proton from an oxidized aqua complex and circumvent formation of a highly acidic (and energetic) intermediate.6,7,26,52–57 Hydrogen-bond acceptors have also been shown to stabilize cobalt hydroxide or hydroperoxo ligands.58,59 Our efforts to isolate or spectroscopically identify intermediates during water oxidation using stoichiometric chemical oxidants and bases were unsuccessful. However, the marked difference in water oxidation activity between 1 and 2, along with the deuterium isotope effect, indicate the potential role of intramolecular proton acceptors in facilitating redox catalysis.
Conclusions
The oxidation of water to oxygen is the vital half reaction to carbon dioxide reduction in photosynthesis. As a result, synthetic catalysts for this reaction are key to artificial photosynthetic schemes. We establish that appropriately positioned pendant bases can enable water oxidation activity in this system. Although the overall stability is an issue with this ligand set, our study underscores design principles for synthetic microenvironments that can be incorporated into more oxidatively stable catalysts.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This material is based on work supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0012150. XPS work was performed at the UC Irvine Materials Research Institute (IMRI) using instrumentation funded in part by the National Science Foundation Major Research Instrumentation Program under grant No. CHE-1338173.Notes and references
- L. Vogt, D. J. Vinyard, S. Khan and G. W. Brudvig, Curr. Opin. Chem. Biol., 2015, 25, 152–158 CrossRef CAS PubMed.
- J. P. McEvoy and G. W. Brudvig, Phys. Chem. Chem. Phys., 2004, 6, 4754–4763 RSC.
- H. J. Hwang, P. Dilbeck, R. J. Debus and R. L. Burnap, Biochemistry, 2007, 46, 11987–11997 CrossRef CAS PubMed.
- T. J. Meyer, M. H. V. Huynh and H. H. Thorp, Angew. Chem., Int. Ed., 2007, 46, 5284–5304 CrossRef CAS PubMed.
- B. A. Barry, U. Brahmachari and Z. Guo, Acc. Chem. Res., 2017, 50(8), 1937–1945 CrossRef CAS PubMed.
- D. K. Dogutan, R. McGuire and D. G. Nocera, J. Am. Chem. Soc., 2011, 133, 9178–9180 CrossRef CAS PubMed.
- W. Lai, R. Cao, G. Dong, S. Shaik, J. Yao and H. Chen, J. Phys. Chem. Lett., 2012, 3, 2315–2319 CrossRef CAS PubMed.
- H. Sun, Y. Han, H. Lei, M. Chen and R. Cao, Chem. Commun., 2017, 53, 6195–6198 RSC.
- M. Z. Ertem and C. J. Cramer, Dalton Trans., 2012, 41, 12213–12219 RSC.
- M. Yoshida, M. Kondo, S. Torii, K. Sakai and S. Masaoka, Angew. Chem., Int. Ed., 2015, 54, 7981–7984 CrossRef CAS PubMed.
- R. Matheu, M. Z. Ertem, J. Benet-Buchholz, E. Coronado, V. S. Batista, X. Sala and A. Llobet, J. Am. Chem. Soc., 2015, 137, 10786–10795 CrossRef CAS PubMed.
- Z. Chen, J. J. Concepcion, X. Hu, W. Yang, P. G. Hoertz and T. J. Meyer, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 7225–7229 CrossRef CAS PubMed.
- M. J. Baldwin and V. L. Pecoraro, J. Am. Chem. Soc., 1996, 118, 11325–11326 CrossRef CAS.
- B. Lassalle-Kaiser, C. Hureau, D. A. Pantazis, Y. Pushkar, R. Guillot, V. K. Yachandra, J. Yano, F. Neese and E. Anxolabehere-Mallart, Energy Environ. Sci., 2010, 3, 924–938 CAS.
- M. H. V. Huynh and T. J. Meyer, Chem. Rev., 2007, 107, 5004–5064 CrossRef CAS PubMed.
- M. Amin, L. Vogt, S. Vassiliev, I. Rivalta, M. M. Sultan, D. Bruce, G. W. Brudvig, V. S. Batista and M. R. Gunner, J. Phys. Chem. B, 2013, 117, 6217–6226 CrossRef CAS PubMed.
- J. M. Mayer and I. J. Rhile, Biochim. Biophys. Acta, Bioenerg., 2004, 1655, 51–58 CrossRef CAS PubMed.
- D. J. Vinyard and G. W. Brudvig, Annu. Rev. Phys. Chem., 2017, 68, 101–106 CrossRef CAS PubMed.
- G. W. Brudvig, a. W. F. Beck and J. C. Paula, Annu. Rev. Biophys. Biophys. Chem., 1989, 18, 25–46 CrossRef CAS PubMed.
- N. Cox, D. A. Pantazis, F. Neese and W. Lubitz, Acc. Chem. Res., 2013, 46, 1588–1596 CrossRef CAS PubMed.
- H. Bao, P. L. Dilbeck and R. L. Burnap, Photosynth. Res., 2013, 116, 215–229 CrossRef CAS PubMed.
- A. Klauss, M. Haumann and H. Dau, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 16035–16040 CrossRef CAS PubMed.
- L. I. Krishtalik, Bioelectrochem. Bioenerg., 1990, 23, 249–263 CrossRef CAS.
- C. W. Hoganson and G. T. Babcock, Science, 1997, 277, 1953–1956 CrossRef CAS PubMed.
- L. Vigara, M. Z. Ertem, N. Planas, F. Bozoglian, N. Leidel, H. Dau, M. Haumann, L. Gagliardi, C. J. Cramer and A. Llobet, Chem. Sci., 2012, 3, 2576–2586 RSC.
- X. Sala, S. Maji, R. Bofill, J. García-Antón, L. Escriche and A. Llobet, Acc. Chem. Res., 2014, 47, 504–516 CrossRef CAS PubMed.
- W. A. Hoffert, M. T. Mock, A. M. Appel and J. Y. Yang, Eur. J. Inorg. Chem., 2013, 2013, 3846–3857 CrossRef CAS.
- R. Zong and R. P. Thummel, J. Am. Chem. Soc., 2005, 127, 12802–12803 CrossRef CAS PubMed.
- H. Yamazaki, T. Hakamata, M. Komi and M. Yagi, J. Am. Chem. Soc., 2011, 133, 8846–8849 CrossRef CAS PubMed.
- J. L. Boyer, D. E. Polyansky, D. J. Szalda, R. Zong, R. P. Thummel and E. Fujita, Angew. Chem., 2011, 123, 12808–12812 CrossRef.
- T. Zhang, C. Wang, S. Liu, J.-L. Wang and W. Lin, J. Am. Chem. Soc., 2014, 136, 273–281 CrossRef CAS PubMed.
- F. Chen, N. Wang, H. Lei, D. Guo, H. Liu, Z. Zhang, W. Zhang, W. Lai and R. Cao, Inorg. Chem., 2017, 56, 13368–13375 CrossRef CAS PubMed.
- A. R. Parent, T. P. Brewster, W. De Wolf, R. H. Crabtree and G. W. Brudvig, Inorg. Chem., 2012, 51, 6147–6152 CrossRef CAS PubMed.
- D. W. Shaffer, Y. Xie, D. J. Szalda and J. J. Concepcion, J. Am. Chem. Soc., 2017, 139, 15347–15355 CrossRef CAS PubMed.
- J. F. Khosrowabadi Kotyk, J. W. Ziller and J. Y. Yang, J. Coord. Chem., 2016, 69, 1990–2002 CrossRef CAS.
- D. Coucouvanis, in Inorg. Synth., John Wiley & Sons, Inc., 2002, pp. 75–121, DOI:10.1002/0471224502.ch2.
- D. C. Lacy, Y. J. Park, J. W. Ziller, J. Yano and A. S. Borovik, J. Am. Chem. Soc., 2012, 134, 17526–17535 CrossRef CAS PubMed.
- J. J. Dannenberg, J. Am. Chem. Soc., 1998, 120, 5604 CrossRef CAS.
- J. H. Kim, J. Britten and J. Chin, J. Am. Chem. Soc., 1993, 115, 3618–3622 CrossRef CAS.
- J.-M. Savéant, Chem. Rev., 2008, 108, 2348–2378 CrossRef PubMed.
- J. M. Savéant, Elements of Molecular and Biomolecular Electrochemistry: An Electrochemical Approach to Electron Transfer Chemistry, Wiley, 2006 Search PubMed.
- C. Tsay and J. Y. Yang, J. Am. Chem. Soc., 2016, 138(43), 14174–14177 CrossRef CAS PubMed.
- M. Grau, A. Kyriacou, F. Cabedo Martinez, I. M. de Wispelaere, A. J. P. White and G. J. P. Britovsek, Dalton Trans., 2014, 43, 17108–17119 RSC.
- S. Kim, J. W. Ginsbach, J. Y. Lee, R. L. Peterson, J. J. Liu, M. A. Siegler, A. A. Sarjeant, E. I. Solomon and K. D. Karlin, J. Am. Chem. Soc., 2015, 137, 2867–2874 CrossRef CAS PubMed.
- D. M. S. Mosha and D. Nicholls, Inorg. Chim. Acta, 1980, 38, 127–130 CrossRef CAS.
- Z. Chen, J. J. Concepcion, H. Luo, J. F. Hull, A. Paul and T. J. Meyer, J. Am. Chem. Soc., 2010, 132, 17670–17673 CrossRef CAS PubMed.
- J. J. Stracke and R. G. Finke, J. Am. Chem. Soc., 2011, 133, 14872–14875 CrossRef CAS PubMed.
- V. Artero and M. Fontecave, Chem. Soc. Rev., 2013, 42, 2338–2356 RSC.
- C. J. Hawkins, A. M. Sargeson and G. H. Searle, Aust. J. Chem., 1964, 17, 598–600 CrossRef CAS.
- R. Gilson and M. C. Durrant, Dalton Trans., 2009, 10223–10230, 10.1039/b911593e.
- W. M. Haynes, T. J. Bruno and D. R. Lide, CRC handbook of chemistry and physics, CRC Press, [Boca Raton, Florida], 2015 Search PubMed.
- D. J. Wasylenko, H. M. Tatlock, L. S. Bhandari, J. R. Gardinier and C. P. Berlinguette, Chem. Sci., 2013, 4, 734–738 RSC.
- D. Wang and J. T. Groves, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 15579–15584 CrossRef CAS PubMed.
- W. Rüttinger and G. C. Dismukes, Chem. Rev., 1997, 97, 1–24 CrossRef.
- V. K. Yachandra, K. Sauer and M. P. Klein, Chem. Rev., 1996, 96, 2927–2950 CrossRef CAS PubMed.
- S. Berardi, S. Drouet, L. Francas, C. Gimbert-Surinach, M. Guttentag, C. Richmond, T. Stoll and A. Llobet, Chem. Soc. Rev., 2014, 43, 7501–7519 RSC.
- M. D. Kärkäs, O. Verho, E. V. Johnston and B. Åkermark, Chem. Rev., 2014, 114, 11863–12001 CrossRef PubMed.
- A. S. Borovik, Acc. Chem. Res., 2005, 38, 54–61 CrossRef CAS PubMed.
- C. M. Wallen, L. Palatinus, J. Bacsa and C. C. Scarborough, Angew. Chem., Int. Ed., 2016, 55, 11902–11906 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental details, synthetic methods, characterization, electrochemical data, gas chromatography, cif files. CCDC 1586338–1586341. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc04960a |
| This journal is © The Royal Society of Chemistry 2018 |