Open Access Article
Open Access ArticleSelective lithium ion recognition in self-assembled columnar liquid crystals based on a lithium receptor†
Yuan
Luo
 ,
Nicolas
Marets
,
Nicolas
Marets
 and
Takashi
Kato
and
Takashi
Kato
 *
*
Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: kato@chiral.t.u-tokyo.ac.jp
First published on 1st November 2017
Abstract
Lithium is recognized as being significantly important due to its various applications in different areas especially in energy technology. In the present study, self-assembled nanostructured liquid-crystalline (LC) materials, that selectively bind lithium cations, have been developed for the first time. Wedge-shaped crown ether derivatives bearing dibenzo-14-crown-4 (DB14C4) or 12-crown-4 moieties are able to act as LC lithium-selective receptors. We have found that complexation of these receptors with lithium perchlorate induces liquid-crystalline columnar phases, while sodium perchlorate is immiscible with both receptors. Remarkably, a receptor consisting of DB14C4 as an effective lithium-selective ligand exhibits high selectivity for LiCl over NaCl, KCl, RbCl and CsCl. The lithium selectivity was demonstrated and investigated by 1H NMR, 1H COSY and FT-IR spectroscopic measurements. The preferred coordination number of four and the ideal cavity geometry of the DB14C4 moiety of the receptor are shown to be key factors for the high lithium selectivity. This new design of LC lithium-selective receptors opens unexplored paths for the development of methods to fabricate nanostructured materials for efficient selective lithium recognition.
Introduction
Lithium has been attracting considerable attention due to its extensive applications in modern battery technology, glass and ceramics processing, lubricants and pharmaceuticals.1–4 The majority of lithium currently produced comes from salt-lake brines by solar evaporation processes.5 As the demand for lithium has begun to grow dramatically after the turn of the century, mining lithium in a more efficient and economically-feasible way is of great interest.6,7 Many efforts have been devoted to developing new methodologies/techniques for extracting lithium.8–13 However, lithium cations are still difficult targets for selective recognition and purification because of their large hydration energy and the coexistence of other similar alkali metal cations.14,15 The design of new functional receptor systems may contribute to the development of efficient selective lithium recognition.Nature has been a source of inspiration for the development of artificial functional systems.16–21 The extraordinary selective and efficient transport of specific ions across the cell membrane is common and essential to many of life’s processes.22,23 This ion-transport ability is owed to ionic channels with characteristic architectures and coordination geometries created by membrane proteins.23–28 Inspired by these discoveries in biological systems, artificial ionic channels that are capable of transporting protons or alkaline metal ions efficiently have been developed.18,29–36 But lithium cation selective ionic channels have not yet been achieved.
Liquid-crystalline (LC) self-assembly is a promising platform to construct nanochannels with various characteristic architectures and coordination geometries.37–45 The tunable nanostructure and functionality of a LC assembly37–47 enables it to become an outstanding candidate for the design of nanostructured ion receptors or transporters.48–59 We previously developed several nanostructured lithium ion-transport materials based on LC assemblies.59–63 Recently, Sijbesma et al. developed sodium and potassium ion-selective nanoporous films upon a templated LC complex.64 However, lithium ion-selective LC materials have not yet been reported to the best of our knowledge.37,51,65,66
Our strategy here is to obtain lithium-selective crown ether (CE) based liquid crystals. A variety of liquid crystals having CE moieties have been prepared.65–68 Herein we report on the development of nanostructured materials based on LC crown ether receptors for the selective recognition of lithium cations. We designed and synthesized CE derivatives 1, 2 and 3 with wedge-shaped LC mesogenic parts (Fig. 1).
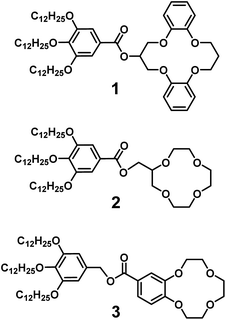 | ||
| Fig. 1 Molecular structures of liquid-crystalline crown ether receptors: 1 composed of dibenzo-14-crown-4 (DB14C4), 2 composed of 12-crown-4 (12C4) and 3 composed of benzo-12-crown-4 (B12C4). | ||
Results and discussion
Material design
Crown ethers are well-known ionophores towards certain metal ions and their selectivity can be controlled by tuning the number of coordination sites and the cavity size.69–73 Wedge-shaped crown ether derivatives 1, 2 and 3 are designed to act as liquid-crystalline ion-selective receptors. The ion-selective moieties of 1, 2 and 3 consist of dibenzo-14-crown-4 (DB14C4), 12-crown-4 (12C4) and benzo-12-crown-4 (B12C4), respectively (Fig. 1). It is known that the most favourable coordination numbers for lithium cations (Li+) are expected to be 4, 5 and 6.74 Additionally 12- to 14-membered CE rings (cavity size: 1.2–1.8 Å) are most selective to Li+, having an ionic diameter of about 1.4 Å.15,73,74 Hence, DB14C4 (1.8 Å) is chosen as a selective ligand because of its well matched geometry towards lithium coordination.75–80 The DB14C4 macrocycle could provide a rigid coordination conformation for ideal Li+ complexation.81,82 12C4 and B12C4 are common and well-known lithium complexing ligands, which have been used to develop lithium-selective materials.83,84 The introduction of LC mesogenic moieties to the CE derivatives could develop a new class of ion-active LC self-assembled functional materials.66 Taking advantage of LC self-assembly, liquid-crystalline CE derivatives could form well-defined interconnected ionic channels suitable for selective ion transport.85–89 Previously, Beginn and co-workers reported LC materials containing sodium ion-selective channels based on the lyotropic LC assemblies of a LC 15-crown-5 derivative and sodium trifluoromethanesulfonate (NaSO3CF3) in a methacrylate mixture.90–92 However, LC materials exhibiting lithium-selective properties have not yet been developed. Thus, we considered that the complexation of 1, 2 or 3 with Li+ would lead to the development of new, nanostructured lithium ion-selective LC materials (Fig. 2).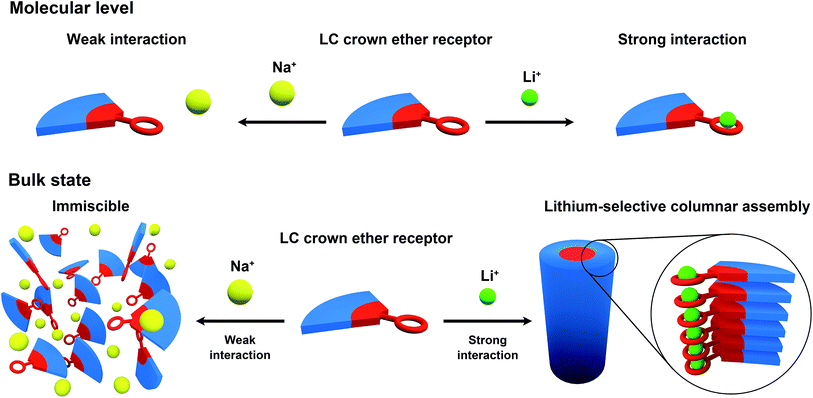 | ||
| Fig. 2 Schematic illustration of the strategy and material design of liquid-crystalline lithium-selective ion receptors. | ||
Liquid-crystalline properties
The thermal properties of the mesogenic compounds 1–3 and their mixtures with lithium perchlorate (LiClO4) and sodium perchlorate (NaClO4) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 equimolar mixture) were studied by polarized optical microscopy (POM), differential scanning calorimetry (DSC) and X-ray diffraction (XRD) (see the ESI†). The 1
1 equimolar mixture) were studied by polarized optical microscopy (POM), differential scanning calorimetry (DSC) and X-ray diffraction (XRD) (see the ESI†). The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures were prepared by adding stoichiometric amounts of the THF solutions of LiClO4 and NaClO4 to the crown ether derivatives, followed by the complete evaporation of the solvent to yield the anhydrous mixtures.
1 mixtures were prepared by adding stoichiometric amounts of the THF solutions of LiClO4 and NaClO4 to the crown ether derivatives, followed by the complete evaporation of the solvent to yield the anhydrous mixtures.
The phase transition behaviours of the samples are presented in Table 1. Fig. 3 shows the POM images of the mixtures of 1/Li+, 1/Na+ and 2/Li+. The POM images and XRD patterns suggest that complex 1/Li+ exhibits a columnar rectangular (Colr) phase (Fig. 3a and S2†) and complex 2/Li+ shows a columnar hexagonal (Colh) phase (Fig. 3c and S4†). The single components of compounds 1–3 show only crystal and isotropic phases. For 1 and 2, liquid-crystalline columnar phases are induced after the complexation with LiClO4. The introduction of LC mesophases and significant increases in the isotropization temperatures for both complexes 1/Li+ and 2/Li+ may be related to the intermolecular ion-dipolar interactions between the CE moieties and lithium cations.87,89,93 The formation of supramolecular complexes composed of CE moieties and lithium cations may induce well-packed core structures while the aliphatic chains are still mobile. The change in the volume fraction of the polar and nonpolar parts may also contribute to the introduction of LC mesophases. In contrast, NaClO4 salt is immiscible with both compounds 1 and 2. Liquid–solid phase separations for 1/Na+ and 2/Na+ are observed above the isotropization temperature by POM measurements (Fig. 3b and S6a†). The DSC thermograms of the mixtures of 1/Na+ and 2/Na+ are analogous to those of the single components of compounds 1 and 2 (Fig. S1c and S3c†). This observation also suggests that phase separations occur in the mixtures of 1/Na+ and 2/Na+. It is believed that sufficient supramolecular interactions are essential to generate stable LC phases. Thus, these results imply that the nanostructured lithium-selective ionic channels are formed within the LC assemblies by the selective interactions between Li+ and the CE moieties.
| Sample | Phase transition behavioura | ||||
|---|---|---|---|---|---|
| a Transition temperatures (°C) were determined by DSC on a first cooling cycle at a scan rate of 10 K min−1. G, glassy; Cr, crystal; Iso, isotropic; Colr, columnar rectangular phase; Colh, columnar hexagonal phase. b The sample shows monotropic liquid-crystalline Colr phase upon cooling from the isotropic state. c Observed by POM measurements above the isotropization temperature of the single component compounds 1–3. | |||||
| 1 | Iso | −10 | Cr | ||
| 1/Li+ | Iso | 109 | Colrb | 73 | G |
| 1/Na+ | Phase separationc | ||||
| 2 | Iso | 2 | Cr | ||
| 2/Li+ | Iso | 156 | Colh | 22 | Cr |
| 2/Na+ | Phase separationc | ||||
| 3 | Iso | 14 | Cr | ||
| 3/Li+ | Iso | −10 | Cr | ||
| 3/Na+ | Phase separationc | ||||
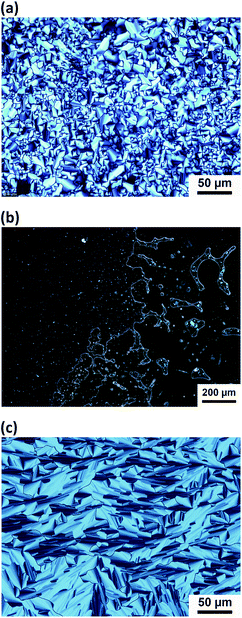 | ||
| Fig. 3 Polarizing optical microscope images of (a) 1/Li+ at 95 °C; (b) 1/Na+ at 95 °C and (c) 2/Li+ at 130 °C. | ||
1H NMR binding studies
Induction of the LC phases has been observed for 1 and 2 by selective interaction with lithium ions. In order to examine the interactions between mesogenic receptors 1–3 and alkali cations (Li+ and Na+), 1H NMR measurements of receptors 1–3 treated with excess LiClO4 and NaClO4 in CDCl3/CD3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution were performed. Comparison of the 1H NMR spectra of 1, 2 (Fig. 4) and 3 (Fig. S11†) treated with excess Li+ and Na+ reveals the different nature of the interactions between the receptors and the cations. Upon exposure to excess Li+, all of the related crown ether proton resonances (Ha–h) of 1 underwent considerable downfield shifts (Fig. 4a). In contrast, proton resonances Hb and He–h showed less of a downfield shift in response to the addition of Na+, while the methylene protons Hc and Hd may exhibit peak splitting (Hc1, Hc2, Hd1, and Hd2). This might indicate a weaker interaction between 1 and Na+, and a conformational change of the DB14C4 ring of 1 (Fig. 4a). The 1H NMR spectra of 2 with Li+ and Na+ showed similar but smaller shifts compared to those of 1 (Fig. 4b). Addition of Li+ induced an apparent downshift movement of Ha–i of 2, and adding Na+ caused only a slight shift of these proton resonances. However, the 1H NMR spectra of 3 showed almost the same downfield shift upon the addition of Li+ and Na+ (Fig. S11†). These resonance changes are rationalized in terms of the deshielding and shielding effects that may result from the cation-oxygen atom coordination and conformational changes induced upon different cation complexation.
1, v/v) solution were performed. Comparison of the 1H NMR spectra of 1, 2 (Fig. 4) and 3 (Fig. S11†) treated with excess Li+ and Na+ reveals the different nature of the interactions between the receptors and the cations. Upon exposure to excess Li+, all of the related crown ether proton resonances (Ha–h) of 1 underwent considerable downfield shifts (Fig. 4a). In contrast, proton resonances Hb and He–h showed less of a downfield shift in response to the addition of Na+, while the methylene protons Hc and Hd may exhibit peak splitting (Hc1, Hc2, Hd1, and Hd2). This might indicate a weaker interaction between 1 and Na+, and a conformational change of the DB14C4 ring of 1 (Fig. 4a). The 1H NMR spectra of 2 with Li+ and Na+ showed similar but smaller shifts compared to those of 1 (Fig. 4b). Addition of Li+ induced an apparent downshift movement of Ha–i of 2, and adding Na+ caused only a slight shift of these proton resonances. However, the 1H NMR spectra of 3 showed almost the same downfield shift upon the addition of Li+ and Na+ (Fig. S11†). These resonance changes are rationalized in terms of the deshielding and shielding effects that may result from the cation-oxygen atom coordination and conformational changes induced upon different cation complexation.
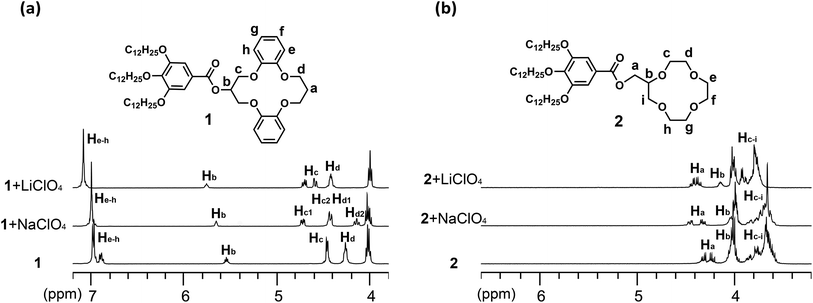 | ||
Fig. 4 Partial 1H NMR spectra (400 MHz, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CDCl3/CD3CN, 298 K) of 1 (a) and 2 (b), showing shifts of the crown ether proton resonances in the presence of excess Li+ and Na+. 1 CDCl3/CD3CN, 298 K) of 1 (a) and 2 (b), showing shifts of the crown ether proton resonances in the presence of excess Li+ and Na+. | ||
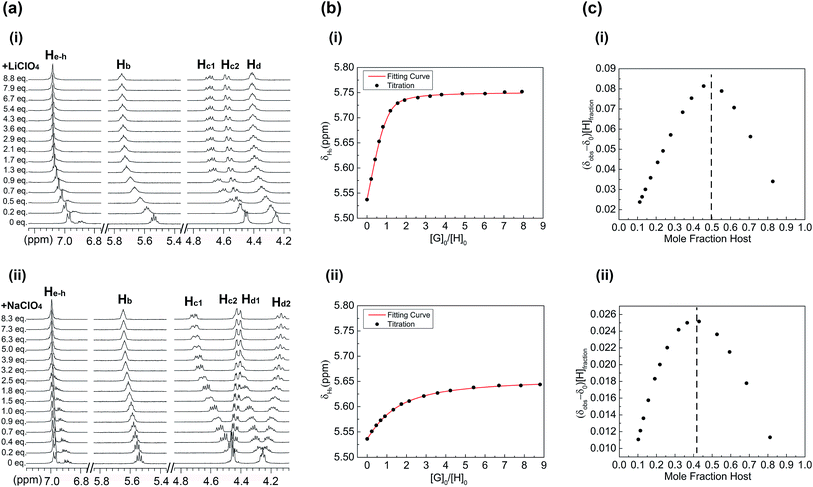
The abilities of 1, 2 and 3 to bind lithium and sodium cations in solution were further examined via multiple 1H NMR spectroscopic titrations using CDCl3/CD3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as solvent (Fig. 5 and ESI†). Cation binding studies of 1, 2 and 3 were performed by monitoring the shift movement of the respective CE proton resonances upon the addition of Li+ and Na+. In all cases LiClO4 and NaClO4 were used as cation sources. The titrations reveal that the cation-dependent shifting was observed only for the CE related proton resonances. Other 1H NMR resonances were relatively unaffected by the presence of cationic stimuli.
1, v/v) as solvent (Fig. 5 and ESI†). Cation binding studies of 1, 2 and 3 were performed by monitoring the shift movement of the respective CE proton resonances upon the addition of Li+ and Na+. In all cases LiClO4 and NaClO4 were used as cation sources. The titrations reveal that the cation-dependent shifting was observed only for the CE related proton resonances. Other 1H NMR resonances were relatively unaffected by the presence of cationic stimuli.
Table 2 shows the resulting association constants of multiple 1H NMR spectroscopic titrations that assessed the binding abilities of receptors 1, 2 and 3 towards Li+ and Na+. All binding data were fit to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model and association constants were determined using BindFit v0.5.94 As shown in Table 2, compounds 1 and 2 exhibit a binding preference for Li+ over Na+, while compound 3 shows weak association with both cations and preferentially binds to Na+ rather than Li+. Significantly, 1 exhibits a much higher binding ability and ion selectivity for Li+ than those of 2 and 3. It displayed association constants of 2359 and 228 M−1 towards lithium and sodium cations, respectively. The binding selectivity for Li+/Na+ ions is 10.35. As mentioned in the introduction, the effective selectivity of ion-selective ionophores is owed to their characteristic architectures and coordination geometries. The significant preference of 1 for Li+ could be due to its larger DB14C4 cavity which provides a more ideal geometry for Li+ coordination, whereas the smaller macrocycles 12C4 of 2 and B12C4 of 3 are less favourable. The high selectivity is achieved by locking the lithium cation into the four oxygen coordination sites around the cavity. The cavity that can fit the cation best is preferred. Moreover, the relatively flexible receptor 2 shows higher binding constants in both cases compared to the rigid receptor 3. This also implies that the ligand should be flexible enough to allow sufficient ion binding. These results reveal that significant effects on selective binding could be induced by relatively minor structural alterations of the receptors.93
1 binding model and association constants were determined using BindFit v0.5.94 As shown in Table 2, compounds 1 and 2 exhibit a binding preference for Li+ over Na+, while compound 3 shows weak association with both cations and preferentially binds to Na+ rather than Li+. Significantly, 1 exhibits a much higher binding ability and ion selectivity for Li+ than those of 2 and 3. It displayed association constants of 2359 and 228 M−1 towards lithium and sodium cations, respectively. The binding selectivity for Li+/Na+ ions is 10.35. As mentioned in the introduction, the effective selectivity of ion-selective ionophores is owed to their characteristic architectures and coordination geometries. The significant preference of 1 for Li+ could be due to its larger DB14C4 cavity which provides a more ideal geometry for Li+ coordination, whereas the smaller macrocycles 12C4 of 2 and B12C4 of 3 are less favourable. The high selectivity is achieved by locking the lithium cation into the four oxygen coordination sites around the cavity. The cavity that can fit the cation best is preferred. Moreover, the relatively flexible receptor 2 shows higher binding constants in both cases compared to the rigid receptor 3. This also implies that the ligand should be flexible enough to allow sufficient ion binding. These results reveal that significant effects on selective binding could be induced by relatively minor structural alterations of the receptors.93
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3CN (v/v, 1
CD3CN (v/v, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution at 298 K. All association constants are an average of at least three 1H NMR titrations fit to a 1
1) solution at 298 K. All association constants are an average of at least three 1H NMR titrations fit to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model using BindFit v0.5, and associated errors are <10% unless noted
1 binding model using BindFit v0.5, and associated errors are <10% unless noted
Binding conformation investigation
The results of 1H NMR titration experiments for receptor 1 suggest that Li+ binding is preferred, as a result of both the preferred coordination number of four and the geometry of the DB14C4 cavity (Fig. 5). The analysis of the 1H NMR spectroscopic titration data reveals solution phase structural information about the cationic binding conformations of receptor 1. In titrations of 1 with Li+, the related crown ether proton resonances (Ha–h) of 1 moved downfield throughout the titrations (Fig. 5a,i). Fig. 5b,i shows that the rapid and noticeable downfield shift almost reached a maximum after adding about 1.0 equiv. of Li+, then only a slight shift even up to 8.0 equiv. of Li+. This movement of the CE proton resonances implies the four coordination sites of the DB14C4 ring bind to the lithium cations tightly beyond 1.0 equiv. of Li+. This can also be confirmed by the Job plot of the titrations (Fig. 5c,i). The peak of the Job plot corresponds to a 0.5 mole fraction of the host in the titration solution, which indicates the formation of a stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex of Li+ and 1. The DB14C4 macrocycle provides an ideal coordination geometry for Li+, in which strong supramolecular interactions between the lithium cation and the four ethereal oxygens occur. A different shifting trend was observed for the titrations of 1 with Na+ (Fig. 5a,ii). The proton resonances (Ha–h) of 1 showed not only a downfield shift but also peak splittings and an upfield shift for the methylene protons Hc and Hd. The titration of 1 with Na+ induced only steady changes in the Ha and Hb resonances up to 9.0 equiv. of Na+, revealing the weak affinity of 1 towards Na+ (Fig. 5b,ii). The Job plot of the titrations shows that the equilibrium of the complex formation is at about 0.42 mole fraction of host (Fig. 5c,ii). No stable 1
1 complex of Li+ and 1. The DB14C4 macrocycle provides an ideal coordination geometry for Li+, in which strong supramolecular interactions between the lithium cation and the four ethereal oxygens occur. A different shifting trend was observed for the titrations of 1 with Na+ (Fig. 5a,ii). The proton resonances (Ha–h) of 1 showed not only a downfield shift but also peak splittings and an upfield shift for the methylene protons Hc and Hd. The titration of 1 with Na+ induced only steady changes in the Ha and Hb resonances up to 9.0 equiv. of Na+, revealing the weak affinity of 1 towards Na+ (Fig. 5b,ii). The Job plot of the titrations shows that the equilibrium of the complex formation is at about 0.42 mole fraction of host (Fig. 5c,ii). No stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex of Na+ and 1 was formed according to these results. It is known that Na+ strongly prefers 6-fold coordination.34,72 These results imply the four binding sites of DB14C4 are not sufficient to bind larger cations with a higher coordination number. The high selectivity of 1 towards Li+ is achieved by forming a more stable arrangement of the complex.
1 complex of Na+ and 1 was formed according to these results. It is known that Na+ strongly prefers 6-fold coordination.34,72 These results imply the four binding sites of DB14C4 are not sufficient to bind larger cations with a higher coordination number. The high selectivity of 1 towards Li+ is achieved by forming a more stable arrangement of the complex.
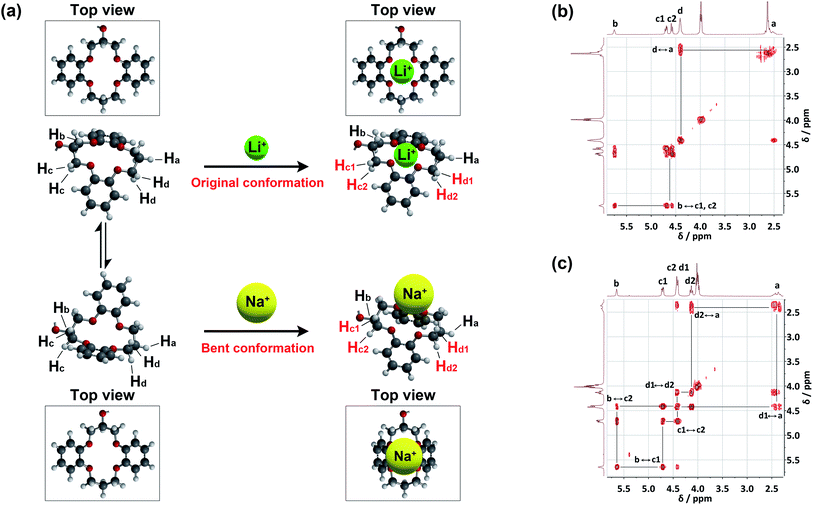
The binding conformations of 1 in response to different guest cations were also confirmed by correlation 1H NMR spectroscopy (COSY) (Fig. 6 and S8†). The 1H COSY spectra of 1/LiClO4 (Fig. 6b) and 1/NaClO4 (Fig. 6c) reveal the correlations of the DB14C4 proton resonances (Ha, Hb, Hc and Hd), confirming that a conformational rearrangement of the DB14C4 ring occurs upon interaction with a sodium cation. The peak splitting of the methylene protons Hc and Hd is shown in the 1H COSY spectra, this demonstrates a bent conformation of the DB14C4 ring. This suggests that the size of the sodium cation is too large to fit into the DB14C4 cavity. The DB14C4 macrocycle has to be bent to interact with the large sodium cation. A proposed schematic drawing of the conformational rearrangements is shown in Fig. 6a. The DB14C4 ring of receptor 1 in the free state exhibits a rapid interconversion on the NMR timescale between two equivalent conformations.95 The smaller lithium cation (1.4 Å) can fit easily into the DB14C4 cavity (1.8 Å) (Fig. 6a).15,73,74 Hence, only the deshielding effects resulting from the lithium cation-oxygen atom interactions were observed for the methylene proton resonances Hc and Hd in the 1H NMR titration and 1H COSY spectra of 1 with Li+ (Fig. 5a,i and 6b). On the other hand, the interactions with a larger sodium cation (2.0 Å)15 induces a bent conformation of the DB14C4 ring (1.8 Å) of 1 (Fig. 6a). Not only the deshielding effects from the sodium cation-oxygen atom interactions, but also the shielding effects from the structural changes of the bent conformation, were observed (Fig. 5a,ii and 6c). The methylene proton resonances Hc2 and Hd2 thus shift to upfield due to the higher electron density induced by the bent conformation. These results suggest that the high selectivity of receptor 1 for Li+ results from both the preferred coordination number of four and the favoured geometry of the DB14C4 cavity.
Lithium selectivity over other alkali metal chloride salts
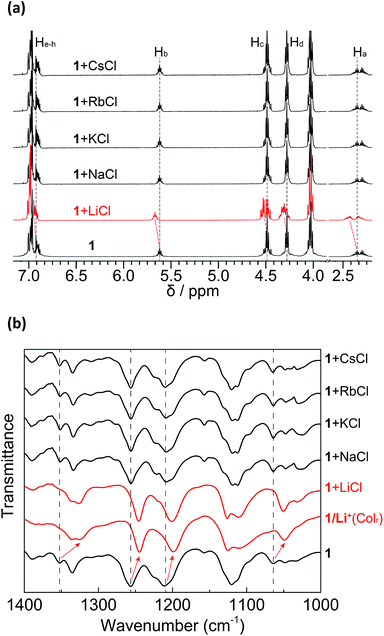
In order to evaluate the lithium-selective binding ability of 1 towards different alkali metal chloride salts in solution, 1H NMR measurements of 1 with excess alkali chloride salts were performed using a mixture of CDCl3 and CD3OD (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the solvent (Fig. 7a). Exposing 1 to excess LiCl induced distinctive changes in the 1H NMR spectrum. These changes in the DB14C4 proton resonances (Ha–h) are analogous to those seen in the titrations with LiClO4. In contrast, no appreciable chemical shift changes were observed in the 1H NMR spectra of the same solutions of 1 after exposure to excess NaCl, KCl, RbCl and CsCl, even after leaving overnight. We conclude that 1 is capable of binding LiCl with high selectivity over other alkali salts in the solution state.
1, v/v) as the solvent (Fig. 7a). Exposing 1 to excess LiCl induced distinctive changes in the 1H NMR spectrum. These changes in the DB14C4 proton resonances (Ha–h) are analogous to those seen in the titrations with LiClO4. In contrast, no appreciable chemical shift changes were observed in the 1H NMR spectra of the same solutions of 1 after exposure to excess NaCl, KCl, RbCl and CsCl, even after leaving overnight. We conclude that 1 is capable of binding LiCl with high selectivity over other alkali salts in the solution state.
The selective interactions between 1 and LiCl were also examined by FT-IR measurements in the solid state. After mixing 1 with excess LiCl, NaCl, KCl, RbCl and CsCl salts in CH3Cl/CH3OH (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution, the solvent was removed completely under reduced pressure and the resulting mixtures were dried and analysed with FT-IR spectroscopy (Fig. 7b). Only the mixture with LiCl showed shifts in the ester and ethereal C–O stretching vibration bands from 1355, 1258, 1211 and 1062 cm−1 to 1328, 1246, 1201 and 1047 cm−1, respectively. These shifts to lower wavenumbers are attributable to the formation of a complex between 1 and Li+ that causes more restriction in the C–O vibrations of the DB14C4 cavity. In contrast, none of the other alkali cations showed interactions with 1. FT-IR measurement was also performed on 1/Li+ at 95 °C in Colr LC phase, which is denoted as 1/Li+(Colr). The IR spectrum of 1/Li+(Colr) is analogous to that of 1 + LiCl. The LC nanostructure has a minor negative effect on the selectivity of 1 towards lithium ions. These results demonstrate that the selective lithium-ion interactions of 1 towards LiCl in the solid state are the same as those of 1/Li+ in LC assembly.
1, v/v) solution, the solvent was removed completely under reduced pressure and the resulting mixtures were dried and analysed with FT-IR spectroscopy (Fig. 7b). Only the mixture with LiCl showed shifts in the ester and ethereal C–O stretching vibration bands from 1355, 1258, 1211 and 1062 cm−1 to 1328, 1246, 1201 and 1047 cm−1, respectively. These shifts to lower wavenumbers are attributable to the formation of a complex between 1 and Li+ that causes more restriction in the C–O vibrations of the DB14C4 cavity. In contrast, none of the other alkali cations showed interactions with 1. FT-IR measurement was also performed on 1/Li+ at 95 °C in Colr LC phase, which is denoted as 1/Li+(Colr). The IR spectrum of 1/Li+(Colr) is analogous to that of 1 + LiCl. The LC nanostructure has a minor negative effect on the selectivity of 1 towards lithium ions. These results demonstrate that the selective lithium-ion interactions of 1 towards LiCl in the solid state are the same as those of 1/Li+ in LC assembly.
Conclusions
In conclusion, we developed nanostructured LC materials, formed by the co-assembly of wedge-shaped CE derivatives (1 or 2) and LiClO4. To the best of our knowledge, compounds 1 and 2 are the first lithium ion-selective receptors capable of forming stable LC nanostructures. The system we reported here can be applied to the fabrication of various LC-based ion-recognition materials, and opens new pathways for the development of new techniques for efficient lithium-selective extraction. It is shown that the self-assembly of the LC columnar structures is driven by the selective supramolecular interactions between the CE moieties and lithium cations. The lithium selectivity of the compounds was characterized by 1H NMR and FT-IR spectroscopy. Remarkably, compound 1 with a DB14C4 moiety shows high selectivity towards lithium salts over the corresponding alkali metal salts. We have found that the high selectivity of 1 for Li+ is due to the preferred coordination number of four and the ideal cavity geometry of the DB14C4 moiety.Experimental
The synthesis and characterization of compounds 1–3 are described in the ESI.†Preparation of the mixtures
Compounds 1–3, LiClO4 and NaClO4 were dried under vacuum at 60 °C for at least 8 h before the preparation. All the materials and solvents for the preparation of the mixtures were dried before use. Mixtures of the receptors and salts were prepared by adding the appropriate volume of a THF solution of LiClO4 or NaClO4 (0.056 M) to a weighed amount of CE derivatives 1, 2 and 3 (10–20 mg) in a microtube. The solution was homogeneously dispersed by sonication and then the solvent was slowly removed by rotary evaporation. The samples were dried under vacuum at 60 °C for 8 h before their study.NMR titrations
A 5 mM host stock solution of respective receptors 1, 2 or 3 was prepared using a mixture of CDCl3 and CD3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the solvent. The guest stock solutions (100 mM) were prepared by dissolving respective alkali metal salts using the as-prepared host stock solutions as the solvent to maintain a constant host concentration throughout the titration. In each titration, 500 μL of the host solution was transferred to an NMR tube via a Hamilton gas tight microsyringe. An appropriate volume of the guest solutions was added via a Hamilton gas tight microsyringe to the host solution in the NMR tube for titration, and a spectrum was obtained via a JEOL JNM-ECX400 NMR spectrometer at 298 K after thorough mixing. Association constants (Ka) were calculated by non-linear curve fitting of the obtained titration isotherms fit to a 1
1, v/v) as the solvent. The guest stock solutions (100 mM) were prepared by dissolving respective alkali metal salts using the as-prepared host stock solutions as the solvent to maintain a constant host concentration throughout the titration. In each titration, 500 μL of the host solution was transferred to an NMR tube via a Hamilton gas tight microsyringe. An appropriate volume of the guest solutions was added via a Hamilton gas tight microsyringe to the host solution in the NMR tube for titration, and a spectrum was obtained via a JEOL JNM-ECX400 NMR spectrometer at 298 K after thorough mixing. Association constants (Ka) were calculated by non-linear curve fitting of the obtained titration isotherms fit to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model using BindFit v0.5.94 The reported association constants were calculated from the downfield shifting of all of the distinguishable related crown ether proton resonances for cation titrations. All titrations were performed at least in triplicate.
1 binding model using BindFit v0.5.94 The reported association constants were calculated from the downfield shifting of all of the distinguishable related crown ether proton resonances for cation titrations. All titrations were performed at least in triplicate.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JST, CREST, JPMJCR1422.Notes and references
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- R. O. Bach, Lithium, current applications in science, medicine, and technology, Wiley, New York, 1985 Search PubMed.
- J. R. Geddes, S. Burgess, K. Hawton, K. Jamison and G. M. Goodwin, Am. J. Psychiatry, 2004, 161, 217–222 CrossRef PubMed.
- J. M. Tarascon, Nat. Chem., 2010, 2, 510 CrossRef CAS PubMed.
- S. E. Kesler, P. W. Gruber, P. A. Medina, G. A. Keoleian, M. P. Everson and T. J. Wallington, Ore Geol. Rev., 2012, 48, 55–69 CrossRef.
- J. Speirs, M. Contestabile, Y. Houari and R. Gross, Renewable Sustainable Energy Rev., 2014, 35, 183–193 CrossRef.
- H. Vikström, S. Davidsson and M. Höök, Appl. Energy, 2013, 110, 252–266 CrossRef.
- B. Swain, Sep. Purif. Technol., 2017, 172, 388–403 CrossRef CAS.
- P. Meshram, B. D. Pandey and T. R. Mankhand, Hydrometallurgy, 2014, 150, 192–208 CrossRef CAS.
- D. J. Cram, T. Kaneda, R. C. Helgeson, S. B. Brown, C. B. Knobler, E. Maverick and K. N. Trueblood, J. Am. Chem. Soc., 1985, 107, 3645–3657 CrossRef CAS.
- J. M. Mahoney, A. M. Beatty and B. D. Smith, Inorg. Chem., 2004, 43, 7617–7621 CrossRef CAS PubMed.
- J. V. Gavette, J. Lara, L. L. Reling, M. M. Haley and D. W. Johnson, Chem. Sci., 2013, 4, 585–590 RSC.
- Q. He, Z. Zhang, J. T. Brewster, V. M. Lynch, S. K. Kim and J. L. Sessler, J. Am. Chem. Soc., 2016, 138, 9779–9782 CrossRef CAS PubMed.
- B. Swain, J. Chem. Technol. Biotechnol., 2016, 91, 2549–2562 CrossRef CAS.
- Y. Marcus, Biophys. Chem., 1994, 51, 111–127 CrossRef CAS.
- E. R. Kay, D. A. Leigh and F. Zerbetto, Angew. Chem., Int. Ed., 2007, 46, 72–191 CrossRef CAS PubMed.
- C. Y. Cheng, P. R. McGonigal, S. T. Schneebeli, H. Li, N. A. Vermeulen, C. F. Ke and J. F. Stoddart, Nat. Nanotechnol., 2015, 10, 547–553 CrossRef CAS PubMed.
- C. Lang, W. F. Li, Z. Y. Dong, X. Zhang, F. H. Yang, B. Yang, X. L. Deng, C. Y. Zhang, J. Y. Xu and J. Q. Liu, Angew. Chem., Int. Ed., 2016, 55, 9723–9727 CrossRef CAS PubMed.
- P. Li, G. Xie, X.-Y. Kong, Z. Zhang, K. Xiao, L. Wen and L. Jiang, Angew. Chem., Int. Ed., 2016, 55, 15637–15641 CrossRef CAS PubMed.
- A. C. C. Esteves, Y. Luo, M. W. P. van de Put, C. C. M. Carcouet and G. de With, Adv. Funct. Mater., 2014, 24, 986–992 CrossRef CAS.
- X. Xie, G. A. Crespo, G. Mistlberger and E. Bakker, Nat. Chem., 2014, 6, 202–207 CrossRef CAS PubMed.
- G. Eisenman and R. Horn, J. Membr. Biol., 1983, 76, 197–225 CrossRef CAS PubMed.
- E. Gouaux and R. MacKinnon, Science, 2005, 310, 1461–1465 CrossRef CAS PubMed.
- H. Li, J. S. Francisco and X. C. Zeng, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 10851–10856 CrossRef CAS PubMed.
- D. A. Kopfer, C. Song, T. Gruene, G. M. Sheldrick, U. Zachariae and B. L. de Groot, Science, 2014, 346, 352–355 CrossRef PubMed.
- D. Krauss, B. Eisenberg and D. Gillespie, Eur. Biophys. J., 2011, 40, 775–782 CrossRef CAS PubMed.
- D. Yernool, O. Boudker, Y. Jin and E. Gouaux, Nature, 2004, 431, 811–818 CrossRef CAS PubMed.
- D. A. Doyle, J. M. Cabral, R. A. Pfuetzner, A. Kuo, J. M. Gulbis, S. L. Cohen, B. T. Chait and R. MacKinnon, Science, 1998, 280, 69–77 CrossRef CAS PubMed.
- L. O. Essen and U. Koert, Annu. Rep. Prog. Chem., Sect. C: Phys. Chem., 2008, 104, 165–188 RSC.
- G. W. Gokel and A. Mukhopadhyay, Chem. Soc. Rev., 2001, 30, 274–286 RSC.
- S. Bhosale, A. L. Sisson, P. Talukdar, A. Fürstenberg, N. Banerji, E. Vauthey, G. Bollot, J. Mareda, C. Röger, F. Würthner, N. Sakai and S. Matile, Science, 2006, 313, 84–86 CrossRef CAS PubMed.
- B. Soberats, M. Yoshio, T. Ichikawa, S. Taguchi, H. Ohno and T. Kato, J. Am. Chem. Soc., 2013, 135, 15286–15289 CrossRef CAS PubMed.
- A. Yamashita, M. Yoshio, B. Soberats, H. Ohno and T. Kato, J. Mater. Chem. A, 2015, 3, 22656–22662 CAS.
- X. J. Gong, J. C. Li, K. Xu, J. F. Wang and H. Yang, J. Am. Chem. Soc., 2010, 132, 1873–1877 CrossRef CAS PubMed.
- I. M. Bennett, H. M. V. Farfano, F. Bogani, A. Primak, P. A. Liddell, L. Otero, L. Sereno, J. J. Silber, A. L. Moore, T. A. Moore and D. Gust, Nature, 2002, 420, 398–401 CrossRef CAS PubMed.
- T. Muraoka, T. Endo, K. V. Tabata, H. Noji, S. Nagatoishi, K. Tsumoto, R. Li and K. Kinbara, J. Am. Chem. Soc., 2014, 136, 15584–15595 CrossRef CAS PubMed.
- Handbook of Liquid Crystals, ed. J. W. Goodby, P. J. Collings, T. Kato, C. Tschierske, H. Gleeson and P. Raynes, Wiley-VCH, Weinheim, Germany, 2nd edn, 2014 Search PubMed.
- T. Kato, N. Mizoshita and K. Kishimoto, Angew. Chem., Int. Ed., 2006, 45, 38–68 CrossRef CAS PubMed.
- D. J. Broer, C. M. W. Bastiaansen, M. G. Debije and A. P. H. J. Schenning, Angew. Chem., Int. Ed., 2012, 51, 7102–7109 CrossRef CAS PubMed.
- T. Wohrle, I. Wurzbach, J. Kirres, A. Kostidou, N. Kapernaum, J. Litterscheidt, J. C. Haenle, P. Staffeld, A. Baro, F. Giesselmann and S. Laschat, Chem. Rev., 2016, 116, 1139–1241 CrossRef PubMed.
- T. Kato, Science, 2002, 295, 2414–2418 CrossRef CAS PubMed.
- T. Kato, T. Matsuoka, M. Nishii, Y. Kamikawa, K. Kanie, T. Nishimura, E. Yashima and S. Ujiie, Angew. Chem., Int. Ed., 2004, 43, 1969–1972 CrossRef CAS PubMed.
- T. Kato, T. Yasuda, Y. Kamikawa and M. Yoshio, Chem. Commun., 2009, 729–739 RSC.
- K. Goossens, K. Lava, C. W. Bielawski and K. Binnemans, Chem. Rev., 2016, 116, 4643–4807 CrossRef CAS PubMed.
- A. P. H. J. Schenning, Y. C. Gonzalez-Lemus, I. K. Shishmanova and D. J. Broer, Liq. Cryst., 2011, 38, 1627–1639 CrossRef CAS.
- J. Yoshida, S. Tamura, G. Watanabe, Y. Kasahara and H. Yuge, Chem. Commun., 2017, 53, 5103–5106 RSC.
- Y. Gao, J. M. Slattery and D. W. Bruce, New J. Chem., 2011, 35, 2910–2918 RSC.
- T. Kato, M. Yoshio, T. Ichikawa, B. Soberats, H. Ohno and M. Funahashi, Nat. Rev. Mater., 2017, 2, 17001 CrossRef.
- W. Pisula, X. Feng and K. Müllen, Adv. Mater., 2010, 22, 3634–3649 CrossRef CAS PubMed.
- C. Tschierske, Chem. Soc. Rev., 2007, 36, 1930–1970 RSC.
- B. M. Rosen, C. J. Wilson, D. A. Wilson, M. Peterca, M. R. Imam and V. Percec, Chem. Rev., 2009, 109, 6275–6540 CrossRef CAS PubMed.
- T. Kato, Angew. Chem., Int. Ed., 2010, 49, 7847–7848 CrossRef CAS PubMed.
- T. Hatano and T. Kato, Chem. Commun., 2006, 1277–1279 RSC.
- M. Henmi, K. Nakatsuji, T. Ichikawa, H. Tomioka, T. Sakamoto, M. Yoshio and T. Kato, Adv. Mater., 2012, 24, 2238–2241 CrossRef CAS PubMed.
- D. Hogberg, B. Soberats, R. Yatagai, S. Uchida, M. Yoshio, L. Kloo, H. Segawa and T. Kato, Chem. Mater., 2016, 28, 6493–6500 CrossRef CAS.
- B. Soberats, M. Yoshio, T. Ichikawa, X. B. Zeng, H. Ohno, G. Ungar and T. Kato, J. Am. Chem. Soc., 2015, 137, 13212–13215 CrossRef CAS PubMed.
- B. Soberats, E. Uchida, M. Yoshio, J. Kagimoto, H. Ohno and T. Kato, J. Am. Chem. Soc., 2014, 136, 9552–9555 CrossRef CAS PubMed.
- M. Yoshio, T. Mukai, H. Ohno and T. Kato, J. Am. Chem. Soc., 2004, 126, 994–995 CrossRef CAS PubMed.
- T. Ichikawa, M. Yoshio, A. Hamasaki, S. Taguchi, F. Liu, X. B. Zeng, G. Ungar, H. Ohno and T. Kato, J. Am. Chem. Soc., 2012, 134, 2634–2643 CrossRef CAS PubMed.
- B. Soberats, M. Yoshio, T. Ichikawa, H. Ohno and T. Kato, J. Mater. Chem. A, 2015, 3, 11232–11238 CAS.
- J. Sakuda, E. Hosono, M. Yoshio, T. Ichikawa, T. Matsumoto, H. Ohno, H. S. Zhou and T. Kato, Adv. Funct. Mater., 2015, 25, 1206–1212 CrossRef CAS.
- T. Ichikawa, M. Yoshio, A. Hamasaki, J. Kagimoto, H. Ohno and T. Kato, J. Am. Chem. Soc., 2011, 133, 2163–2169 CrossRef CAS PubMed.
- H. Shimura, M. Yoshio, A. Hamasaki, T. Mukai, H. Ohno and T. Kato, Adv. Mater., 2009, 21, 1591–1594 CrossRef CAS.
- G. M. Bogels, J. A. M. Lugger, O. J. G. M. Goor and R. P. Sijbesma, Adv. Funct. Mater., 2016, 26, 8023–8030 CrossRef.
- M. Kaller, A. Baro and S. Laschat, in Handbook of Liquid Crystals, ed. J. W. Goodby, P. J. Collings, T. Kato, C. Tschierske, H. Gleeson and P. Raynes, Wiley-VCH, Weinheim, Germany, 2nd edn, 2014, vol. 8, ch. 11, pp. 335–377 Search PubMed.
- M. Kaller and S. Laschat, in Liquid Crystals: Materials Design and Self-assembly, ed. C. Tschierske, Springer, Berlin, Heidelberg, 2012, vol. 318, pp. 109–192 Search PubMed.
- R. P. Tuffin, K. J. Toyne and J. W. Goodby, J. Mater. Chem., 1995, 5, 2093–2104 RSC.
- R. P. Tuffin, K. J. Toyne and J. W. Goodby, J. Mater. Chem., 1996, 6, 1271–1282 RSC.
- C. J. Pedersen, J. Am. Chem. Soc., 1967, 89, 2495–2496 CrossRef CAS.
- D. J. Cram and J. M. Cram, Acc. Chem. Res., 1978, 11, 8–14 CrossRef CAS.
- J.-M. Lehn, Angew. Chem., Int. Ed. Engl., 1988, 27, 89–112 CrossRef.
- G. W. Gokel, W. M. Leevy and M. E. Weber, Chem. Soc. Rev., 2004, 104, 2723–2750 CrossRef CAS PubMed.
- C. J. Pedersen, J. Am. Chem. Soc., 1967, 89, 7017–7036 CrossRef CAS.
- U. Olsher, R. M. Izatt, J. S. Bradshaw and N. K. Dalley, Chem. Rev., 1991, 91, 137–164 CrossRef CAS.
- U. Olsher, J. Am. Chem. Soc., 1982, 104, 4006–4007 CrossRef CAS.
- U. Olsher, F. Frolow, G. Shoham, G. S. Heo and R. A. Bartsch, Anal. Chem., 1989, 61, 1618–1621 CrossRef CAS.
- T. Hayashita, J. H. Lee, M. G. Hankins, J. C. Lee, J. S. Kim, J. M. Knobeloch and R. A. Bartsch, Anal. Chem., 1992, 64, 815–819 CrossRef CAS.
- K. Suzuki, H. Yamada, K. Sato, K. Watanabe, H. Hisamoto, Y. Tobe and K. Kobiro, Anal. Chem., 1993, 65, 3404–3410 CrossRef CAS.
- R. E. C. Torrejos, G. M. Nisola, M. J. Park, A. B. Beltran, J. G. Seo, S. P. Lee and W. J. Chung, Desalin. Water Treat., 2015, 53, 2774–2781 CrossRef CAS.
- R. E. C. Torrejos, G. M. Nisola, M. J. Park, H. K. Shon, J. G. Seo, S. Koo and W. J. Chung, Chem. Eng. J., 2015, 264, 89–98 CrossRef CAS.
- G. Shoham, W. N. Lipscomb and U. Olsher, J. Chem. Soc., Chem. Commun., 1983, 208–209 RSC.
- G. Shoham, D. W. Christianson, R. A. Bartsch, G. S. Heo, U. Olsher and W. N. Lipscomb, J. Am. Chem. Soc., 1984, 106, 1280–1285 CrossRef CAS.
- X. B. Luo, B. Guo, J. M. Luo, F. Deng, S. Y. Zhang, S. L. Luo and J. Crittenden, ACS Sustainable Chem. Eng., 2015, 3, 460–467 CrossRef CAS.
- B. Hashemi, M. Shamsipur and Z. Seyedzadeh, New J. Chem., 2016, 40, 4803–4809 RSC.
- V. Percec, G. Zipp, G. Johansson, U. Beginn and M. Moller, Macromol. Chem. Phys., 1997, 198, 265–277 CrossRef CAS.
- U. Beginn, G. Zipp, M. Moller, G. Johansson and V. Percec, Macromol. Chem. Phys., 1997, 198, 2839–2852 CrossRef CAS.
- G. Johansson, V. Percec, G. Ungar and D. Abramic, J. Chem. Soc., Perkin Trans. 1, 1994, 447–459 RSC.
- G. Ungar, S. V. Batty, V. Percec, J. Heck and G. Johansson, Adv. Mater. Opt. Electron., 1994, 4, 303–313 CrossRef CAS.
- V. Percec, G. Johansson, J. Heck, G. Ungar and S. V. Batty, J. Chem. Soc., Perkin Trans. 1, 1993, 1411–1420 RSC.
- U. Beginn, G. Zipp, A. Mourran, P. Walther and M. Moller, Adv. Mater., 2000, 12, 513–516 CrossRef CAS.
- U. Beginn, G. Zipp and M. Moller, Chem.–Eur. J., 2000, 6, 2016–2023 CrossRef CAS.
- U. Beginn, G. Zipp and M. Moller, Adv. Mater., 2000, 12, 510–513 CrossRef CAS.
- M. Kaller, P. Staffeld, R. Haug, W. Frey, F. Giesselmann and S. Laschat, Liq. Cryst., 2011, 38, 531–553 CrossRef CAS.
- P. Thordarson, Chem. Soc. Rev., 2011, 40, 1305–1323 RSC.
- Z. H. Chen and R. A. Sachleben, J. Chem. Soc., Perkin Trans. 2, 1994, 537–541 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details of the synthesis and characterisation of the thermotropic liquid-crystalline properties of all samples. Representative titration spectra and fit binding curves. See DOI: 10.1039/c7sc03652c |
| This journal is © The Royal Society of Chemistry 2018 |