Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
H2O2-responsive and plaque-penetrating nanoplatform for mTOR gene silencing with robust anti-atherosclerosis efficacy†
Wen
Gao‡
a,
Yujie
Zhao‡
a,
Xiang
Li
a,
Yuhui
Sun
a,
Michelle
Cai
b,
Wenhua
Cao
a,
Zhenhua
Liu
a,
Lili
Tong
a,
Guanwei
Cui
a and
Bo
Tang
 *a
*a
aCollege of Chemistry, Chemical Engineering and Materials Science, Collaborative Innovation Center of Functionalized Probes for Chemical Imaging in Universities of Shandong, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Institute of Biomedical Sciences, Shandong Normal University, Jinan 250014, P. R. China. E-mail: tangb@sdnu.edu.cn
bFaculty of Science, Western University, London, Ontario N6A5B7, Canada
First published on 27th October 2017
Abstract
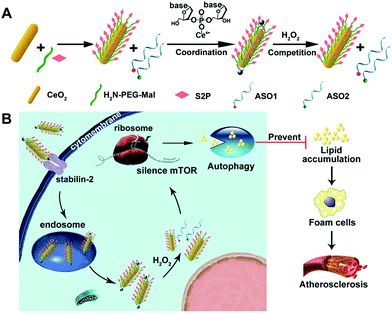
The mammalian target of rapamycin (mTOR) that controls autophagy and lipid metabolism is pivotal for atherosclerosis initiation and progression. Although blocking the mTOR function with rapamycin and its analogs may stimulate autophagy and consequently attenuate lipid storage and atherosclerotic lesions, only limited success has been achieved in clinical applications due to the unsatisfactory efficacy and safety profiles. In this study, we engineered a cerium oxide nanowire (CeO2 NW)-based RNA interference (RNAi) oligonucleotide delivery nanoplatform for the effective silencing of mTOR and treatment of atherosclerosis. This nanoplatform is composed of the following three key components: (i) a stabilin-2-specific peptide ligand (S2P) to improve plaque targeting and penetration; (ii) polyethylene glycosylation (PEGylation) to extend in vivo circulation time; and (iii) a high aspect ratio CeO2 core to facilitate endosome escape and ensure “on-demand” release of the RNAi payloads through competitive coordination of cytosolic hydrogen peroxide (H2O2). Systemic administration of the nanoplatforms efficiently targeted stabilin-2-expressing plaque and suppressed mTOR expression, which significantly rescued the impaired autophagy and inhibited the atherosclerotic lesion progression in apolipoprotein E-deficient (ApoE−/−) mice fed with a high-fat diet. These results demonstrated that this H2O2-responsive and plaque-penetrating nanoplatform can be a potent and safe tool for gene therapy of atherosclerosis.
Introduction
Atherosclerosis is the leading cause of cardiovascular disease and stroke which increasingly threatens human health worldwide.1,2 The initiating event in atherosclerosis is the accumulation of lipids within the arterial wall that leads to the formation of vascular smooth muscle cell (VSMC)/macrophage (Mϕ)-derived foam cells.3–5 Recently, the mammalian target of rapamycin (mTOR) has drawn more attention to atherosclerosis as it has an important role in the regulation of autophagy.6,7 Autophagy is a protective and life-sustaining process by which lipid droplets within the VSMC/Mϕ are sequestered in double-membrane vesicles and degraded via lysosomal acid lipase after fusion with lysosomal compartments.8–10 Inhibiting mTOR function with rapamycin or its derivative everolimus activates the autophagy process and impedes foam cell formation, suggesting a promising target for the treatment of atherosclerosis.11–13 However, only limited success has been achieved in clinical applications of mTOR inhibitors due to their poor solubility and low bioavailability.14,15 Moreover, rapamycin does not perturb all mTOR functions; at least two distinct multiprotein complexes of mTOR exist within cells, only one of which is sensitive to rapamycin.16,17 Therefore, the exploration of more efficient strategies for mTOR inhibition is highly desirable.RNA interference (RNAi) using an antisense oligonucleotide (ASO) or short-interfering RNA (siRNA) is an attractive alternative to inhibiting otherwise intractable therapeutic targets.18,19 Effective knockdown occurs once an ASO or siRNA strand is incorporated into the RNA-induced silencing complex, which is then guided to the target mRNA sequence to be excised.20,21 This ensures that the “nondruggable” target proteins are no longer expressed within the cell.22 Although this strategy has been effective in vitro, delivery of therapeutic ASOs or siRNAs into diseased tissues or cells of patients remains a challenge. For atherosclerosis therapy, the barriers to effective in vivo siRNA delivery mainly include serum nuclease degradation, atherosclerotic plaque targeting, plaque and cell membrane penetration, escape from the endosome, and release of ASOs or siRNAs in the cytoplasm.23 Recent advances in nanotechnology make it possible to overcome the extracellular barrier by packaging ASOs or siRNAs into nanoparticles. Based on their large surface areas, intrinsic properties and nanoscale effects, nanoparticles can accommodate a variety of ASOs or siRNAs with a high loading efficiency24,25 and act as a protective barrier against the enzymatic environment outside of the cells, prolonging their half-life in the circulation.26–29 In addition, the defective and immature nature of the blood vessels surrounding atherosclerotic plaques allows ASO- or siRNA-loaded nanoparticles to extravasate the leaky blood vessels and accumulate in the plaques, relying on the enhanced permeation and retention (EPR) effect.30 However, the efficient delivery and release of the ASOs or siRNAs to the target VSMC/Mϕ against non-specific cellular uptake, subsequent endosomal entrapment and lysosomal degradation remain an intractable problem. In order to successfully inhibit the mTOR function with ASOs or siRNAs in vivo, the nanoparticle delivery platform should be tailored to exhibit the following features: (1) good biocompatibility, (2) a long blood circulation time and high EPR effect, (3) plaque-penetrating ability and VSMC/Mϕ-targeting specificity, (4) an efficient endosomal escape ability and the “on-demand” release of active ASOs or siRNAs into the cytoplasm, and (5) an imaging function for monitoring the release profile and therapeutic efficacy.
Herein, we developed an engineered cerium oxide nanowire (CeO2 NW)-based RNAi oligonucleotide delivery nanoplatform capable of satisfying all of the above-mentioned criteria and demonstrated its high efficiency in suppressing mTOR expression and atherosclerosis progression. This nanoplatform consisted of a high aspect ratio CeO2 NW functionalized with PEG (MW 2000, surface protection moiety31) and a plaque-penetrating peptide (S2P, stabilin-2-specific peptide ligand32). Two different mTOR ASOs naturally wrapped around the S2P-PEGylated CeO2 NW through metal coordination33 between phosphate groups in DNA and Ce ions at the particle surfaces (Scheme 1A). The resulting S2P–CeO2–ASOs nanoplatform exhibited an excellent biocompatibility and prolonged blood circulation lifetime. It also had the ability to specifically target and penetrate stabilin-2-overexpressing VSMCs, both in the culture and in the atherosclerotic plaques of apolipoprotein E-deficient (ApoE−/−) mice. Once inside the cell, the high aspect ratio CeO2 core was able to pierce the endosomal membrane, thus facilitating the loaded ASOs escape from the endosome prior to lysosomal degradation. As demonstrated in previous atherosclerosis studies,34,35 plaque cells exhibit a higher level of cytosolic reactive oxygen species (ROS), particularly hydrogen peroxide (H2O2). In the presence of H2O2, the absorbed ASOs are liberated from the CeO2 surface due to the stronger binding ability between peroxo and Ce4+ compared to that between phosphate oxygen and Ce4+.36,37 Fluorescently tagged ASOs were used to monitor the endosome rupture and cytosolic H2O2-triggered release. Targeted delivery and “on-demand” release of ASOs to high-fat diet-fed ApoE−/− mice efficiently depleted mTOR expression and rescued the impaired autophagy, which led to a dramatic reduction in foam cell formation and atherosclerotic lesion progression (Scheme 1B). These results suggested that the S2P–CeO2–ASOs nanoplatform can be a potential new therapeutic option for atherosclerosis.
Results and discussion
Preparation and characterization of the S2P–CeO2–ASOs nanoplatform
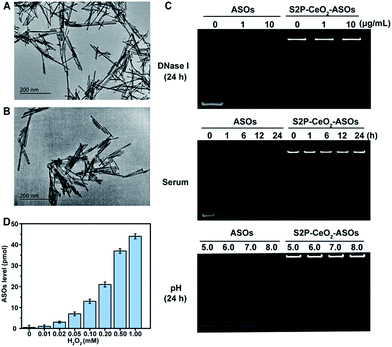
To realize our design, CeO2 NWs were synthesized via a modified hydrothermal method using Ce(NO3)3 as the Ce source.38 A high aspect ratio CeO2 NW with a dimension of 100–150 nm was selected as the core unit, which is the optimal size for EPR-mediated targeting and endosomal escape and allows for maximal RNAi oligonucleotide loading. Transmission electron microscopy (TEM) showed that the obtained CeO2 NWs presented a smooth surface with the length being 130 nm and width being 9 nm (Fig. 1A). Their X-ray diffraction (XRD) pattern in Fig. S1† indicated that these particles were composed of CeO2 of the typical fluorite cubic structure (JCPDS card 34-0394, space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/i_char_0033_0304.gif) m).39 The zeta potential of CeO2 NWs in PBS (pH 7.4) was determined to be −14.2 ± 0.4 mV, which allowed for electrostatic interactions with positively charged maleimide–PEG–amine (MW 2000). The reversed zeta potential of 2.2 ± 0.5 mV indicated that PEG was successfully coated onto the CeO2 core surface. The plaque-penetrating peptide S2P, Cys-Arg-Leu-Thr-Leu-Thr-Val-Arg-Lys-Cys, was further grafted to the PEG-capped CeO2 by thioether bonds between the thiol groups on Cys and maleimide groups on PEG, resulting in a much higher positive zeta potential (4.3 ± 0.3 mV). The S2P-PEGylated CeO2 NW was then wrapped with mTOR-specific ASO1 and ASO2 (sequences are described in Table S1†) via metal coordination between phosphate groups (PO4−) in DNA and Ce ions (Ce4+) at the particle surfaces. The loading efficiency of the ASOs was calculated using a NanoDrop spectrophotometer. The average number of ASOs loaded in S2P-PEGylated CeO2 NWs was estimated to be 435 DNA/NW (Table S2†). We also evaluated the distribution of ASO1 and ASO2 in the coloaded particles by quantitative real-time PCR (qRT-PCR) analysis. In three different batches, the results indicated that coloading of ASO1 and ASO2 in S2P-PEGylated CeO2 NWs occurred almost at equimolar concentration (Fig. S2†). The morphology and size of the resulting S2P–CeO2–ASOs nanoplatform were further confirmed with TEM (Fig. 1B). Electrophoretic gel analysis showed that S2P–CeO2–ASOs, when compared to free ASOs, were resistant to 10 μg mL−1 DNase I treatment and highly stable in 10% FBS supplemented DMEM at 37 °C for 24 h. Importantly, S2P–CeO2–ASOs maintained a high ASO loading upon pH change (Fig. 1C). This is mainly attributed to the steric blocking effect of the PEG layer31 and the stable nature of the Ce4+–phosphate oxygen coordination conjunction,33 which reduced enzymatic degradation and unexpected desorption of the ASOs. Because of the stronger binding ability between Ce4+ and peroxo, approximately 90% of the loaded ASOs was released within 5 min in the presence of 100 μM H2O2 (Fig. S3†). The amount of ASOs released was proportional to the H2O2 concentration (Fig. 1D). Further inspection of the ASOs release caused by other components, such as superoxide anions (O2˙−), hydroxyl radicals (˙OH), cysteine (Cys), glutathione (GSH), ascorbic acid (AA), and adenosine triphosphate (ATP), possibly coexisting with H2O2, revealed that these species did not cause a remarkable release of ASOs (Fig. S3†). These results together indicated that S2P–CeO2–ASOs formed a stable uniform-sized nanoplatform capable of efficient loading and H2O2-specific controlled release of mTOR ASOs.
m).39 The zeta potential of CeO2 NWs in PBS (pH 7.4) was determined to be −14.2 ± 0.4 mV, which allowed for electrostatic interactions with positively charged maleimide–PEG–amine (MW 2000). The reversed zeta potential of 2.2 ± 0.5 mV indicated that PEG was successfully coated onto the CeO2 core surface. The plaque-penetrating peptide S2P, Cys-Arg-Leu-Thr-Leu-Thr-Val-Arg-Lys-Cys, was further grafted to the PEG-capped CeO2 by thioether bonds between the thiol groups on Cys and maleimide groups on PEG, resulting in a much higher positive zeta potential (4.3 ± 0.3 mV). The S2P-PEGylated CeO2 NW was then wrapped with mTOR-specific ASO1 and ASO2 (sequences are described in Table S1†) via metal coordination between phosphate groups (PO4−) in DNA and Ce ions (Ce4+) at the particle surfaces. The loading efficiency of the ASOs was calculated using a NanoDrop spectrophotometer. The average number of ASOs loaded in S2P-PEGylated CeO2 NWs was estimated to be 435 DNA/NW (Table S2†). We also evaluated the distribution of ASO1 and ASO2 in the coloaded particles by quantitative real-time PCR (qRT-PCR) analysis. In three different batches, the results indicated that coloading of ASO1 and ASO2 in S2P-PEGylated CeO2 NWs occurred almost at equimolar concentration (Fig. S2†). The morphology and size of the resulting S2P–CeO2–ASOs nanoplatform were further confirmed with TEM (Fig. 1B). Electrophoretic gel analysis showed that S2P–CeO2–ASOs, when compared to free ASOs, were resistant to 10 μg mL−1 DNase I treatment and highly stable in 10% FBS supplemented DMEM at 37 °C for 24 h. Importantly, S2P–CeO2–ASOs maintained a high ASO loading upon pH change (Fig. 1C). This is mainly attributed to the steric blocking effect of the PEG layer31 and the stable nature of the Ce4+–phosphate oxygen coordination conjunction,33 which reduced enzymatic degradation and unexpected desorption of the ASOs. Because of the stronger binding ability between Ce4+ and peroxo, approximately 90% of the loaded ASOs was released within 5 min in the presence of 100 μM H2O2 (Fig. S3†). The amount of ASOs released was proportional to the H2O2 concentration (Fig. 1D). Further inspection of the ASOs release caused by other components, such as superoxide anions (O2˙−), hydroxyl radicals (˙OH), cysteine (Cys), glutathione (GSH), ascorbic acid (AA), and adenosine triphosphate (ATP), possibly coexisting with H2O2, revealed that these species did not cause a remarkable release of ASOs (Fig. S3†). These results together indicated that S2P–CeO2–ASOs formed a stable uniform-sized nanoplatform capable of efficient loading and H2O2-specific controlled release of mTOR ASOs.
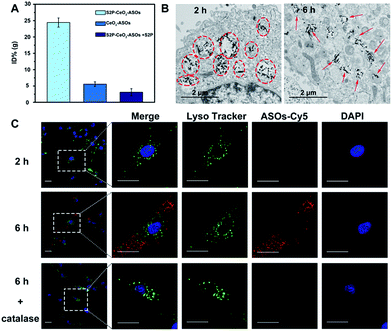
In vitro plaque-targeting ability and mTOR silencing efficacy of S2P–CeO2–ASOs
After acquiring the nanoplatform with a high loading efficiency and H2O2-responsive release property, an inductive coupling plasma atomic emission spectrometer (ICP-AES, based on a total Ce content uptaken per cell) was employed to evaluate its in vitro plaque-targeting ability. The uptake of the S2P–CeO2–ASOs nanoplatform was facilitated through a stabilin-2-mediated endocytosis pathway. Stabilin-2 is a transmembrane protein expressed predominantly in VSMCs of atherosclerotic plaques (Fig. S4†), which can recognize and bind S2P peptides on the nanoplatform. As shown in Fig. S5,† VSMCs incubated with S2P–CeO2–ASOs showed a significant uptake starting at 2 h and saturating at 6 h. This uptake was concentration-dependent with a maximum Ce content per cell at 50 μg mL−1 (Fig. S6†). Importantly, VSMCs uptake of S2P–CeO2–ASOs was significantly higher than that of S2P absent CeO2–ASOs and S2P–CeO2–ASOs with a blocking dose (1 mg mL−1) of S2P peptide (Fig. 2A). In addition, the S2P–CeO2–ASOs was not taken up efficiently by any of the stabilin-2-negative cell lines (Fig. S7†). This targeted uptake by cultured VSMCs demonstrated in vitro that S2P–CeO2–ASOs have a specific and strong binding affinity with stabilin-2 and therefore can target and penetrate stabilin-2-overexpressing VSMCs in the plaque area.Next, the endosomal escape ability of S2P–CeO2–ASOs was examined using TEM. The piercing of the endolysosomal membrane by high aspect ratio CeO2 cores was seen, which led to the release of the S2P–CeO2–ASOs nanoplatform into the cytoplasm after 6 h of incubation (indicated by the red arrows in Fig. 2B). An MTT assay (Fig. S8†) showed that localized damage inflicted on the endosomal membrane did not induce cell death. Red fluorescently (Cy5) tagged ASOs were further used to confirm the endosomal escape and cytosolic H2O2-triggered release. As shown in Fig. 2C, owing to the high quenching ability of CeO2 NWs and excellent pH stability (5–8) of CeO2 NW–DNA coordination complexes,36 there was no red fluorescence signal when S2P–CeO2–ASOs nanoplatforms were localized inside the endosomes/lysosomes at the early time point (2 h). When the nanoplatforms entered the cytoplasm (6 h), the presence of H2O2 (Fig. S9†) induced liberation of the absorbed ASOs from the CeO2 surface as a result of the stronger binding ability between peroxo and Ce4+ in comparison to that between phosphate oxygen and Ce4+. This resulted in a dramatic increase in the red fluorescence signal. The lack of colocalization with LysoTracker staining indicated the effective endosomal escape of S2P–CeO2–ASOs. However, upon addition of a H2O2 scavenger (catalase, 100 μM), a marked decrease in the red fluorescence intensity was observed, further confirming that the nanoplatform allowed for “on-demand” release of ASOs in response to cytosolic H2O2.
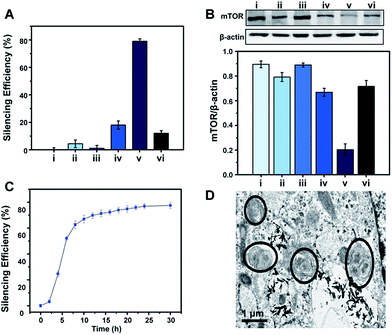
To demonstrate the feasibility of the S2P–CeO2–ASOs nanoplatform toward enhanced mTOR gene silencing, various controls and time-silencing dependence studies were carried out to investigate and optimize the in vitro intracellular mRNA silencing mechanism involved in the mTOR knockdown. Fig. 3A shows the qRT-PCR results of VSMCs after they have been exposed to different conditions and incubated for 24 h in the culture medium. mTOR gene-silencing achieved 79% with S2P–CeO2–ASOs at an 80 ng ASO concentration, as compared to 18% achieved with CeO2–ASOs (Fig. 3A, bar v vs. bar iv), which was ∼4 times lower than S2P-conjugated silencing abilities. It has also been demonstrated that S2P–CeO2–ASOs better facilitate the ASO internalization process, as the nanoplatform offers an 18-fold enhanced silencing when compared to free ASOs in solution at the same concentration (Fig. 3A, bar v vs. bar ii). Furthermore, to ensure that the silencing efficiency was a result of the H2O2-triggered release of ASOs, cells treated with catalase and S2P–CeO2–ASOs and cells treated with S2P–CeO2 cores lacking the ASOs (Fig. 3A, bar vi and bar iii) were also analyzed. These control experiments resulted in negligible mTOR downregulation. In addition, the S2P–CeO2–ASOs showed a fast silencing response (52% after a 6 h treatment), reaching a silencing percentage plateau 24 h post-treatment (Fig. 3C). Western blots (Fig. 3B) and immunofluorescence analysis (Fig. S10 and S11†) further confirmed that the mTOR protein levels were greatly reduced by the nanoplatform at 24 h. In view of these results, there are clear advantages in the efficiency and speed of the mTOR gene knockdown with the S2P–CeO2–ASOs. With the suppressed mTOR expression, the autophagy, measured as the ratio of LC3II/LC3I (Fig. S12†), and the number of double-membrane autophagosomes (Fig. 3D) increased significantly after the treatment with S2P–CeO2–ASOs for 24 h. The S2P–CeO2–ASOs-induced autophagy activation resembled the activation achieved by the direct treatment of cells with rapamycin. Consequently, activation of the autophagy inhibited oxidized low-density lipoprotein (oxLDL)-induced VSMC foam cell formation, which was manifested by a decreased lipid droplet accumulation (Fig. S13A†) and total cholesterol level (Fig. S13B†). This decrease also occurred in the rapamycin treated group.
Pharmacokinetic and in vivo plaque-targeting ability of S2P–CeO2–ASOs
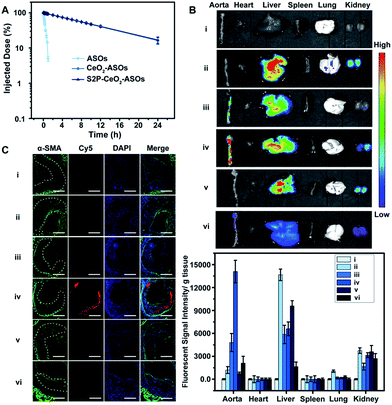
Having established that S2P–CeO2–ASOs can activate autophagy and inhibit VSMC foam cell formation through mTOR gene expression silencing, we assessed their pharmacokinetics and in vivo plaque-targeting ability. The pharmacokinetics of S2P–CeO2–ASOs was examined by intravenous injection of Cy5-tagged ASOs loaded nanoplatforms to healthy ApoE−/− mice (ASO dose: 0.5 mg kg−1, n = 3). As shown in Fig. 4A, the blood half-life (t1/2) of S2P–CeO2–ASOs was around 9.33 h, which was comparable to the t1/2 of CeO2–ASOs (∼9.16 h), but far longer than that of free ASOs (t1/2 < 20 min). This prolonged half-life in the blood circulation was mainly due to the protection of the PEG outer layer and stable nature of the Ce4+–phosphate oxygen coordination conjunction. The in vivo plaque-targeting ability of S2P–CeO2–ASOs was assessed by intravenously injecting Cy5-tagged ASOs loaded nanoplatforms into plaque-bearing ApoE−/− mice (ASO dose: 0.5 mg kg−1, n = 3). Fig. 4B showed the fluorescence images of the aorta and major organs excised 24 h post-injection. With S2P-mediated stabilin-2 (overexpression on plaque, Fig. S14†) targeting and H2O2 (overgeneration in plaque, Fig. S15†) triggered release of ASOs, S2P–CeO2–ASOs showed a high accumulation in the aorta corresponding to the bright fluorescence. When administration of S2P peptide (1 mg kg−1) was performed 1 h before S2P–CeO2–ASOs injection, stabilin-2 blocking significantly reduced the aorta accumulation of S2P–CeO2–ASOs. In comparison, CeO2–ASOs or free ASOs presented a high accumulation in the liver and kidneys and an extremely low accumulation in the aorta. Fig. 4B also showed a marked decrease in the fluorescence intensity in mice treated with S2P–CeO2–ASOs with H2O2 scavenger catalase (1 mg kg−1), further confirming the ability of the nanoplatform to release ASOs “on-demand” in response to H2O2.It has been identified that the blood vessels surrounding atherosclerotic plaques are defective and immature.40,41 Once a large proportion of S2P–CeO2–ASOs accumulated in the aorta, the leaky vasculature allows them to penetrate and be retained in the plaques. To evaluate the plaque-penetrating ability of the S2P–CeO2–ASOs, aortic arches were isolated from mice and their localization was determined by an immunohistochemistry study. Immunofluorescence micrographs of aortic arch cross-sections (Fig. 4C) clearly showed that S2P–CeO2–ASOs can (shown with the red fluorescence of Cy5) easily pass through the arterial wall and become trapped by VSMC in atherosclerotic plaques (shown with the green fluorescence of α-SMA) via S2P mediated stabilin-2 targeting. As expected, non-coated CeO2–ASOs and free ASOs did not show plaque-specific targeting. These results strongly demonstrated the deep plaque-penetrating and H2O2-triggered release characteristics of S2P–CeO2–ASOs.
In vivo mTOR silencing and anti-atherosclerosis efficacy of S2P–CeO2–ASOs
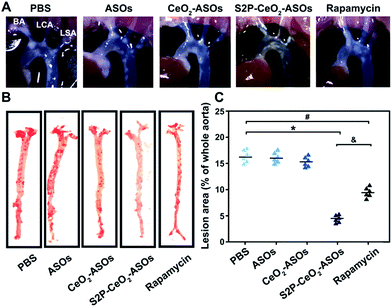
To examine the inhibition of the mTOR expression in aortic atherosclerotic lesions, S2P–CeO2–ASOs nanoplatforms were intravenously administered into ApoE−/− mice fed with a high-fat diet (ASO dose: 0.5 mg kg−1, n = 3). Aortas were harvested three days post-injection and the in vivo mTOR expression was evaluated by western blot analysis. By virtue of plaque-penetration, S2P–CeO2–ASOs induced >75% knockdown of the mTOR expression in the aortas. In contrast, there was <26% knockdown for the CeO2–ASOs and free ASOs controls (Fig. S16A†). As a result, significant autophagy activation was observed in mice treated with S2P–CeO2–ASOs, as evidenced by the increased LC3II/LC3I ratio in the aortic lesions (Fig. S16B†). To evaluate whether mTOR silencing had an anti-atherosclerosis effect, the S2P–CeO2–ASOs nanoplatforms were intravenously injected into the ApoE−/− mice twice a week at a dose of 0.5 mg kg−1 ASOs (n = 6). After 12 weeks, the S2P–CeO2–ASOs treatment had the best therapeutic results in limiting atherosclerotic lesion progression in the ApoE−/− mice on a high-fat diet compared to the controls. Examination of the aortic arch with a stereo dissecting microscope (Fig. 5A) showed a marked decrease in the progression of atherogenesis, and en face preparations (Fig. 5B and C) revealed that the oil red-O-stained area was also reduced by 67.1%. In comparison, a smaller decrease in the progression of atherosclerotic lesions (41.3%) was observed in the rapamycin group. The main reason for this is that rapamycin is water insoluble and lacks specificity. Consequently, it has a low bioavailability and cannot confine its therapeutic effects to atherosclerotic regions as seen with the S2P–CeO2–ASOs treatment. Notably, S2P–CeO2–ASOs showed good biocompatibility, as evidenced by the lack of histological changes in tissues of the heart, liver, spleen, lung, or kidneys and no obvious influence on the mouse body weight (Fig. S17 and S18†).Conclusions
In summary, we have successfully developed a H2O2-responsive and plaque-penetrating S2P–CeO2–ASOs nanoplatform for targeted mTOR ASOs delivery. In vitro and in vivo results demonstrated that this nanoplatform has a long blood circulation time, and can efficiently pass through the arterial wall, penetrating into the plaque through specific recognition between S2P ligands and overexpressed stabilin-2 on VSMCs. With the high aspect ratio CeO2 core and H2O2-based competitive coordination, this nanoplatform can effectively escape from endosomes and release therapeutic ASOs “on-demand” into the cytoplasm, leading to a significant suppression of the mTOR expression and atherosclerotic plaque formation in ApoE−/− mice fed with a high-fat diet. After 12 weeks of treatment, the nanoplatform showed a more effective therapeutic outcome compared to mTOR inhibitor rapamycin. Accordingly, this targeted gene-silencing nanoplatform opens up new opportunities for developing CeO2-based nanotheranostics and possibly a robust atherosclerosis treatment strategy for clinical applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21535004, 21390411, 21775092, 21575082, and 21605097).Notes and references
- C. Weber and H. Noels, Nat. Med., 2011, 17, 1410–1422 CrossRef CAS PubMed.
- A. J. Lusis, Nature, 2000, 407, 233–241 CrossRef CAS PubMed.
- X. Yu, Y. Fu, D. Zhang, K. Yin and C. Tang, Clin. Chim. Acta, 2013, 424, 245–252 CrossRef CAS PubMed.
- Y. V. Bobryshev, Micron, 2006, 37, 208–222 CrossRef CAS PubMed.
- B. H. Li, Y. W. Yin, Y. Liu, Y. Pi, L. Guo, X. J. Cao, C. Y. Gao, L. L. Zhang and J. C. Li, Cell Death Dis., 2014, 5, e1182 CrossRef CAS PubMed.
- W. Martinet and G. R. De Meyer, Circ. Res., 2009, 104, 304–317 CrossRef CAS PubMed.
- X. Wang, L. Li, X. Niu, X. Dang, P. Li, L. Qu, X. Bi, Y. Gao, Y. Hu, M. Li, W. Qiao, Z. Peng and L. Pan, DNA Cell Biol., 2014, 33, 198–204 CrossRef PubMed.
- X. Feng, Y. Yuan, C. Wang, J. Feng, Z. Yuan, X. Zhang, W. Sui, P. Hu, P. Zheng and J. Ye, Lipids Health Dis., 2014, 13, 10 CrossRef PubMed.
- I. M. Aparicio, J. Espino, I. Bejarano, A. Gallardo-Soler, M. L. Campo, G. M. Salido, J. A. Pariente, F. J. Pena and J. A. Tapia, Sci. Rep., 2016, 6, 33647 CrossRef CAS PubMed.
- M. Ouimet, V. Franklin, E. Mak, X. Liao, I. Tabas and Y. L. Marcel, Cell Metab., 2011, 13, 655–667 CrossRef CAS PubMed.
- W. Martinet, H. Loof and G. R. Y. D. Meyer, Atherosclerosis, 2014, 233, 601–607 CrossRef CAS PubMed.
- W. Martinet, S. Verheye and G. R. Y. De Meyer, Autophagy, 2014, 3, 241–244 CrossRef.
- R. Watanabe, L. Wei and J. Huang, J. Nucl. Med., 2011, 52, 497–500 CAS.
- Z. Miao, Y. Deng, H. Du, X. Suo, X. Y. Wang, X. Wang, L. Wang, L. Cui and N. Duan, Exp. Ther. Med., 2015, 9, 941–946 CrossRef CAS PubMed.
- D. Benjamin, M. Colombi, C. Moroni and M. N. Hall, Nat. Rev. Drug Discovery, 2011, 10, 868–880 CrossRef CAS PubMed.
- V. J. Dzau, R. C. Braun-Dullaeus and D. G. Sedding, Nat. Med., 2002, 8, 1249–1256 CrossRef CAS PubMed.
- R. Loewith, E. Jacinto, S. Wullschleger, A. Lorberg, J. L. Crespo, D. Bonenfant, W. Oppliger, P. Jenoe and M. N. Hall, Mol. Cell, 2002, 10, 457–468 CrossRef CAS PubMed.
- J. McClellan and M. C. King, Cell, 2010, 141, 210–217 CrossRef CAS PubMed.
- D. Castanotto and J. J. Rossi, Nature, 2009, 457, 426–433 CrossRef CAS PubMed.
- N. Agrawal, P. V. N. Dasaradhi, A. Mohmmed, P. Malhotra, R. K. Bhatnagar and S. K. Mukherjee, Microbiol. Mol. Biol. Rev., 2003, 67, 657–685 CrossRef CAS PubMed.
- B. Esteban-Fernandez de Avila, C. Angell, F. Soto, M. A. Lopez-Ramirez, D. F. Baez, S. Xie, J. Wang and Y. Chen, ACS Nano, 2016, 10, 4997–5005 CrossRef CAS PubMed.
- J. Soutschek, A. Akinc, B. Bramlage, K. Charisse, R. Constien, M. Donoghue, S. Elbashir, A. Geick, P. Hadwiger, J. Harborth, M. John, V. Kesavan, G. Lavine, R. K. Pandey, T. Racie, K. G. Rajeev, I. Röhl, I. Toudjarska, G. Wang, S. Wuschko, D. Bumcrot, V. Koteliansky, S. Limmer, M. Manoharan and H. P. Vornlocher, Nature, 2004, 432, 173–178 CrossRef CAS PubMed.
- J. Wang, Z. Lu, M. G. Wientjes and J. L. S. Au, AAPS J., 2010, 12, 492–503 CrossRef CAS PubMed.
- H. Xu, Q. Li, L. Wang, Y. He, J. Shi, B. Tang and C. Fan, Chem. Soc. Rev., 2014, 43, 2650–2661 RSC.
- H. Pei, X. Zuo, D. Zhu, Q. Huang and C. Fan, Acc. Chem. Res., 2014, 47, 550–559 CrossRef CAS PubMed.
- R. Huschka, A. Barhoumi, Q. Liu, J. A. Roth, L. Ji and N. J. Halas, ACS Nano, 2012, 6, 7681–7691 CrossRef CAS PubMed.
- N. Li, H. Yang, Z. Yu, Y. Li, W. Pan, H. Wang and B. Tang, Chem. Sci., 2017, 8, 2816–2822 RSC.
- C. A. Hong, A. A. Eltoukhy, H. Lee, R. Langer, D. G. Anderson and Y. S. Nam, Angew. Chem., Int. Ed., 2015, 54, 6740–6744 CrossRef CAS PubMed.
- X. Xu, J. Wu, Y. Liu, M. Yu, L. Zhao, X. Zhu, S. Bhasin, Q. Li, E. Ha and J. Shi, Angew. Chem., Int. Ed., 2016, 55, 7091–7094 CrossRef CAS PubMed.
- S. Barua and S. Mitragotri, Nano Today, 2014, 9, 223–243 CrossRef CAS PubMed.
- W. Ngamcherdtrakul, J. Morry, S. Gu, D. J. Castro, S. M. Goodyear, T. Sangvanich, M. M. Reda, R. Lee, S. A. Mihelic, B. L. Beckman, Z. Hu, J. W. Gray and W. Yantasee, Adv. Funct. Mater., 2015, 25, 2646–2659 CrossRef CAS PubMed.
- G. Y. Lee, J. H. Kim, G. T. Oh, B. H. Lee, I. C. Kwon and I. S. Kim, J. Controlled Release, 2011, 155, 211–217 CrossRef CAS PubMed.
- H. Liu, J. Phys. Chem. C, 2009, 113, 3116–3119 CAS.
- M. Ekstrand, M. Gustafsson Trajkovska, J. Perman-Sundelin, P. Fogelstrand, M. Adiels, M. Johansson, L. Mattsson-Hulten, J. Boren and M. Levin, PLoS One, 2015, 10, e0130898 Search PubMed.
- C. H. Byon, J. M. Heath and Y. Chen, Redox Biol., 2016, 9, 244–253 CrossRef CAS PubMed.
- W. Gao, X. Wei, X. Wang, G. Cui, Z. Liu and B. Tang, Chem. Commun., 2016, 52, 3643–3646 RSC.
- B. Liu, Z. Sun, P. J. Huang and J. Liu, J. Am. Chem. Soc., 2015, 137, 1290–1295 CrossRef CAS PubMed.
- H. X. Mai, L. D. Sun, Y. W. Zhang, R. Si, W. Feng, H. P. Zhang, H. C. Liu and C. H. Yan, J. Phys. Chem. B, 2005, 109, 24380–24385 CrossRef CAS PubMed.
- Z. Ji, X. Wang, H. Zhang, S. Lin, H. Meng, B. Sun, S. George, T. Xia, A. E. Nel and J. I. Zink, ACS Nano, 2012, 6, 5366–5380 CrossRef CAS PubMed.
- V. Amirbekian, M. J. Lipinski, K. C. Briley-Saebo, S. Amirbekian, J. G. S. Aguinaldo, D. B. Weinreb, E. Vucic, J. C. Frias, F. Hyafil, V. Mani, E. A. Fisher and Z. A. Fayad, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 961–966 CrossRef CAS PubMed.
- M. E. Lobatto, C. Calcagno, A. Millon, M. L. Senders, F. Fay, P. M. Robson, S. Ramachandran, T. Binderup, M. P. Paridaans and S. Sensarn, ACS Nano, 2015, 9, 1837–1847 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, supporting tables and figures. See DOI: 10.1039/c7sc03582a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |