Open Access Article
Open Access ArticleSynthesis of stable polymetalated aromatic complexes through metal–macrocycle capsule-triggered cyclization†
Xin
He
 a,
Yang
Xue
a,
Cui-Cui
Li
a,
Yuechao
Wang
b,
Hong
Jiang
a,
Yang
Xue
a,
Cui-Cui
Li
a,
Yuechao
Wang
b,
Hong
Jiang
 b and
Liang
Zhao
b and
Liang
Zhao
 *a
*a
aThe Key Laboratory of Bioorganic Phosphorus Chemistry & Chemical Biology (Ministry of Education), Department of Chemistry, Tsinghua University, Beijing 100084, China. E-mail: zhaolchem@mail.tsinghua.edu.cn
bBeijing National Laboratory for Molecular Sciences, State Key Laboratory of Rare Earth Materials Chemistry and Applications, Institute of Theoretical and Computational Chemistry, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China
First published on 4th December 2017
Abstract
Polymetalated aromatic compounds are of great interest because of their intermediate roles in many organic transformations. However, they are elusive and synthetically challenging. In this study, a dynamic coordination capsule constructed by a flexible macrocycle and silver(I) ions is applied to trigger one-step or cascade cyclization reactions for various alkyne substrates, finally leading to five unprecedented polysilver heteroaromatic intermediates (including indole, quinoline, benzocarbazole and 2,2′-biindole). The acquired heteroaromatic species is doubly charged, particularly at vicinal positions, and each is surrounded by a tetrasilver aggregate. The metal–macrocycle capsule holds a great potential of flexibly adjusting its conformation to adapt different polysilver heteroaromatic species. DFT calculations further reveal that metal-perturbed aromaticity and multi-centered bonding both contribute to stabilization of the polysilver heteroaromatic complexes.
Introduction
Inspired by enzymes' remarkable ability to control and manipulate chemical reactions by means of steric confinement and precisely positioned functional groups, in the past decades chemists have made many attempts to mimic desirable properties of protein catalysts by constructing a confined environment via a molecular container.1 Besides covalently linked cyclic or cage-like molecules,2 recent preparation of molecular containers has evolved to more complex and elegant self-assembled architectures relying on the association of easily accessible modular components via e.g. metal–ligand coordination.3 The container architectures can encage guest molecules within a confined space, dynamically harness multiple non-covalent interactions in a synergistic manner to stabilize highly reactive transition states of substrate molecules, and accelerate otherwise sluggish chemical reactions. A variety of reactions (e.g. Diels–Alder reaction,4 electrocyclization,5 photoaddition6 and reductive elimination7) have been performed within metallacage hosts, yielding products with unusual selectivity and/or enhanced activity. However, yet to date there are few precedent examples of supramolecular coordination capsules that exhibit sufficient structural flexibility to adapt diverse substrates by adjusting cavity size.8 In addition, structurally well-defined reaction intermediates in the cavity of supramolecular coordination capsules are rarely isolated.On the other hand, polymetalated organometallic compounds are of great interest to synthetic chemists due to their promising potential for the synthesis of multi-substituted aromatic compounds,9 and their special role in the comprehension of reaction mechanisms provides inspiration for developing new synthetic methodologies.10 However, these kinds of compounds are challenging synthetic goals because of the difficulty of generating polyanionic species by direct electrophilic metalation or halogen–lithium–metal exchange reactions. Particularly, the 1,2-dimetalated aromatic compounds are very rare.
We herein attempt to trap polymetalated organometallic compounds inside supramolecular coordination capsules. Theoretically, flexible supramolecular capsules have good ability to adapt diverse substrates by adjusting cavity size. However, the use of flexible donor units in coordination self-assembly often causes a dilemma of yielding a mixture of multi-component self-assemblies. In this regard, inclusion of metal–metal interaction in the process of coordination self-assembly to facilitate the formation of a single discrete capsule structure may provide a solution. Metallophilic interaction as a kind of attractive interaction between metal atoms with closed-shell electronic configuration has been substantiated theoretically11 and experimentally.12 The well-known aurophilic and argentophilic interactions have been extensively observed in a number of polynuclear cluster compounds.13–15 In addition, Au(I) and Ag(I) as a linear bridging unit were frequently applied in the construction of coordination cycles and cages.16,17 In this work, we report the dynamic feature of a silver(I)-involved coordination capsule that is constructed by coordination-driven molecular folding of a flexible macrocyclic ligand octamethylazacalix[8]pyridine (Py[8]). The dynamic coordination capsule can trigger a one-step or cascade cyclization transformation for various alkynyl substrates with structural diversity (1a–1e), leading to successful isolation of unprecedented polysilver intermediates (2a–2e) of four heteroaromatics, including indole, quinoline, 2,2′-biindole, and benzocarbazole (Scheme 1). Single crystal X-ray diffraction analysis reveals that the macrocycle-based capsule structure provides optimal confined space to stabilize the elusive polymetalated aromatic intermediates. Computational studies indicate that metal-perturbed aromaticity and Ag2–C(sp2) multi-centered bonding both contribute to stabilization of the polysilver heteroaromatic complexes.
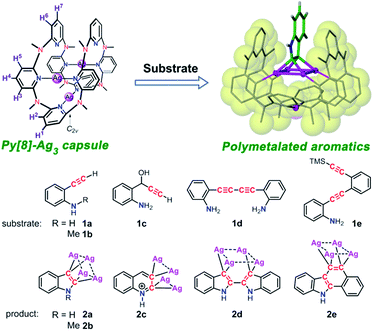 | ||
| Scheme 1 Molecular structures of substrates and polysilver-bonded heteroaromatics formed within a metal–macrocycle capsule. | ||
Results and discussion
Our recent work has shown that Py[8] can bind three silver atoms to construct a capsule-shaped crystalline complex [Ag3(Py[8])(CF3SO3)](CF3SO3)2 (Py[8]-Ag3).18 Interestingly, the metal ion binding process of Py[8] exhibited a significant cooperative coordination effect. 1H NMR monitoring18 and electrospray ionization mass spectroscopy (ESI-MS) (see Fig. S1 in the ESI† for details) revealed the formation of Py[8]-Ag3 even under metal ion deficient condition. Fitting of UV-vis titration results gave a large Ka1 ((1.66 ± 0.17) × 105 M−1) and Ka3 ((1.58 ± 0.16) × 105 M−1) relative to a small Ka2 ((1.90 ± 0.19) × 104 M−1) in the three-step metal ion binding process (Fig. S2†). This result rationalizes the ready formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 product Py[8]-Ag3 upon the presence of deficient silver(I) ions, and indicates that Py[8] helps the gathering of silver(I) ions by cooperative coordination effect.19 Furthermore, structural characterization by variable temperature 1H NMR uncovered a dynamic feature of the Py[8]-Ag3 coordination capsule. At room temperature 1H NMR spectrum of Py[8]-Ag3 exhibited two sets of broad signals corresponding to the pyridyl β- and γ-protons of Py[8] (Fig. 1). Upon lowering the temperature from 293 to 213 K, the signals turned into a series of sharp peaks, which can be properly assigned to seven kinds of pyridyl hydrogen atoms as shown in Py[8]-Ag3 (Scheme 1). These results indicate that at low temperature the conformation of Py[8]-Ag3 is fixed as a rigid form while at room temperature many possible conformations dynamically interconvert.
3 product Py[8]-Ag3 upon the presence of deficient silver(I) ions, and indicates that Py[8] helps the gathering of silver(I) ions by cooperative coordination effect.19 Furthermore, structural characterization by variable temperature 1H NMR uncovered a dynamic feature of the Py[8]-Ag3 coordination capsule. At room temperature 1H NMR spectrum of Py[8]-Ag3 exhibited two sets of broad signals corresponding to the pyridyl β- and γ-protons of Py[8] (Fig. 1). Upon lowering the temperature from 293 to 213 K, the signals turned into a series of sharp peaks, which can be properly assigned to seven kinds of pyridyl hydrogen atoms as shown in Py[8]-Ag3 (Scheme 1). These results indicate that at low temperature the conformation of Py[8]-Ag3 is fixed as a rigid form while at room temperature many possible conformations dynamically interconvert.
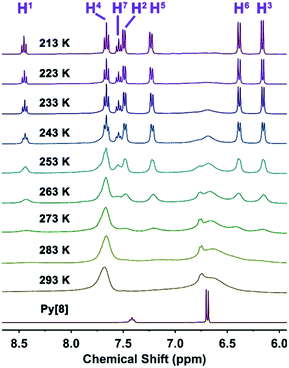 | ||
| Fig. 1 Variable temperature 1H NMR spectra of Py[8]-Ag3. 1H NMR spectrum of Py[8] at room temperature is plotted for comparison. | ||
The remarkable dynamic feature of the Py[8]-Ag3 capsule makes it as a unique flexible molecular flask to conduct organic transformations of diverse substrates. We purposefully selected several alkynyl substrates as Py[8] has been previously utilized by us as an outer template to realize controllable synthesis of silver acetylide clusters.20 In order to guarantee quantitative construction of Py[8]-Ag3, over three equivalents silver triflate were herein employed to react with Py[8]. Treatment of o-ethynylaniline (1a) with a CH2Cl2/CH3OH (v/v 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
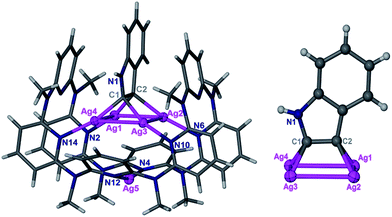
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution of Py[8]-Ag3 and additional silver triflate resulted in the occurrence of an intramolecular cyclization, finally yielding a polymetalated indole complex 2a by crystallization. 2a represents the first structurally well-defined organosilver intermediate for silver(I)-involved cyclizations of aminoalkyne derivatives.21 Single-crystal X-ray diffraction showed that the indole ring in 2a is negatively charged at two vicinal carbon atoms, and the resulting dianionic indole is stabilized by a coplanar Ag4 rectangle (Fig. 2). This argentophilic12d interaction-based tetrasilver rectangle is composed of two long edges (Ag1–Ag4: 3.093(2) Å, Ag2–Ag3: 3.085(2) Å) and two short ones (Ag1–Ag2: 2.831(2) Å, Ag3–Ag4: 2.831(2) Å). Each anionic carbon center is in an unusual pseudo-tetrahedral bonding fashion to connect with a short Ag–Ag edge (Ag–C: 2.06(2)–2.16(2) Å) and the neighboring nitrogen and carbon atoms. In addition, the planar indole ring is perpendicular to the Ag4 rectangle. Py[8] in 2a adopts a semi-open bowl-shaped conformation with a large cavity to accommodate the Ag4 aggregate via four-fold Ag–N coordination (2.12(2)–2.21(2) Å). The bottom of the bowl is sealed by another silver atom. The Py[8]-Ag3 capsule-triggered cyclization is also applicable for the homolog substrate 1b. The acquired tetrametalated indole complex 2b is isostructural with 2a (Fig. S3†). Interestingly, when 1a was mixed with 4 equiv. AgCF3SO3 at room temperature without adding Py[8], the 13C NMR monitoring did not show any signal change for the C
1) solution of Py[8]-Ag3 and additional silver triflate resulted in the occurrence of an intramolecular cyclization, finally yielding a polymetalated indole complex 2a by crystallization. 2a represents the first structurally well-defined organosilver intermediate for silver(I)-involved cyclizations of aminoalkyne derivatives.21 Single-crystal X-ray diffraction showed that the indole ring in 2a is negatively charged at two vicinal carbon atoms, and the resulting dianionic indole is stabilized by a coplanar Ag4 rectangle (Fig. 2). This argentophilic12d interaction-based tetrasilver rectangle is composed of two long edges (Ag1–Ag4: 3.093(2) Å, Ag2–Ag3: 3.085(2) Å) and two short ones (Ag1–Ag2: 2.831(2) Å, Ag3–Ag4: 2.831(2) Å). Each anionic carbon center is in an unusual pseudo-tetrahedral bonding fashion to connect with a short Ag–Ag edge (Ag–C: 2.06(2)–2.16(2) Å) and the neighboring nitrogen and carbon atoms. In addition, the planar indole ring is perpendicular to the Ag4 rectangle. Py[8] in 2a adopts a semi-open bowl-shaped conformation with a large cavity to accommodate the Ag4 aggregate via four-fold Ag–N coordination (2.12(2)–2.21(2) Å). The bottom of the bowl is sealed by another silver atom. The Py[8]-Ag3 capsule-triggered cyclization is also applicable for the homolog substrate 1b. The acquired tetrametalated indole complex 2b is isostructural with 2a (Fig. S3†). Interestingly, when 1a was mixed with 4 equiv. AgCF3SO3 at room temperature without adding Py[8], the 13C NMR monitoring did not show any signal change for the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C moiety even after ten hours (Fig. S4†). Fourier transform infrared (FT-IR) spectra of the 1a–AgCF3SO3 mixture exhibited a C
C moiety even after ten hours (Fig. S4†). Fourier transform infrared (FT-IR) spectra of the 1a–AgCF3SO3 mixture exhibited a C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C stretching vibration at 1999 cm−1 (Fig. S5†). The downward shift relative to 1a (v(C
C stretching vibration at 1999 cm−1 (Fig. S5†). The downward shift relative to 1a (v(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) = 2096 cm−1) is possibly due to the formation of a polynuclear cluster aggregate C
C) = 2096 cm−1) is possibly due to the formation of a polynuclear cluster aggregate C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CAgn.20
CAgn.20
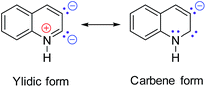
We next investigated the transformation of substrate 1c to study the selectivity of different cyclization modes within the coordination capsule. Previous studies have shown that 1c preferred a 5-exo-dig cyclization to produce indole derivatives.22 However, reaction of 1c with the Py[8]-Ag3 capsule experienced a new 6-endo-dig cyclization pathway, finally producing a Ag4-bonded quinoline ring in 2c (Fig. 3). The 1c-to-2c transformation should arise from intramolecular nucleophilic attack of an amino group on a C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C group followed by aromatization-driven dehydration. The resulting quinoline ring is also doubly charged and each anionic carbon is bonded to a Ag–Ag edge. Py[8] adopts a similar bowl-shaped structure with a cavity to accommodate the quinoline–Ag4 aggregate. It is noteworthy that the heterocyclic species in 2c is in a quinolinium form. The composition of this unique structure has been substantiated by elemental analysis and ESI-MS (Fig. S6†). This quinolinium species has two resonance structures including the ylidic and carbene form as shown below, which were only reported in previous ruthenium carbonyl clusters of N-methylquinolin-3-yl-2-ylidene.23 Since the Ag–C bond distances for two anionic carbon atoms in 2c are comparable (2.163(8)–2.218(8) Å), we suppose that the heterocyclic skeleton in 2c actually takes the ylidic form.
C group followed by aromatization-driven dehydration. The resulting quinoline ring is also doubly charged and each anionic carbon is bonded to a Ag–Ag edge. Py[8] adopts a similar bowl-shaped structure with a cavity to accommodate the quinoline–Ag4 aggregate. It is noteworthy that the heterocyclic species in 2c is in a quinolinium form. The composition of this unique structure has been substantiated by elemental analysis and ESI-MS (Fig. S6†). This quinolinium species has two resonance structures including the ylidic and carbene form as shown below, which were only reported in previous ruthenium carbonyl clusters of N-methylquinolin-3-yl-2-ylidene.23 Since the Ag–C bond distances for two anionic carbon atoms in 2c are comparable (2.163(8)–2.218(8) Å), we suppose that the heterocyclic skeleton in 2c actually takes the ylidic form.
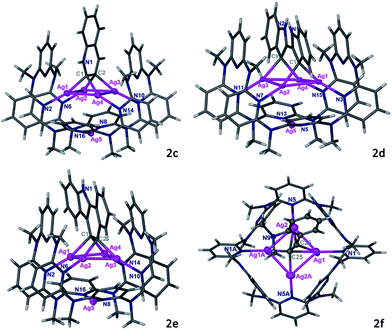
The Py[8]-Ag3 capsule-triggered cyclization was applicable to inner alkyne substrates as well. Reaction of substrate 1d with Py[8]-Ag3 led to the construction of a 2,2′-biindole skeleton in 2d through a two-step nucleophilic cyclization (Fig. 3). The 3,3′-positions of the biindole are both negatively charged, and each anionic carbon atom is bonded to two contacted silver atoms (Ag⋯Ag: 2.766(1)–2.775(1) Å) as similar as in 2a–2c. Due to the large separation of two anionic carbon centers (C1⋯C9: 3.353 Å), the resulting tetrasilver aggregate in 2d adopts a parallelogram shape rather than a rectangle in 2a–2c. Consequently, the bowl-shaped Py[8] undergoes a significant expansion at open side to adaptively encircle the resulting 2,2′-biindole–Ag4 organometallic cluster. Besides four alternate coordinative pyridines of Py[8] to support the Ag4 aggregate as shown in 2a–2c, in 2d there are two more pyridine rings connecting with the Ag4 parallelogram via longer Ag–N coordination (avg. 2.71 Å). In this way, the conformation of Py[8] in 2d is fixed by the encapsulated Ag4 parallelogram guest, finally leading to a well-resolved NMR spectrum. NMR monitoring showed that in the presence of Py[8]-Ag3 1d can be quantitatively transformed into 2d within five hours (Fig. S7†).
Substrate 1e was then attempted to construct a more complex and extended aromatic species within the capsule. As reported in literatures,24 the trialkylsilyl-protected alkynyl group in 1e is likely to undergo a silver(I)-induced desilylation to generate a terminal alkyne. When 1e was mixed with Py[8]-Ag3, it went through a cascade reaction pathway to produce a tetrasilver-bonded benzo[a]carbazole ring in 2e, which is still encapsulated within a Py[8]-Ag bowl (Fig. 3). The newly constructed benzo[a]carbazole ring is supported by a Ag4 rectangle through Ag–C bonding of two vicinal carbon atoms of a six-membered ring. This result indicates that the anionic carbon atom generated by the first cyclization of the inner alkyne species in 1e has sufficient nucleophilicity to attack the Ag-activated ethynyl group to complete the second cyclization step. To the best of our knowledge, this is the first example of silver-catalyzed or -mediated cyclization toward the construction of an extended benzo[a]carbazole ring.
Above successful isolation of polysilver complexes of different heteroaromatics including fused, biaryl and extended polycyclic rings highlights the great potential of the Py[8]-based capsule structure to encapsulate various polymetalated guests. This extraordinary capability arises from the remarkable structural adjustment ability of Py[8], which varies from its primitive planar parallelogram structure in crystalline state25 to the curved ball-like conformation in Py[8]-Ag3, and the bowl-shaped ones in 2a–2e. With this in mind, we hypothesize that in the synthetic process of 2a–2e the dynamic Py[8]-Ag3 capsule enables the silver atoms to flexibly partake in silver-acetylide bonding and then initiates the cyclization reaction. To prove this assumption, we employed substrate 1f, which is an analog of 1a but bearing a moderate nucleophilic group NMe2, to react with Py[8]-Ag3. The reaction finally generated an acetylide-centered Ag4 cluster surrounded by a 1,2-alternate Py[8] macrocycle in 2f (Fig. 3). We therefore conceive that the polymetallic gathering in Py[8]-Ag3 increases the effective concentration of silver ions and thus promotes the formation of a silver acetylide cluster inside Py[8]. Furthermore, the Py[8]-based capsule dynamically harnesses multiple coordination interactions to stabilize acquired polymetalated heteroaromatic species.
Previous studies have proved that organosilver complexes have limited stability.26 However, complexes 2a–2e are quite stable upon exposure to air and moisture. Preliminary reactivity studies revealed that reaction of 2a with CF3COOD gave rise to 2,3-deuterated indole in high yield, suggesting the nucleophilic nature of the Ag–C bonds. In solution, they can also keep their structures intact as evidenced in ESI-MS and NMR (Fig. S8–S18†). The pyridyl proton signals of Py[8] in 2a, 2b, 2c and 2e all gave very broad peaks, which can be ascribed to the interconversion of many possible fluxional conformations of Py[8] during the NMR time scale.20 The NMR spectrum of the exceptional example 2d contrarily exhibited a set of well-resolved peaks (Fig. S16†) because the conformation of Py[8] is fixed by the encapsulated Ag4 parallelogram guest as mentioned above.
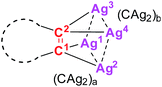
In order to comprehend the reasons for the excellent stability of 2a–2e, we next carried out density functional theory (DFT) calculations to investigate the electronic structure and bonding of 2a, 2c, 2d, and 2e. In the calculated model complexes (denoted as 2a′, 2c′, 2d′, and 2e′, respectively), each coordinative pyridine ring of Py[8] was simplified to a 2,6-diaminopyridine. The calculated Mayer bond order results are summarized in Table 1. For model complex 2a′, the sum of Ag–C bond orders for each CAg2 species is larger than one. This result suggests that in addition to the donation via sp2 orbital of each anionic carbon, the pπ orbital of the carbon also participates in bonding with two silver atoms. The inclusion of pπ orbital in Ag–C bonding is further confirmed by calculated molecular orbital (MO) diagrams (Fig. S19†). Furthermore, bond-order calculation of 2a′ revealed significant argentophilic interaction between two silver atoms (Table 1 and Fig. S20†), in good agreement with the short Ag⋯Ag distances in the crystal structure of 2a. In addition, an interesting three-centered bond among the anionic carbon and two bonded silver atoms was observed in MO analysis (Fig. S21†). Multi-centered bond indices (MBI) calculation gave the values of 0.0362 and 0.0343 for two CAg2 species in 2a′, suggesting the existence of a multi-centered bond. The bond order and multi-centered bond index calculation of 2c′, 2d′ and 2e′ also indicate the dominant presence of sp2 and pπ electron donation and the multi-centered bond (Fig. S22–S29†).
| 2a′ | 2c′ | 2d′ | 2e′ | ||
|---|---|---|---|---|---|
| MBI | (CAg2)a | 0.0362 | 0.0706 | 0.0501 | 0.0463 |
| (CAg2)b | 0.0343 | 0.0491 | 0.0557 | 0.0436 | |
| Mayer bond order | C1–Ag1 | 0.5631 | 0.5099 | 0.5323 | 0.6067 |
| C1–Ag2 | 0.5751 | 0.5416 | 0.5561 | 0.5463 | |
| Ag1–Ag2 | 0.2128 | 0.3374 | 0.3067 | 0.2728 | |
| C2–Ag3 | 0.5787 | 0.5743 | 0.6128 | 0.5887 | |
| C2–Ag4 | 0.5666 | 0.5219 | 0.5551 | 0.5576 | |
| Ag3–Ag4 | 0.2185 | 0.2795 | 0.2637 | 0.2774 | |
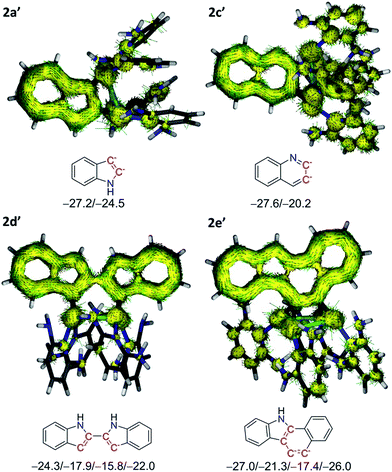
The aromaticity of heterocyclic moieties in 2a′, 2c′, 2d′ and 2e′ was evaluated by nucleus-independent chemical shift (NICS) computations27 (Fig. 4). As to 2a′, the acquired NICS(1)zz values for both the five-(5MR) and six-membered ring (6MR) are −24.5 and −27.2 ppm, respectively, which are comparable with the values of an indole ring (−28.7 and −28.8 ppm, respectively). For other model complexes 2c′, 2d′ and 2e′, the calculated NICS values of the silver-bonded 5MR or 6MR are all negative, substantiating the aromatic nature of these rings. It is notable that the NICS values of the silver-bonded rings are less negative than other rings in the same heterocyclic skeleton, implying a lesser aromatic feature. This result can be rationalized by the above bond-order calculation, wherein pπ electron of the anionic carbon donates to silver atoms and thus lowers aromaticity. Aromatic nature of the pseudo-tetrahedrally bonded heterocyclic rings in model complexes was further manifested by anisotropy of current-induced density (ACID) analysis.28 In 2a′ and 2c′ (Fig. 4), the current density vectors plotted on the isosurface show a strong diatropic ring current in the π system. Similarly, the benzo[a]carbozole skeleton in 2e′ formed a large conjugation system with the diatropic ring current crossing along the rim of the whole skeleton. In 2d′, the current is localized within only one of the two indole rings, suggesting the presence of two independent aromatic systems. The theoretical calculations substantiate that such polysilver-bonded heterocyclic rings in 2a–2e are all aromatic although the anionic carbon atoms each is pseudo-tetrahedrally bonded with two silver atoms.
Conclusions
In conclusion, we have demonstrated that a macrocycle-based dynamic coordination capsule can trigger cyclization reactions for various alkyne substrates under mild conditions. In the presence of the dynamic coordination capsule, some unconventional reaction pathways for silver-mediated cyclizations (such as 6-endo-dig cyclization and cascade reaction) can be conducted. More importantly, several unprecedented polysilver complexes of four heteroaromatics, including indole, quinoline, 2,2′-biindole and benzocarbazole, have been successfully isolated and fully characterized. Theoretical analysis has revealed the presence of sp2 and pπ electron donation and multi-centered bonding in the polysilver aromatic complexes, which substantiated the important role of metal-perturbed aromaticity in stabilization of the polysilver aromatic complexes. The present study showcases a promising approach to synthesize intricate organometallics by taking advantage of dynamic supramolecular capsule. Structure and reactivity comprehension of polymetalated organometallics may deepen our understanding of bonding nature and mechanistic studies, which is conducive to the advancement of more efficient and versatile synthetic methods. This study is still in progress.Experimental
Materials and methods
All commercially available chemicals were used without further purification. Octamethylazacalix[8]pyridine (Py[8]) was synthesized according to the literature method by the [3 + 5] fragment coupling protocol between a terminal dibrominated linear trimer and a terminal diaminated linear pentamer.25 The solvents used in this study were processed by standard procedures. 1H and 13C NMR experiments were carried out on a JEOL ECX-400 MHz instrument. Elemental analyses were recorded on a Thermo FlashEA 1112 elemental analyzer. Mass spectra were obtained using a Thermo Scientific Exactive Orbitrap instrument. Infrared spectra were recorded on a Perkin-Elmer spectrum 100 FT-IR spectrometer.Synthesis of complexes 2a–2f
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 (v:v) = 1
CH2Cl2 (v:v) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of crude product. Yield: 46% (17.9 mg) based on Py[8]. When the amount of silver triflate was raised to 10 equivalents, the yield was enhanced to 72% (28.1 mg). Anal. calcd for 2a (C59H53Ag5F9N17O9S3): C, 36.33; H, 2.74; N, 12.21. Found: C, 35.96; H, 2.78; N, 12.57.
1) of crude product. Yield: 46% (17.9 mg) based on Py[8]. When the amount of silver triflate was raised to 10 equivalents, the yield was enhanced to 72% (28.1 mg). Anal. calcd for 2a (C59H53Ag5F9N17O9S3): C, 36.33; H, 2.74; N, 12.21. Found: C, 35.96; H, 2.78; N, 12.57.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 (v:v) = 1
CH2Cl2 (v:v) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 2e ([Ag5(C16NH9)(Py[8])](CF3SO3)3): 25.0 mg, 61% based on Py[8]. Elemental analysis for 2e·CH3OH (C68H61Ag5F9N17O10S3), found (calcd.): C, 39.00 (39.21); H, 3.02 (2.95); N, 11.04 (11.43). 2f ([Ag4(C8NH6)(Py[8])](CF3SO3)2(CF3CO2)·2CH3OH): 31.1 mg, 83% based on Py[8]. Elemental analysis for 2f (C61H58Ag4F9N17O9S3), found (calcd.): C, 39.49 (39.14); H, 3.34 (3.12); N, 12.48 (12.72).
1). 2e ([Ag5(C16NH9)(Py[8])](CF3SO3)3): 25.0 mg, 61% based on Py[8]. Elemental analysis for 2e·CH3OH (C68H61Ag5F9N17O10S3), found (calcd.): C, 39.00 (39.21); H, 3.02 (2.95); N, 11.04 (11.43). 2f ([Ag4(C8NH6)(Py[8])](CF3SO3)2(CF3CO2)·2CH3OH): 31.1 mg, 83% based on Py[8]. Elemental analysis for 2f (C61H58Ag4F9N17O9S3), found (calcd.): C, 39.49 (39.14); H, 3.34 (3.12); N, 12.48 (12.72).
X-ray crystallography
Single-crystal X-ray data for complexes 2a–2f were collected at 173 K with Mo Kα radiation (λ = 0.71073 Å) on a Rigaku Saturn 724/724+ CCD diffractometer or Cu Kα radiation (λ = 1.54178 Å) on a Rigaku Oxford Diffraction SuperNova diffractometer. The selected crystal was mounted onto a nylon loop in polyisobutene and immersed in a low-temperature (173 K) stream of dry nitrogen gas during data collection. All structures were solved by direct methods, and non-hydrogen atoms were located from difference Fourier maps. Non-hydrogen atoms, otherwise noticed, were subjected to anisotropic refinement by full-matrix least-squares on F2 by using the SHELXTL program29 and Olex2 program.30 All figures were drawn by using X-seed program.31 CCDC numbers for reported complexes are 1558954 (2a in space group Cmcm), 1558955 (2a in space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ), 1558956 (2b), 1558958 (2c), 1558960 (2d), 1558959 (2e) and 1558963 (2f). The refinement details and crystal data for 2a–2f are summarized in the ESI.†
), 1558956 (2b), 1558958 (2c), 1558960 (2d), 1558959 (2e) and 1558963 (2f). The refinement details and crystal data for 2a–2f are summarized in the ESI.†
Computational details
Theoretical calculations were performed using the Gaussian 09 program.32 Initial structures of model clusters 2a′, 2e′, 2f′, and 2g′ were obtained from crystal structures. The Py[8] ligand was simplified to four 2,6-diamino pyridines and the silver atoms not linked to the cluster core were omitted. All four structures were optimized at the TPSS level33 of density functional theory with “Empirical Dispersion = GD3”34 to describe the dispersion corrections. Furthermore, the frequency analyses were performed to confirm the characteristics of the calculated structures as minima. In the calculations, LanL2DZ basis set was used to describe the Ag atom, whereas the 6-31G(d) basis set for the C, N, and H atoms.35 The NICS values were calculated at the TPSS-GIAO level.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support by National Natural Science Foundation of China (21522206, 21772111 and 21661132006) is gratefully acknowledged. We are grateful to Profs. Mei-Xiang Wang (THU), Sam C. K. Hau (HKBU), De-Xian Wang (ICCAS) and Han-Yuan Gong (BNU) for helpful discussions.References
- For recent reviews, see: (a) P. Ballester, M. Fujita and J. Rebek Jr, Chem. Soc. Rev., 2015, 44, 392 RSC, and other review articles in this theme issue; (b) A. Diaz-Moscoso and P. Ballester, Chem. Commun., 2017, 53, 4635 RSC.
- For recent reviews, see: (a) M. Raynal, P. Ballester, A. Vidal-Ferran and P. W. N. M. van Leeuwen, Chem. Soc. Rev., 2014, 43, 1734 RSC; (b) D. Zhang, A. Martinez and J.-P. Dutasta, Chem. Rev., 2017, 117, 4900 CrossRef CAS PubMed.
- For reviews, see: (a) T. R. Cook and P. J. Stang, Chem. Rev., 2015, 115, 7001 CrossRef CAS PubMed; (b) K. Harris, D. Fujita and M. Fujita, Chem. Commun., 2013, 49, 6703 RSC; (c) M. J. Wiester, P. A. Ulmann and C. A. Mirkin, Angew. Chem., Int. Ed., 2011, 50, 114 CrossRef CAS PubMed; (d) C. J. Brown, F. D. Toste, R. G. Bergman and K. N. Raymond, Chem. Rev., 2015, 115, 3012 CrossRef CAS PubMed; (e) A. J. McConnell, C. S. Wood, P. P. Neelakandan and J. R. Nitschke, Chem. Rev., 2015, 115, 7729 CrossRef CAS PubMed; (f) L.-J. Chen, H.-B. Yang and M. Shionoya, Chem. Soc. Rev., 2017, 46, 2555 RSC.
- (a) M. Yoshizawa, M. Tamura and M. Fujita, Science, 2006, 312, 251 CrossRef CAS PubMed; (b) Y. Inokuma, S. Yoshioka and M. Fujita, Angew. Chem., Int. Ed., 2010, 49, 8912 CrossRef CAS PubMed.
- C. J. Hastings, M. D. Pluth, R. G. Bergman and K. N. Raymond, J. Am. Chem. Soc., 2010, 132, 6938 CrossRef CAS PubMed.
- (a) M. Yoshizawa, Y. Takeyama, T. Kusukawa and M. Fujita, Angew. Chem., Int. Ed., 2002, 41, 1347 CrossRef CAS; (b) N. Vallavoju and J. Sivaguru, Chem. Soc. Rev., 2014, 43, 4084 RSC.
- (a) D. M. Kaphan, M. D. Levin, R. G. Bergman, K. N. Raymond and F. D. Toste, Science, 2015, 350, 1235 CrossRef CAS PubMed; (b) M. D. Levin, D. M. Kaphan, C. M. Hong, R. G. Bergman, K. N. Raymond and F. D. Toste, J. Am. Chem. Soc., 2016, 138, 9682 CrossRef CAS PubMed.
- A. M. Lifschitz, M. S. Rosen, C. M. McGuirk and C. A. Mirkin, J. Am. Chem. Soc., 2015, 137, 7252 CrossRef CAS PubMed.
- L. Ilies, M. Isomura, S. Yamauchi, T. Nakamura and E. Nakamura, J. Am. Chem. Soc., 2017, 139, 23 CrossRef CAS PubMed.
- C. H. Winter, K. N. Seneviratne and A. Bretschneider-hurley, Comments Inorg. Chem., 1996, 19, 1 CrossRef CAS.
- (a) P. Pyykkö, Chem. Rev., 1997, 97, 597 CrossRef; (b) P. Pyykkö, Angew. Chem., Int. Ed., 2002, 41, 3573 CrossRef; (c) P. Pyykkö, Angew. Chem., Int. Ed., 2004, 43, 4412 CrossRef PubMed.
- (a) H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2008, 37, 1931 RSC; (b) S. Sculfort and P. Braunstein, Chem. Soc. Rev., 2011, 40, 2741 RSC; (c) H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2012, 41, 370 RSC; (d) H. Schmidbaur and A. Schier, Angew. Chem., Int. Ed., 2015, 54, 746 CrossRef CAS PubMed.
- (a) M. C. Gimeno and A. Laguna, Chem. Soc. Rev., 2008, 37, 1952 RSC; (b) A. Laguna, T. Lasanta, J. M. Lopez-de-Luzuriaga, M. Monge, P. Naumov and M. E. Olmos, J. Am. Chem. Soc., 2010, 132, 456 CrossRef CAS PubMed; (c) T. Lasanta, M. E. Olmos, A. Laguna, J. M. Lopez-de-Luzuriaga and P. Naumov, J. Am. Chem. Soc., 2011, 133, 16358 CrossRef CAS PubMed.
- (a) L.-Y. Yao, F. K.-W. Hau and V. W.-W. Yam, J. Am. Chem. Soc., 2014, 136, 10801 CrossRef CAS PubMed; (b) L.-Y. Yao and V. W.-W. Yam, J. Am. Chem. Soc., 2015, 137, 3506 CrossRef CAS PubMed.
- (a) S. C. K. Hau, M. C.-L. Yeung, V. W.-W. Yam and T. C. W. Mak, J. Am. Chem. Soc., 2016, 138, 13732 CrossRef CAS PubMed; (b) Y.-P. Xie, J.-L. Jin, G.-X. Duan, X. Lu and T. C. W. Mak, Coord. Chem. Rev., 2017, 331, 54 CrossRef CAS.
- N. Sinha and F. E. Hahn, Acc. Chem. Res., 2017, 50, 2167 CrossRef CAS PubMed.
- (a) D. L. Reger, R. F. Semeniuc and M. D. Smith, Inorg. Chem., 2003, 42, 8137 CrossRef CAS PubMed; (b) C. Khin, A. S. K. Hashmi and F. Rominger, Eur. J. Inorg. Chem., 2010, 1063 CrossRef CAS; (c) Q. Chen, F. Jiang, D. Yuan, G. Lyu, L. Chen and M. Hong, Chem. Sci., 2014, 5, 483 RSC.
- X. He, X.-B. Xu, X. Wang and L. Zhao, Chem. Commun., 2013, 49, 7153 RSC.
- G. Ercolani and L. Schiaffino, Angew. Chem., Int. Ed., 2011, 50, 1762 CrossRef CAS PubMed.
- (a) C.-Y. Gao, L. Zhao and M.-X. Wang, J. Am. Chem. Soc., 2011, 133, 8448 CrossRef CAS PubMed; (b) C.-Y. Gao, L. Zhao and M.-X. Wang, J. Am. Chem. Soc., 2012, 134, 824 CrossRef CAS PubMed; (c) X. He, C.-Y. Gao, M.-X. Wang and L. Zhao, Chem. Commun., 2012, 48, 10877 RSC.
- B. C. J. van Esseveldt, F. L. van Delft, J. M. M. Smits, R. de Gelder, H. E. Schoemaker and F. P. J. T. Rutjesa, Adv. Synth. Catal., 2004, 346, 823 CrossRef CAS.
- Y. K. Kumar, G. R. Kumar, T. J. Reddy, B. Sridhar and M. S. Reddy, Org. Lett., 2015, 17, 2226 CrossRef CAS PubMed.
- J. A. Cabeza, I. del Río, E. Pérez-Carreño, M. G. Sánchez-Vega and D. Vázquez-García, Angew. Chem., Int. Ed., 2009, 48, 555 CrossRef CAS PubMed.
- U. Halbes-Letinois, J.-M. Weibel and P. Pale, Chem. Soc. Rev., 2007, 36, 759 RSC.
- H.-Y. Gong, X.-H. Zhang, D.-X. Wang, H.-W. Ma, Q.-Y. Zheng and M.-X. Wang, Chem.–Eur. J., 2006, 12, 9262 CrossRef CAS PubMed.
- B. K. Tate, A. J. Jordan, J. Bacsa and J. P. Sadighi, Organometallics, 2017, 36, 964 CrossRef CAS , and references therein.
- (a) P. v. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao and N. J. R. v. E. Hommes, J. Am. Chem. Soc., 1996, 118, 6317 CrossRef CAS PubMed; (b) H. Fallah-Bagher-Shaidaei, C. S. Wannere, C. Corminboeuf, R. Puchta and P. v. R. Schleyer, Org. Lett., 2006, 8, 863 CrossRef CAS PubMed.
- (a) R. Herges and D. Geuenich, J. Phys. Chem. A, 2001, 105, 3214 CrossRef CAS; (b) D. Geuenich, K. Hess, F. Kçhler and R. Herges, Chem. Rev., 2005, 105, 3758 CrossRef CAS PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339 CrossRef CAS.
- J. L. Atwood and L. J. Barbour, Cryst. Growth Des., 2003, 3, 3 CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- J. M. Tao, J. P. Perdew, V. N. Staroverov and G. E. Scuseria, Phys. Rev. Lett., 2003, 91, 146401 CrossRef PubMed.
- S. Grimme, J. Antony, S. Ehrlichn and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 299 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Supporting figures, high-resolution ESI-MS, NMR, crystal refinement details and computational results. CCDC 1558954–1558956, 1558958–1558960, 1558963. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc04503d |
| This journal is © The Royal Society of Chemistry 2018 |