A framework for understanding student nurses’ experience of chemistry as part of a health science course
Kerrie
Boddey
and
Kevin
de Berg
 *
*
Avondale College of Higher Education, New South Wales, Australia. E-mail: kdeberg@avondale.edu.au
First published on 5th March 2018
Abstract
Twenty-seven first-year nursing students, divided across six focus groups formed on the basis of their past chemistry experience, were interviewed about their chemistry experience as a component of a Health Science unit. Information related to learning and academic performance was able to be established from student conversations resulting in three themes (and associated categories): Connectivity (curriculum, application, and social interaction); Reductivity (nature of chemistry, exposition, and control of learning); and Reflexivity (confidence, anxiety, and goal orientation). The framework proved useful in portraying relationships between themes for conversations related to tutorial sessions, prior knowledge, and chemistry in nursing. The focus groups were representative of the total cohort of students in terms of gender, age, working hours, academic performance, enjoyment level of chemistry, and the extent of the relevance of chemistry to nursing. Implications for chemistry educators, especially those supporting novices, are considered.
Introduction
In Australia undergraduate students of professions such as nursing, engineering, veterinary science, optometry and forensics, which demand some knowledge of the sciences, are usually required to undertake introductory chemistry as part of their course. Such students will come to chemistry with a variety of academic skills, a diverse chemistry background, and different orientations and attitudes to chemistry as a subject. Some students have been known to exhibit the symptoms of chemophobia, an inherent desire to have nothing to do with chemistry (Billington et al., 2008). It is not surprising then why chemistry educators have devoted their research skills towards exploring ways in which interest and academic performance in introductory chemistry at the tertiary level can be enhanced. A sample of the research effort from 2003 to 2016 is shown in Table 1.| Research work | Key concepts studied | Methodology |
|---|---|---|
| Abbreviations: Quant = quantitative; Qual = qualitative; CAEQ = chemistry attitudes and experiences questionnaire; SE = self-efficacy; EFA = evolving factor analysis; CFA = confirmatory factor analysis; SC = self-concept; PVOR = purdue visualisation of rotations test; VSEPR = valence shell electron pair repulsion test; ANOVA = analysis of variance; SEM = structural equation modelling; CCSS = college chemistry self-efficacy scale; MSLQ = motivated strategies for learning questionnaire; Gen Chem = general chemistry; Org Chem = organic chemistry; ACT = American College-entrance Test; HLM = Hierarchical Linear Model. | ||
| Quantitative only | ||
| Zusho et al. (2003) | Motivation: self-efficacy; task value belief; goal orientation; interest; anxiety. | Quant: 3 survey instruments at 3 times (n = 458). |
| Chemistry achievement | Qual: — | |
| Turner and Lindsay (2003) | Gen Chem grade; Org Chem grade; ACT math score; science reasoning score; spatial visual score; confidence; anxiety; effectance motivation; usefulness | Quant: spatial visual test (PVOR); chemistry attitudes inventory. [n = 93 (1999); n = 100 (2000)] |
| Qual: — | ||
| Uzuntiryaki and Aydin (2009) | Chemistry self-efficacy for cognitive, psychomotor, everyday applications | Quant: CCSS: EFA (n = 363); CFA (n = 353) |
| Qual: — | ||
| Seery (2009) | Prior chemistry knowledge; aptitude; academic performance; attendance; study; interest | Quant: questionnaire on student perceptions; correlations; regression (n = 89) |
| Qual: — | ||
| Lewis et al. (2009) | Self concept; academic performance; enjoyment | Quant: SC inventory; construct and content validated (n = 411) |
| Qual: — | ||
| Aydin et al. (2011) | Chemistry anxiety; chemistry self-efficacy; attitude to math; task value; rehearsal; elaboration; organisation; motivation; metacognitive self-regulation | Quant: MSLQ; CCSS scale; chemistry anxiety scale; attitude to Math scale (n = 518) |
| Qual: — | ||
| Bell and Volckmann (2011) | Confidence; Gen Chem knowledge; exam performance | Quant: knowledge survey pre- and post (n = 164) |
| Qual: — | ||
| Merchant et al. (2012) | Spatial orientation; self-efficacy; learner characteristics; 3D virtual environment | Quant: SEM; VSEPR test; PVOR test; CFA (n = 204) |
| Qual: — | ||
| Xu et al. (2013) | Attitudes to chemistry; chemistry achievement; math ability; prior conceptual knowledge | Quant: SEM (n = 963) |
| Qual: — | ||
| Villafane et al. (2014) | Chemistry self-efficacy; cultural background; gender | Quant: CAEQ (n = 409); multilevel modelling |
| Qual: — | ||
| Gonzalez and Paolini (2015) | Chemistry grade; autonomy-support; expectancy; importance; value; utility; interest; metacognitive strategies (planning, monitoring, evaluating) | Quant: SEM (n = 503) |
| Qual: — | ||
| Ferrell et al. (2016) | Achievement motivation; chemistry performance; self-efficacy; interest; effort beliefs | Quant: multiple regression; path analysis (n = 170) |
| Qual: — | ||
| Qualitative only | ||
| Brown et al. (2012) | Chemistry concepts relevant to nursing clinical practice; anxiety of failure; math ability; prior science background; academic skills; cognitive load; instructor teaching style | Quant: — |
| Qual: person to person semi-structured interviews with 4 chem instructors, 6 nurse educators, 4 nursing graduates (n = 14) | ||
| Quantitative and qualitative | ||
| Dalgety et al. (2003) | Attitudes; chemistry self-efficacy; chemistry learning experiences | Quant: development of CAEQ (n = 669). |
| Qual: semi-structured interviews with faculty, graduate students relating face validity of items on questionnaire. | ||
| Dalgety and Coll (2006) | Chemistry self-efficacy; intention to enrol in more chemistry; prior experience and achievement; effort; exam stress | Quant: SE section of CAEQ 3 times (n1 = 126; n2 = 102; n3 = 84) |
| Qual: interviews with 19 students; 14 reinterviewed at end of sem 1; 9 at the end of sem 2. Data analysed using a thematic approach | ||
| El-Farargy (2009) | Previous experience; college experience; teaching strategies; study techniques; lab work; student materials; attitudes; relevance; future intentions; chemistry curriculum; applications-based chemistry | Quant: Likert style questionnaire (n = 23) |
| Qual: two focus group interviews (30–40 min) (n = 8, 10) | ||
| Taasoobshirazi and Glynn (2009) | Problem solving; problem conceptualization; problem solution; chemistry self-efficacy | Quant: SEM (n = 101) |
| Qual: explanations in writing; some asked orally | ||
| Brooks and Koretsky (2011) | Group discussion; confidence; achievement | Quant: confidence scale (n = 64) |
| Qual: written explanations of answers coded; group discussion (self-select 2/3 students) between initial and final answers | ||
| Schmid et al. (2012) | Bridging course; prior chemistry experience; self-efficacy; chemistry achievement | Quant: surveys (n = 216 week 3, 144 week 13) |
| Qual: interviews (n = 12) | ||
| Ferrell and Barbera, (2015) | Chemistry self-efficacy; interest; effort beliefs | Quant: SE scale from CCSS; interest scale (Harackiewicz); effort beliefs scale (Jones); CFA (n1 = 373; n2 = 294) |
| Qual: interviews with students to determine readability of scale items; validity of items | ||
| Boddey and de Berg (2015) | Prior chemistry experience; academic performance of nursing students | Quant: t-tests; chi-square; ANOVA (n = 101) |
| Qual: focus group interviews (n = 27) | ||
| Sinapuelas and Stacy (2015) | Learning approaches for exam preparation outside the classroom | Quant: survey (n = 428); HLM |
| Qual: individual interviews at 3 stages (n = 61) | ||
Fourteen of the twenty-two studies listed in Table 1 focus on self-efficacy or the more general term, confidence. Chemistry self-efficacy has been defined as “students’ beliefs about the extent to which they are capable of performing specific chemistry tasks” (Cheung, 2015, pp. 102–103). One of the reasons for its popularity amongst researchers is that, as indicated by at least three of the studies in Table 1, chemistry self-efficacy is either a major predictor of chemistry achievement or is highly correlated with it (Ferrell et al., 2016; Merchant et al., 2012; Zusho et al., 2003). Typically, quantitative measures of chemistry self-efficacy derived from the Chemistry Attitudes and Experiences Questionnaire (CAEQ), developed by Dalgety et al. (2003), and the College Chemistry Self-Efficacy Scale (CCSS), developed by Uzuntiryaki and Aydin (2009), have been used. Bandura (1997) cautions researchers to distinguish between the concepts self-efficacy and self-concept: self-efficacy being a belief in one's capability to perform a task while self-concept being a judgment of worth based on a comparison with significant others. One of the references in Table 1 (Lewis et al., 2009) uses a self-concept inventory developed by Bauer (2005) to report that chemistry students with a high self-concept score achieved a grade about 5% higher than predicted on the ACS (American Chemical Society) exam. But most of the studies have focussed on self-efficacy.
Not all studies of chemistry achievement consider the impact of chemistry self-efficacy. In a model constructed by Xu et al. (2013) concerned with factors affecting the chemistry achievement of 963 general chemistry students in a large public university, it was found that 69% of the variance in chemistry achievement could be attributed to prior conceptual knowledge, math ability, and attitude towards chemistry. The relationships are shown in Fig. 1. All three predictors have a significant impact on chemistry achievement with prior knowledge having the biggest impact and attitude towards chemistry having the smallest impact according to the path coefficients. The three predictors are positively correlated with each other indicating the benefits of focussing on multiple aspects of learning in the classroom as a way of supporting student performance.
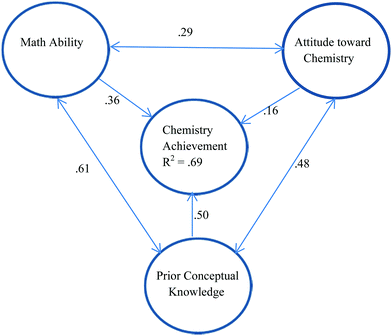 | ||
| Fig. 1 Model for chemistry achievement based on Xu et al. (2013) which explains 69% of the variance in achievement. Prior Conceptual Knowledge is shown to have the biggest impact on chemistry achievement and Attitudes toward Chemistry the least impact. Single-headed arrows relate to standardized path coefficients while double-headed arrows relate to correlation coefficients (r). | ||
Gonzalez and Paolini (2015) considered a different model (Fig. 2) for chemistry achievement which involved examining the impact of metacognitive learning strategies, motivation variables, and perception of teaching style on the grade in chemistry. Fifty-seven percent of the variance in chemistry achievement could be attributed to the model with the motivational variables of importance and utility, and the metacognitive learning strategies of planning, monitoring, and evaluating having direct impact. The impact of the teaching style, described as autonomy-support (i.e. student-centred style), on the chemistry grade was mediated through the motivational and metacognitive variables, and the impact of the motivational variables of expectancy and interest on grade was mediated through the metacognitive variables. Self-efficacy was not considered in this model.
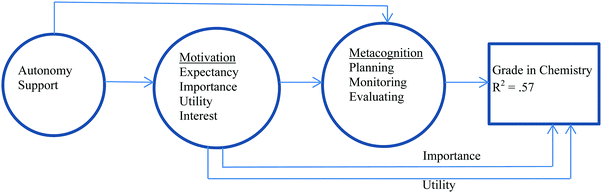 | ||
| Fig. 2 A condensed model of chemistry achievement according to Gonzalez and Paolini (2015) in which 57% of the variance in grade can be explained by the model. | ||
Aydin et al. (2011) chose a structural model that focuses not on chemistry achievement but on chemistry self-efficacy and chemistry anxiety and looks at the impact of cognitive, metacognitive learning strategies, and task value on chemistry self-efficacy and anxiety. Metacognitive self-regulation (MSR) directly and positively impacts chemistry self-efficacy while chemistry anxiety directly and negatively impacts chemistry self-efficacy. The model shows that when students learn to plan, monitor, and regulate their own study programs, self-efficacy can increase directly or through decreasing anxiety. Task value, that is, the degree of importance attached to learning by the student, and the cognitive strategies of rehearsing, elaborating, and organising, indirectly impact self-efficacy through metacognitive self-regulation strategies. However only 19.5% of the variance in Chemistry Anxiety and only 34.6% of the variance in Chemistry Self-efficacy can be explained by this model. The model is illustrated in Fig. 3.
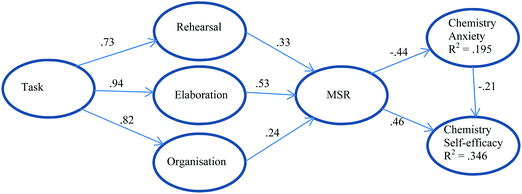 | ||
| Fig. 3 A model for chemistry self-efficacy and chemistry anxiety based on Aydin et al. (2011). Standardized path coefficients are shown. The model explains 34.6% of the variance in self-efficacy and 19.5% of the variance in anxiety. | ||
It is not possible for a single model to include all the variables represented in Table 1. The researcher chooses which variables to consider and then sets about establishing a relationship between the variables commonly using statistical modelling. While such an approach adds an element of rigour, the approach has inherent limitations in that one has to limit experimentally which variables are to be considered. This limitation was recognized by Xu et al. (2013, p. 197) after having developed the model shown in Fig. 1: “More work is needed to capture the broad range of factors that are potentially important to understand chemistry learning”. Four of the studies listed in Table 1 (Ferrell et al., 2016; Gonzalez and Paolini, 2015; Merchant et al., 2012; Villafane et al., 2014) recognize the limitations of a statistical quantitative approach to understanding the student experience of introductory chemistry and suggest that qualitative studies be implemented to further our understanding.
For example, after a quantitative study of chemistry self-efficacy across different student groups, Villafane et al. (2014, p. 123) comment as follows: “Third, the findings are based on quantitative data only, which limits the understanding of the differences in chemistry self-efficacy beliefs among the different groups of students……interviews and observations would be a helpful and important aspect of future studies in this area”. Twelve of the twenty-two studies shown in Table 1 are purely quantitative studies and none of the ten studies using a qualitative investigation attempted an analysis of student experiences of introductory chemistry in order to derive a qualitative framework or model purely on the basis of conversational data. Two of the qualitative studies in Table 1 (Dalgety et al., 2003; Ferrell and Barbera, 2015) conducted interviews for the purpose of determining the reliability and validity of questionnaire items. One of the qualitative studies (Brown et al., 2012) did not focus on data from undergraduate students. In the study by Schmid et al. (2012), twelve first-year chemistry students were interviewed after their exam for the purpose of gauging their level of understanding of chemistry concepts. Sixty-one students were individually interviewed in the study by Sinapuelas and Stacy (2015) to ascertain the learning approach used when studying for exams.
El-Faragy (2009) did analyse the data obtained from two focus group interviews (8 to 10 students each) with 18 nursing students studying introductory chemistry. The data were analysed using data reduction techniques into the broad categories: previous experiences, college experiences, teaching strategies, study techniques, lab work, student materials, attitudes, relevance and difficulties, and future intentions. The El-Faragy study, while valuable in its own right, was not concerned with establishing a framework or model using these categories. This paper reports on an investigation of nursing students’ conversations about their experiences with introductory chemistry as part of a Health Science unit in order to determine if a qualitative framework or model emerges from such an investigation. The content of the paper is a qualitative subset of a much broader mixed method study. The conversations are based on some of the key concepts that have occupied the previous studies shown in the middle column of Table 1 in both quantitative and qualitative studies. The study has been approached with two research questions in mind:
1. What relationships, if any, between key concepts such as chemistry self-efficacy, anxiety, and prior chemistry experience emerge from nursing student conversations about their experiences of introductory chemistry and what other factors and relationships might emerge?
2. Can a generalised framework be established which encompasses the rich and complex nature of the relationships?
Theoretical foundations
As students engage in conversation about their learning experiences of introductory chemistry, important foundational principles proposed by Bandura (1986, 1997) and Johnstone (1997, 2000, 2006) can be used to help interpret the data. These are Social Cognitive Theory for accessing important ideas in the role of personal factors, behaviour, and the environment in learning and the Chemistry Triplet for accessing important ideas in the nature of chemical knowledge.Social cognitive theory
Albert Bandura's Social Cognitive Theory (SCT) appeared in the late 1970s and was originally applied to phobias. It has since been used to explain behaviour in many fields including sport and the workplace and increasingly in education, particularly with respect to academic motivation (Pajares, 1996). According to SCT, “human agency [i.e. intentional acts] operates within a triadic interdependent causal structure” (Bandura, 1997, p. 6) where internal personal factors (cognitive, affective and biological), behaviour and environmental events have a bidirectional influence on each other, referred to as ‘reciprocal causation’, the relative strength of which will vary according to circumstances (see Fig. 4). It may take time for the causal effect to be felt and there will be personal variation in the interpretation of and reaction to the three components. Consequently, individuals are proactive rather than simply reactive to environmental forces and are viewed as both products and producers of their environment. Through self-referent processing of social influences and accomplishments, individuals can exercise some control over their thoughts, feelings and actions. The extent of the control will depend on whether the environment is imposed, selected or created and how modifiable it is. In the context of education, teachers can assist students by acting on any of the triadic factors (Pajares, 2002).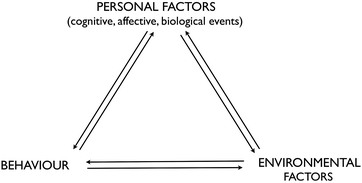 | ||
| Fig. 4 Relationship between the three major classes of determinants in Bandura's triadic reciprocality (Bandura, 1986, as cited in Bandura, 1997). | ||
People's judgments of their capabilities to exercise control over life events play a major role in determining behaviour. Self-efficacy, then, is embedded in social cognitive theory and plays a paramount role in how an individual will organise, create and manage the environment in order to bring about desired changes. Perceived self-efficacy will influence choices, effort expended, duration of perseverance, emotional reactions and accomplishment (Bandura, 1997). Bandura asserts that without belief in the power to achieve, a person will not make an attempt. A highly efficacious student will perceive difficult tasks as a challenge and remain relatively calm, set challenging goals, maintain commitment and effort in the face of adversity or failure, rebound from setbacks and attribute failure to insufficient effort or lack of acquirable skills. However, a highly efficacious student may show a lack of persistence if the task is believed to be too easy (Salomon, 1984). In contrast, a student who lacks confidence in his/her ability to accomplish a challenging task will dwell on personal deficiencies, display low commitment, give up quickly, believe things are tougher than they really are, view failure as a deficiency in aptitude, be more susceptible to stress, anxiety and depression and simply try to avoid the task (Bandura, 1994; Pajares, 1996).
Bandura (1994, 1997), recognising that self-efficacy is responsive to changes, has hypothesised the existence of four antecedents of self-efficacy beliefs. He explains that it is the way information is interpreted through cognitive processing and reflective thought from these antecedents that is of significance. The most influential source is enactive mastery experiences. Successful experiences that come as a result of persistent effort will build robust self-efficacy resulting in participation in subsequent tasks. Failure undermines self-efficacy, particularly if a strong sense of self-efficacy has not yet been established. Judgment of perceived competence will be revised based on attainment in such a task. Mastery experiences provide the most authentic evidence of one's capabilities.
Secondly, self-efficacy can be affected by vicarious experiences provided by social models. Observing others similar to oneself succeed in a task by sustained effort enhances the belief that one can also succeed in that task. Social models assessed as being more capable than oneself are usually discounted as irrelevant. While this is a much weaker source of self-belief than mastery experiences, it can be very significant when there is limited prior experience and the role model possesses similar characteristics to the learner.
Self-efficacy beliefs are also informed by social persuasion that occurs when a respected person considered credible and trustworthy, verbally or non-verbally persuades the student that they possess the capabilities to master the task. In an educational setting, teachers play a crucial role as credible persuaders in providing evaluative feedback in the form of encouragement and suggestions for improvement. As with vicarious experiences, this source will be more powerful if experience is limited.
Finally, emotional and physiological states contribute to efficacy about oneself. Feelings such as anxiety, stress, fatigue and mood that occur as a result of contemplation or engagement are interpreted in light of the complexity of the task and existing self-efficacy. It is not so much the strength of these reactions, but how they are perceived and interpreted that is important. Arousal may be viewed as energizing or debilitating depending on whether it is perceived as originating from a common reaction or personal inadequacies, facilitating judgments of confidence accordingly.
Hence, self-efficacy is not merely a “mechanical audit of one's performances,” (Bandura, 1997, p. 81) but is also moulded by socially mediated experiences such as mastery experience, vicarious experience, social persuasion and emotional and physiological states. It is the cognitive processing of selection, interpretation, and integration of information through reflective thought that determines self-efficacy judgments. As such, judgments of personal efficacy can be used to assess instructional intervention along with differences between individuals and groups.
The nature of chemistry: the chemistry triplet
Johnstone (1991) envisaged the macroscopic, the sub-microscopic, and the representational or symbolic levels of chemistry as occupying the three corners of a triangle. When a student is embedded within the triangle, cognitive overload can easily happen if a student has to think simultaneously of the ‘macro’, the ‘sub-micro’, and the different ways of representing a substance at these levels. Difficulties in learning chemistry can be understood if one realizes that a chemist or a chemistry curriculum will often move from a macroscopic observable substance level to a sub-microscopic invisible atomic/molecular level without any warning. What adds to the difficulty is that our formulaic representations of a substance, NaCl for common salt for example, can be used to represent the macroscopic or the sub-microscopic form. The word ‘substance’ as taught in introductory chemistry is more than likely taken to mean ‘pure substance’ and this is already a major abstraction from the real-life experience of students with material substances that are more than likely not pure. Taber (2013, p. 159) warns us that “the conceptual demand is high even at the ‘macroscopic’ corner of the subject”. According to Johnstone (2000, p. 9), “It is psychological folly to introduce learners to ideas at all three levels simultaneously. Herein lies the origins of many misconceptions. The trained chemist can keep these three in balance, but not the learner”.Course of study
This paper relates to a study of the chemistry experiences of nursing students undertaking a Bachelor of Nursing degree at a College of Higher Education in New South Wales Australia. Health Science I is a core unit taught in the first semester of the degree program made up of approximately 60% chemistry, the remainder being microbiology. The chemistry component was delivered face-to-face in the first part of the semester, consisting of 20 lectures, 7 tutorials, and 4 two-hour laboratory sessions over a period of 7 weeks. Laboratory and theoretical work contributed 25% and 75% respectively to the final assessment. Topics covered included basic atomic structure and ion formation; writing chemical formulae; ionic and covalent bonding; basic organic molecules and polarity; solutions including concentration, diffusion and osmosis; acids and bases; equilibrium and buffers; biomolecules and reaction rates. While these topics may also constitute, at least in part, the content of a first-year university general and organic chemistry curriculum, the emphasis in this unit is the nursing context where appropriate. For example, in the section on organic and biomolecules, the emphasis is on the recognition and properties of carbohydrates, lipids, proteins, and nucleic acids which are relevant to a nurse's study of anatomy and physiology. Osmolarity calculations are performed in the context of the impact of osmosis on the integrity of red blood cells. Acid–Base-Buffer chemistry is studied with an emphasis on respiratory and metabolic acidosis and alkalosis. Laboratory sessions included the performance of chemical tests on foods for detecting the presence of carbohydrates, lipids, and proteins and the construction of models of molecules using a molecular model kit. There is no prerequisite for the unit so no prior chemistry knowledge is assumed.A 3 day chemistry bridging course was offered on a voluntary basis to students who had not taken chemistry in senior high school. The course was conducted in the week prior to the start of the first semester. It comprised seven 50 minute lectures each followed by a 50 minute tutorial where students worked through exercises related to the preceding lecture material. In addition, an 80 minute laboratory session was conducted on the second and third days. The bridging course focussed primarily on the following concepts at a basic level: atomic structure, chemical formulae, bonding, the periodic table, acids and bases, the mole, and organic molecules. These concepts were repeated in Health Science I but at a faster presentation pace and in most instances, at a higher conceptual level.
Method
In this project the recording and evaluating of student conversations about their experiences of introductory chemistry was an approach based partly on grounded theory (Glaser, 1992; Charmaz, 2006) and partly on phenomenography (Marton, 1981). The grounded theory component related to the fact that the methodology was an inductive one based on the emergence of conceptual categories from the recorded data of student ideas expressed in conversation. The approach was not purely a grounded theory one in that the interview protocol was partly guided by categories already published in the literature. The phenomenographic component related to the fact that the world of chemistry as the learner describes it rather than how the scientist observes it is taken seriously (Richardson, 1999). It is understood that the approach here is not purely phenomenographic in that the researcher inherently brings their own experience of chemistry to bear on interpreting student experiences, but every effort was made to give priority to the students’ experience of chemistry and their interpretation of it. Student conversations took place in the context of focus group interviews and individual interviews.Embedded in the design of the interview sessions was the concept of the teacher as researcher (Cochrane-Smith and Lytle, 1999), in particular, the teacher–researcher as one who contemplates the learning of chemistry in and as part of a community of first-year nursing students. While such a design always raises the question of researcher bias, every effort was made to include students across the broad spectrum of academic achievement, level of enjoyment of chemistry, and prior chemistry experience in the interview sessions. The potential for researcher bias was addressed as part of a submission to the institution's research ethics committee from which approval was granted for the research project.
Participants
Demographic data was collected by survey from 101 volunteer participants in the overall mixed method study from Health Science I and from this group of participants, six focus groups were formed based on prior chemistry experience. The idea of forming focus groups containing participants with a common background and shared experience is based on the observations made by Barbour (2008) and Creswell (2008). That is, focus groups work well when interviewees have something in common and are more likely to cooperate with each other. The 101 participants fell into three groups based on previous exposure to chemistry as shown in Table 2.| Prior chemistry experience | Classification of prior chemistry experience groups | Number of students |
|---|---|---|
| Senior chemistry SC | Students who completed chemistry in Years 11 or 12 | 26 |
| Bridging chemistry BC | Students who did not complete Year 11 or 12 chemistry but completed the 3-day chemistry bridging course | 31 |
| Poor chemistry PC | Students who did not complete Year 11 or 12 chemistry nor the chemistry bridging course | 44 |
Two focus groups for each prior chemistry experience category; senior chemistry SC, bridging chemistry BC, poor chemistry background PC; each consisting of 3–5 students was used to create six focus groups (N = 27). Using information supplied by the survey, further selection criteria reflecting the range in age, gender, and results in class Tests 1 and 2 were employed to ensure a level of diversity in each group. Academic performance was based on student performance on two class chemistry tests and an end of semester chemistry examination. The contribution of each to the final mark was: Test 1 (28.6%), Test 2 (14.3%), and the Final Exam (50.1%). The tests and exam consisted of multiple-choice and short-answer questions. For the purpose of this research a laboratory mark (worth 25% of the semester grade) was not included in the academic performance mark as students received a lot of assistance in the laboratory sessions including the writing of their reports. Further, laboratory marks did not correlate well with test marks. Seery (2009) also observed a weak correlation between laboratory marks and theory exam marks in tertiary chemistry. Student academic performance was categorised as low L (<45%), average Av (45–69%), or high H (≥70%).
Each of the six focus group interviews lasted between 25 and 45 minutes and was audio and video recorded in a relaxed and informal environment. An interview protocol was created to guide the discussion (see Fig. 5). In addition, focus group students were asked to answer some questions about the level of difficulty experienced in studying chemistry, and the whole cohort of students was asked to complete survey items dealing with the level of enjoyment when studying chemistry, the level of applicability of chemistry to nursing, and the grade they expected to receive in chemistry. These items are shown in Fig. 6. Participants were assigned pseudonyms consistent with their prior chemistry experience group in order to simplify the identification of comments reported. SC student pseudonyms begin with ‘S’ (e.g. Sarina), BC students begin with ‘B’ (e.g. Bella), and PC students begin with ‘P’ (e.g. Paul).
The group interviews were conducted after the completion of the chemistry component of Health Science I. Following analysis of group interviews, one member from each focus group was selected for an individual interview to allow for a deeper investigation into issues raised within the group interviews and to check the researcher's analysis of the interview data for validity. This individual was selected on the basis that they appeared very comfortable sharing ideas and had the capacity to verbally articulate their ideas. It is understood that selecting one student for an interview can bias the orientation of the research. Again, there was an attempt to ensure that the students selected for individual interviews reflected the diversity within the focus groups with respect to gender, academic performance and enjoyment level of chemistry.
To minimise focus group bias there was an attempt to ensure that the diversity in the focus groups reflected as closely as possible the diversity in the prior chemistry experience groups in Table 2. Focus group data for gender, age, work hours, academic performance, enjoyment level of chemistry, and degree of importance of chemistry for nursing are given in Table 3 and data showing how this compares with the total cohort of students are given in Table 4. It can be seen in Table 3 that there is a diverse representation across all categories within each prior experience group and the data in Table 4 indicate that the SC, BC, and PC students in the focus groups reasonably reflect the diversity in the total cohort of SC, BC, and PC student groups. That is, not all students in the focus groups were high achievers enthusiastic about chemistry and its importance for nursing. The whole range of gender, age, working hours, academic performance, enjoyment, and importance categories are represented.
| Student | Gender | Age | Work | AP | Enjoy 1 | Enjoy 2 | Import 1 | Import 2 |
|---|---|---|---|---|---|---|---|---|
| Age categories: 1 = 17–18; 2 = 19–20; 3 = 21–24; 4 = 25–34; 5 = 35+. Work: average number of hours worked each week during the chemistry component of Health Science I. AP = academic performance: L = low (<45%); Av = average (45–69%); H = high (70+%). Enjoy 1 = level of enjoyment of chemistry at the beginning of the semester: 0 = hated it, 4 = loved it; Enjoy 2 = level of enjoyment of chemistry at the conclusion of the chemistry component of Health Science I: 0 = hated it, 4 = loved it. Import 1 = level of importance chemistry is to nursing at the beginning of the semester: 0 = not at all important, 4 = essential. Import 2 = level of importance chemistry is to nursing at the conclusion of the chemistry component of Health Science I: 0 = not at all important, 4 = essential. | ||||||||
| Paige | F | 1 | 6 | H | 2 | 4 | 3 | 2 |
| Pam | F | 5 | 27 | L | 3 | 0 | 4 | 0 |
| Paris | F | 3 | 30 | L | 1 | 2 | 2 | 2 |
| Paul | M | 3 | 12 | H | 2 | 3 | 4 | 4 |
| Paula | F | 4 | 0 | L | 1 | 1 | 4 | 1 |
| Phebe | F | 1 | 35 | L | 0 | 1 | 1 | 1 |
| Pierce | M | 3 | 0 | Av | 2 | 3 | 4 | 3 |
| Pippa | F | 1 | 10 | Av | 2 | 3 | 3 | 3 |
| Polly | F | 3 | 6 | L | 2 | 3 | 3 | 3 |
| Prue | F | 1 | 1 | Av | 2 | 4 | 3 | 3 |
| Becky | F | 2 | 0 | H | 3 | 4 | 2 | 3 |
| Bella | F | 1 | 12 | H | 3 | 3 | 2 | 2 |
| Bernice | F | 2 | 0 | Av | 3 | 2 | 3 | 1 |
| Beryl | F | 5 | 20 | H | 2 | 4 | 4 | 4 |
| Beth | F | 5 | 0 | H | 4 | 3 | 4 | 4 |
| Bree | F | 1 | 10 | L | 3 | 3 | 4 | 4 |
| Brett | M | 1 | 4 | H | 2 | 4 | 3 | 3 |
| Bridget | F | 1 | 0 | Av | 0 | 0 | 1 | 1 |
| Brittney | F | 1 | 0 | Av | 3 | 3 | 2 | 2 |
| Bronte | F | 5 | 40 | L | 2 | 4 | 2 | 2 |
| Samuel | M | 3 | 2 | Av | 4 | 3 | 3 | 3 |
| Sandy | F | 4 | 20 | H | 3 | 4 | 4 | 4 |
| Sarina | F | 3 | 27 | L | 3 | 3 | 3 | 1 |
| Simon | M | 2 | 5 | H | 3 | 3 | 3 | 3 |
| Sofia | F | 1 | 0 | H | 0 | 3 | 2 | 2 |
| Sonia | F | 1 | 0 | Av | 0 | 2 | 1 | 1 |
| Soraya | F | 1 | 0 | H | 0 | 3 | 2 | 4 |
| Category | Prior chemistry category | |||||
|---|---|---|---|---|---|---|
| SC | BC | PC | ||||
| FG | All | FG | All | FG | All | |
| SC = students studied senior chemistry; BC = students who did the bridging course; PC = students who did not study senior chemistry or attend the bridging course; AP = academic performance; H = high performance (≥70%); Av = average performance (45–69%); L = low performance (<45%); Enjoy 1 = enjoyment of chemistry at the beginning of the semester: 0 = hated it, 4 = loved it; Enjoy 2 = enjoyment of chemistry at the conclusion of the chemistry component of Health Science I: 0 = hated it, 4 = loved it; Import 1 = importance chemistry is to nursing at the beginning of the semester: 0 = not at all important, 4 = essential; Import 2 = importance chemistry is to nursing at the conclusion of the chemistry component of Health Science I: 0 = not at all important, 4 = essential. | ||||||
| No. students | 7 | 26 | 10 | 31 | 10 | 44 |
| No. males | 2 | 2 | 1 | 2 | 2 | 5 |
| Age: % ≤20 | 57 | 77 | 70 | 63 | 40 | 43 |
| Working: ≥10 h per week | 29 | 19 | 40 | 43 | 50 | 52 |
| AP: % H | 57 | 46 | 50 | 37 | 20 | 21 |
| AP: % Av | 29 | 42 | 30 | 30 | 30 | 27 |
| AP: % L | 14 | 12 | 20 | 33 | 50 | 52 |
| Enjoy 1: range | 0–4 | 0–4 | 0–4 | 0–4 | 0–3 | 0–3 |
| Enjoy 2: range | 2–4 | 1–4 | 0–4 | 0–4 | 0–4 | 0–4 |
| Import 1: range | 1–4 | 0–4 | 1–4 | 0–4 | 1–4 | 1–4 |
| Import 2: range | 1–4 | 1–4 | 1–4 | 1–4 | 0–4 | 0–4 |
Data analysis
Creswell (2008) points out that there is no single set approach to the analysis of interview data, particularly since the analyst brings his or her own experience to the process. In order to maximise engagement with the data (Mertens, 2010), interviews were personally transcribed by the researcher. Transcripts, including group dynamics for the focus group interviews, were read several times “to immerse oneself in the data and gain a sense of the possibilities” (Lodico et al., 2006, p. 304) for classification. The inductive line-by-line process of coding for a BC focus group transcript is shown in Fig. 7. As suggested by Williams and Katz (2001), core insights, common phrases and words, mood and non-verbal clues were all considered. Scanning through the transcript in Fig. 7, one can see ideas emerging related to the impact of the teacher on anxiety, the nature of chemistry, the importance of understanding, and the impact of relating chemistry to everyday examples.After initial coding and memoing (writing reflective notes about the data) on paper, the transcripts were entered into NVivo. This was to facilitate the processes of locating and comparing codes and categories (Patton, 2002). It also provided further opportunity to re-examine and refine the emerging code framework and determine its resilience (Mertens, 2010). Additional memos were written during this constant comparative phase. Once initial analysis of all focus group transcripts was done, participant comments were systematically scrutinised (Barbour, 2008), yielding additional insights which resulted in further amendment and coalescence of codes and categories. Numerous sections of text incorporated more than one code and the overlap of codes was useful in determining important relationships.
The approach used in this study was more cyclic than linear with initial codes progressively placed into categories as patterns and relationships surfaced. The codes and categories were clustered into themes and relationships were then explored to determine if a general framework could be developed. The relationships and general framework which emerged from the analysis of the transcripts will form the basis of the Results and discussion section.
The trustworthiness of the codes, themes, categories, and relationships deduced was enhanced by inviting a chemistry educator with experience in teaching the Health Science I unit to apply the coding frame to two randomly selected transcripts. This was then compared with that produced by the researcher. A few codes/categories required clarification (e.g. the nature of chemistry), and an additional code, ‘effort to learn’, was suggested which was subsequently adopted. An individual interviewee from each focus group was asked to comment on the legitimacy of the codes, categories, themes, and relationships presented in the final framework from their point of view. This helped to provide some indication of the extent to which the framework can be generally applied and also helped to shed some credibility on the proposed framework.
Results and discussion
The mean and proportion scores for the answers to the questions in Fig. 6 for the focus group participants will act as important background information for the student conversations and are given in Table 5. One can see that 93% of students in the focus groups rated chemistry as demanding more than an average mental effort to achieve an understanding of the subject. The mean for mental effort in chemistry (7.04) was significantly higher (p < 0.01) than the mean for mental effort in psychology and sociology (5.00). Eighty-one percent of focus group students regarded chemistry content as of above average difficulty. By the conclusion of the chemistry component of Health Science I over 50% of the focus group students rated their level of enjoyment of chemistry as above average, the level of relevance of chemistry to nursing as above average, and their expected grade as equal to a credit or above. The results in Table 5 will continue to guide the discussion that follows.| Question | SC initial | SC final | BC initial | BC final | PC initial | PC final | Mean (n = 27) | p ≥ 6 (n = 27) | p ≥ 3 (n = 27) |
|---|---|---|---|---|---|---|---|---|---|
| SC = students studied senior chemistry; BC = students did the bridging course; PC = students did not study senior chemistry or attend the bridging course; mean and proportion scores are available for the beginning and end of the semester for B, C, and D questions. Questions labelled A were asked only at the end of the semester. Questions labelled A were scored from ‘1’ (none/not at all) to ‘9’ (extremely high); B was scored from ‘0’ (hated it) to ‘4’ (loved it); C was scored from ‘0’ (not at all important) to ‘4’ (essential); and D was scored from ‘1’ (fail) to ‘2’ (pass) to ‘3’ (credit) to ‘4’ (distinction) to ‘5’ (high distinction). | |||||||||
| A1: mental effort in chemistry | 6.29 | 6.90 | 7.70 | 7.04 | 0.93 | ||||
| A2: mental effort in psychology and sociology | 5.43 | 5.20 | 4.50 | 5.00 | 0.30 | ||||
| A3: difficulty of chemistry content | 5.71 | 6.70 | 7.10 | 6.59 | 0.81 | ||||
| A4: difficulty learning chemistry | 4.86 | 6.30 | 7.20 | 6.26 | 0.59 | ||||
| B: level of enjoyment of chemistry | 1.86 | 3.00 | 2.50 | 3.00 | 1.70 | 2.40 | 2.04; 2.78 | 0.41; 0.74 | |
| C: relevance of chemistry to nursing | 2.57 | 2.57 | 2.70 | 2.60 | 3.10 | 2.20 | 2.81; 2.44 | 0.63; 0.52 | |
| D: expected grade in chemistry | 2.86 | 3.43 | 2.90 | 3.00 | 2.40 | 2.70 | 2.70; 3.00 | 0.56; 0.67 | |
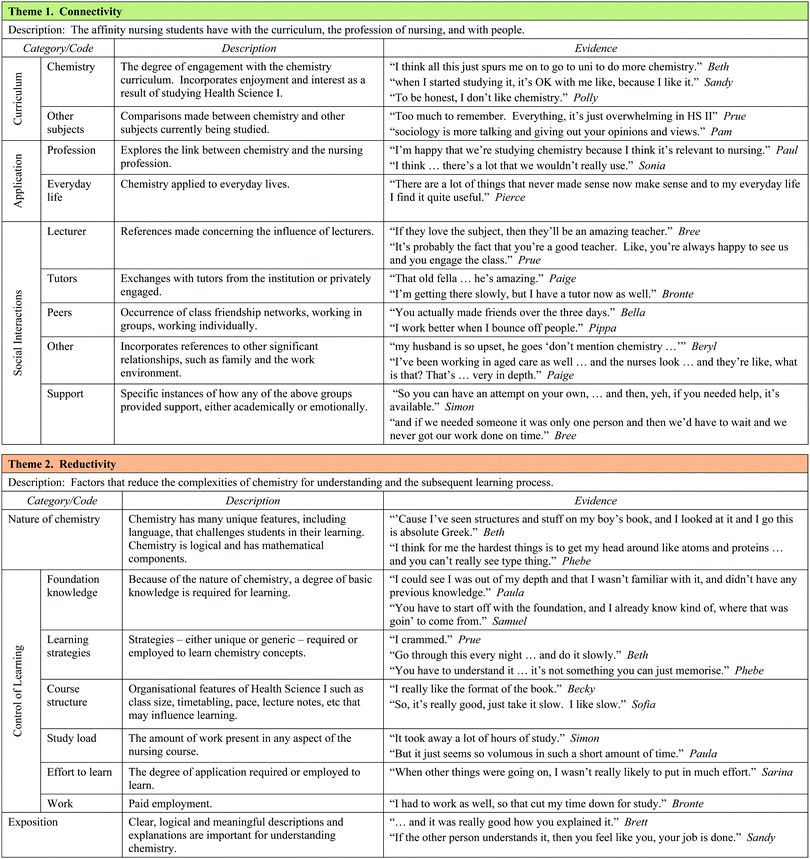
The clustering of categories and codes and discussions with colleagues facilitated the formation of three major themes, each of which was constructed of three categories. The themes are Connectivity (with categories Curriculum, Application, and Social Interaction), Reductivity (with categories Nature of Chemistry, Exposition, and Control of Learning), and Reflexivity (with categories Confidence, Anxiety, and Goal Orientation). The three themes and their categories, along with the sub-categories or codes for each category, are summarised in Table 6. A sample of student comments for each category has been provided as evidence in Table 6. The following discussion of the three themes should be read in conjunction with the information in Tables 5 and 6.
Theme 1: Connectivity
Connectivity represents the affinity that nursing students have with three aspects of their experience: the curriculum, the applications of chemistry and their social interactions. ‘Curriculum’ includes both chemistry and other units of study in the nursing degree. Students discussed the extent to which they could relate to chemistry and embedded in these comments was the notion of enjoyment.Brittney: …but I quite enjoyed it (the chemistry of health Science I) so I’m kind of upset I didn’t pick it at school.
For many students, the degree to which they struggled with chemistry seemed to affect the connection they could make with the subject.
Pippa: Like, “this is going to be so hard”, but once we got into it, it was alright and I actually liked it.
Inevitably, chemistry was compared with other subjects studied by students, particularly with respect to enjoyment and required effort. Table 5 shows that the required mental effort demanded by chemistry significantly exceeded (p < 0.01) that required for psychology and sociology.
Secondly, for chemistry learning to be relevant, students must be able to see a degree of ‘application’ to the profession of nursing, and to everyday life. A number of participants alluded to the correlation between their degree of connection with chemistry and the degree of relevance they perceived chemistry has to nursing. Students identified numerous specific examples from both lectures and the health-care workplace of how chemistry could be applied to the profession of nursing. In Pippa's case the connection with nursing practice grew her confidence.
Pippa: I was really confident when I saw topics like osmosis and oedema that applied to nursing.
Bronte saw how chemistry and anatomy and physiology informed each other in the nursing course.
Bronte: Since we’ve been doing Anatomy & Physiology more…I understand how important it is to have chemistry with nursing. The buffers, the pH levels, now it's all starting to fit into place.
However, according to Table 5, the level of application of chemistry to nursing did not significantly grow across the semester. This presents a challenge to chemistry educators working within nursing courses. While applications of chemistry to everyday life were not the focus of Health Science I, many students were still able to see the relevance of chemistry in their daily lives. Again, this influenced their level of connection with the curriculum.
The third category, ‘social interactions’, incorporates relationships with people in their circle of influence such as the lecturer, tutorial and laboratory staff, class peers and others such as family and workplace colleagues. Both the academic and emotional support received by students from each of these relationships was discussed. The interplay between the social category with curriculum and application was also evident. For example, there was a strong link between the experience of a tutorial and students’ connection with chemistry, particularly amongst those who entered the degree with a poor chemistry background. Paige expressed her frustration as follows.
Paige: And then I went to the first tutorial and it was really frustrating and lots of crying and screaming on the phone, “This is ridiculous. Why are we doing all this chemistry?”, like at my mum, and it's ridiculous.
There was also evidence from students working in the nursing sector of the positive and negative influence that work colleagues had on their level of connection with chemistry and in turn, the link students made with chemistry in nursing. When Paige was working in an Aged Care facility, a well-meaning nursing colleague passed the following judgment on the chemistry she was learning.
Nursing colleague: I understand that (chemistry) is definitely required, but there's things in there that are really, really full on and seem unnecessary.
In summary, this theme embraces both the connection and disconnection students have with the curriculum (particularly chemistry) in their degree, the nursing profession and various social associations with reciprocal relationships between the three categories clearly evident.
Theme 2: Reductivity
Exemplified in the learning of chemistry is the idea of reductivity. According to the responses to questions A1 to A4 in Table 5, chemistry is perceived as being difficult and its concepts and processes can be challenging for the novice, easily leading to cognitive overload. Statements by students like Sofia also confirm that chemistry is considered to be difficult even by those who have studied senior chemistry.Sofia: My anxiety levels were higher, definitely higher, for chemistry than psychology and sociology. Chemistry has always been a hard subject in my mind.
Moreover, many nursing students will never be required to operate at a high, abstract level in this discipline. It is therefore important to reduce the complexities involved in the learning of chemistry to a level manageable by all potential nurses. The reductivity theme consists of three main categories: nature of chemistry, control of learning and exposition. Aspects of Johnstone's model of the nature of chemistry (the Chemistry Triplet) (Johnstone, 2006) were revealed by students with poor chemistry backgrounds.
Beth: When I first went to the chemistry course, it was like stepping into another world. It (chemistry) was absolute Greek.
Phebe: Biology is more practical than chemistry because it is based on things you can see like animals and plants. In chemistry, the hardest thing to get my head around was atoms, proteins, things you really cannot see.
Pam: So you have to imagine what atoms are like in your head without physically touching them.
Paula: Once the chemical equations started to roll in, I lost it.
The unique language, often referred to by PC and BC students as alien or foreign, the cumulative nature of concept development, the logical and mathematical facets of the subject, along with the multidimensional conceptual levels encountered in chemistry, require a reductivist approach not only to learning but also when teaching this sometimes challenging subject.
In addition to problems associated with the ‘nature of chemistry’, students indicated the need to have some sense of ‘control over their learning’ in order to reduce the complexities of learning chemistry, and identified a number of aspects that play a role in this. Fundamental to this inquiry was the notion of foundation knowledge. Many PC students expressed the difficulty they experienced in the early lectures because they lacked a level of fundamental foundation knowledge. For the BC students, the material covered in the bridging course proved to be valuable in their early experiences with Health Science I.
Bella: It's sort of like when you did the bridging course, you already got the basis for when you got into class. You sort of actually knew some things, it's sort of like, we did that in the bridging course.
Students also discussed several learning strategies they applied to chemistry concepts, not all of which were effective. Organisational features of the course such as tutorials, laboratories, class size and the provision of worksheet-type lecture notes affected the degree of control students felt they had over their learning environment. High study loads in other subjects and engaging in significant levels of paid work (50% of PC students worked at least 10 hours per week according to Table 3) reduced the sense of control for many. This was influenced by the extent of foundation knowledge which, in turn, affected the amount of effort that students could put into the learning process.
Finally, because of the conceptual nature of chemistry, the exposure to clear, logical and meaningful explanations – ‘exposition’ – emerged as an essential component in reducing the complexities of chemistry and the promotion of increased levels of understanding.
Theme 3: Reflexivity
Originally named self-reflectivity, reflexivity implies action as a response to incoming data, rather than just reflecting upon it, and considers ways in which students engage in bidirectional, self-referent thought. It incorporates three key categories: confidence, anxiety and goal orientation. Students possess preconceived ideas about chemistry, derived from prior academic experiences largely drawn from school memories, and these coloured the interpretation of experiences in Health Science I, particularly early in the semester.Sofia: ….and my (high school) teacher, she wasn’t very helpful, like I just found it really confusing, everything, and then I forgot the basics, so it was just like a big muddle for me in my head, like all through senior high school.
This past experience was probably responsible for Sofia's score of zero (Table 3) on the enjoyment scale at the beginning of the semester. It was not surprising to find the constructs of confidence and anxiety emerge as categories since students were asked specifically about them in interviews. The confidence category includes any reflection of perceived ability, along with circumstances that may have facilitated changes in confidence. Anxiety incorporates expressions of worry, stress or nervousness and is not restricted to just chemistry anxiety.
Students also talked about other components of motivation such as ‘goal orientation’ and ‘expectancy’ and indicated the influence of both extrinsic and intrinsic goals. Of all the themes, the categories in reflexivity are the most closely affiliated with each other because their roots can be found in various motivation theories. Students explained how worry and anxiety affected their perceived levels of ability. Conversely, students who lacked confidence experienced anxiety. Research has demonstrated the predictive role of confidence in goal setting (Pajares, 1996), with expectations being partly determined by beliefs (Bandura, 1986). It is here that the role of mastery and vicarious experiences elaborated in social cognitive theory has a strong bearing on confidence, anxiety, and goal orientation.
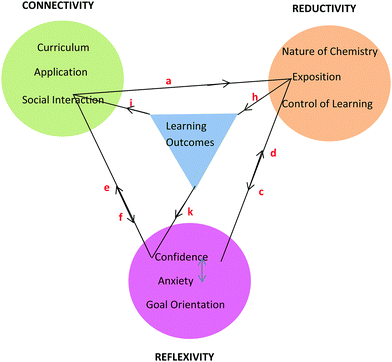
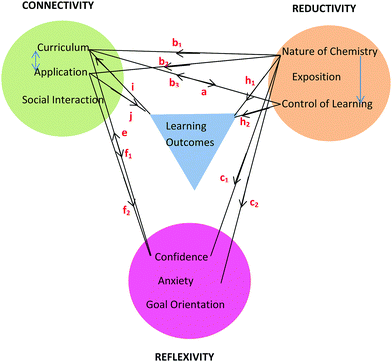
Framework
The learning process is multiplex and the model that has emerged from conversational data is just one representation of some of the complexities involved in the teaching and learning of chemistry for first-year nursing students. As stated by Lincoln and Guba (2007, p. 17), “the best an inquirer can do…is to establish inferences about the patterns and webs”. The model or framework shows the three themes and their categories clustered around learning outcomes and held together by linkages as shown in Fig. 8. The colour coding matches that used in Table 6 with learning outcomes coded in blue. It should be noted that the phrase, ‘learning outcomes’ has a dual meaning in this paper. Firstly, it refers to outcomes like ‘understanding’ and ‘possible test outcomes’ as perceived by the student in conversation but not measured objectively. Secondly, it refers to academic achievement as measured objectively in a test. The context of the conversation will reveal which of these two meanings applies. The initial purpose of the linkages in Fig. 8 was simply to hold the themes and learning outcomes together but it will be seen that the linkages can be used to depict relationships between themes arising in conversation around various topics. This is illustrated here for topics related to tutorial work, prior knowledge, and chemistry in nursing.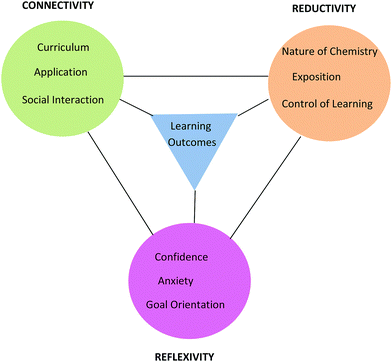 | ||
| Fig. 8 Framework showing three themes each with three categories derived from student nurses’ conversations about chemistry and its learning outcomes. | ||
Conversations about tutorial sessions
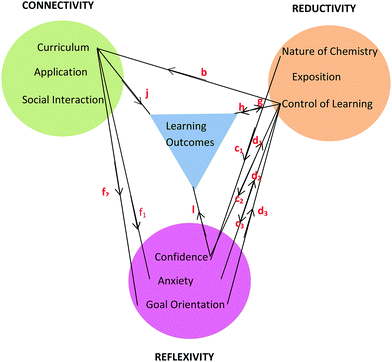
Working in groups for the tutorial sessions (Connectivity) proved vital in learning how to solve problems (Reductivity) which in turn enhanced confidence, lowered anxiety (Reflexivity) and enhanced learning outcomes. In addition, social cohesion wrought through group work (Connectivity) and the adoption of pedagogical tasks by students within a group helped to reinforce chemistry concepts (Reductivity). The comments by Brett, Sofia, and Pierce are informative in this regard and can be visualised through the relationships shown in Fig. 9 built upon the basic framework of Fig. 8. The relationships in Fig. 9 are shown using red letters and are reproduced in the following conversations.
Brett: I started helping people around me in the calculations and stuff, so that definitely boosted my confidence… . So I understood enough to be able to teach them
. So I understood enough to be able to teach them  . (Tutorials) took away my anxiety because my confidence increased….
. (Tutorials) took away my anxiety because my confidence increased…. so if I can explain it (chemistry) to another person, then I can probably do it in an exam
so if I can explain it (chemistry) to another person, then I can probably do it in an exam  . Learning off each other…and teaching other students how to do it (chemistry) reinforces it in my brain and I then understood
. Learning off each other…and teaching other students how to do it (chemistry) reinforces it in my brain and I then understood  . That was a big point in my academic performance.
. That was a big point in my academic performance.
Sofia: My confidence was built (in tutorials) and I felt confident to teach her (a fellow student) things as well and to be able to talk (together) about what we were studying  .
.
Pierce: When someone in the tutes explains it (chemistry) to you, and then someone else doesn’t know it, and then you explain it to them, you are reinforcing it to yourself  .
.
The flow of relationships commences with Social Interaction endemic to tutorial group work. For tutorials the class was divided into groups of no more than twelve students so the opportunity for engagement between students and between students and tutor was enhanced. When students like Brett, Sofia, and Pierce take on a pedagogical role in the group,  , confidence increases and anxiety decreases either directly,
, confidence increases and anxiety decreases either directly,  , or indirectly through the observation that students taught seem to have progressed in their learning,
, or indirectly through the observation that students taught seem to have progressed in their learning,  and
and  . In turn, a growth in confidence can lead to a student engaging in more pedagogical tasks,
. In turn, a growth in confidence can lead to a student engaging in more pedagogical tasks,  , in the knowledge that this activity will increase the chances of one being able to answer an exam question correctly,
, in the knowledge that this activity will increase the chances of one being able to answer an exam question correctly,  , according to Brett. An inverse relationship between confidence and anxiety in the Reflexivity theme is borne out by Brett's comment that an increase in his confidence level was responsible for reducing his anxiety.
, according to Brett. An inverse relationship between confidence and anxiety in the Reflexivity theme is borne out by Brett's comment that an increase in his confidence level was responsible for reducing his anxiety.
Sofia intimates that enhanced confidence can be the result not only of direct teaching but also through the sharing of understandings and experiences in the social group as demonstrated by  in Fig. 9. SCT recognises the importance of vicarious experiences in addition to mastery experiences in building confidence and improved academic outcomes. Both Brett and Sofia note a growth in confidence as a result of tutorial group work and this is reflected in their nominated expected increase in chemistry grade (3 to 4, credit to distinction) by the end of the semester. Both were rated as high performing students H (≥70%) by the end of the semester.
in Fig. 9. SCT recognises the importance of vicarious experiences in addition to mastery experiences in building confidence and improved academic outcomes. Both Brett and Sofia note a growth in confidence as a result of tutorial group work and this is reflected in their nominated expected increase in chemistry grade (3 to 4, credit to distinction) by the end of the semester. Both were rated as high performing students H (≥70%) by the end of the semester.
It is interesting to note that Sinapuelas and Stacy (2015) associate cooperative learning or learning through social interaction with a deep learning approach (Level 4) by introductory chemistry students in a large university. While students in the study reported here adopted a variety of learning approaches from basic memorization and repetition through to more analytical approaches (see below), it is clear that a deeper learning approach akin to social constructivism was used during tutorials.
Phebe: Chemistry is a subject you have to understand; you can’t just memorize it.
Polly: When I studied chemistry, I would just go for memorizing, not reasoning things out.
Bernice: There were just so many little minute details (in chemistry). For learning I just lay it (chemistry concepts) out, one by one, step by step by step. I need to go through the steps again and again-repetition to get it into my head.
Samuel: I’d say it is less the actual information and knowledge, and more the way of incorporating the knowledge. Like, actually thinking about it analytically, symbolically and building those kinds of ways of thinking…you know being scientifically literate and actually being able to think about things in that kind of abstract, analytical kind of way. So you build that over several years or even more. It's not just something you can learn in a couple of days.
The pedagogical role our students can play within social groups and its impact as a learning strategy are worthy of further exploration.
Conversations related to prior knowledge
Students have no hesitation in explaining their academic performance in terms of foundation knowledge and the impact this has on the learning process. The following includes statements from students across the prior chemistry experience spectrum and the relationships are shown in Fig. 10. Some students who had studied chemistry to senior high school level like Sarina did not achieve as good a grade as she was expecting (Expected 2-pass but got 1-fail).
Sarina: It was more like you knew you would understand it (having studied senior chemistry in high school). Whereas some people who haven’t done it (senior chemistry), they don’t know if they’re ever going to understand it  .
.
Sarina (on being questioned about her low academic performance L): I just had so many other things going on, and I realised afterwards I should have studied more. Maybe I was a bit too confident 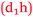 .
.
This poor outcome led Sarina to suggest that, perhaps, poor study habits had led to this,  , because she may have been too confident,
, because she may have been too confident, 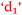 . On reflection, previous experience with chemistry led to a mistaken high confidence level,
. On reflection, previous experience with chemistry led to a mistaken high confidence level, 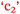 , which led to poor study habits,
, which led to poor study habits, 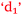 , and poor academic outcomes,
, and poor academic outcomes,  .
.
Motivation and complacency (Goal Orientation) seemed to make a difference to academic performance for students with a sound chemistry background (SC and BC students). At the beginning of the semester the cognitive load was not high for these students being largely revision of work previously encountered.
Bree: Doing the bridging course made it easier because you’ve already got the basics and then you’re like, Oh, I can take a breath because I already know this stuff  .
.
Bella: Walking into the first chemistry class of the semester (in Health Science I) it was like, Suck eggs. I know everything. My confidence was there having done the 3 day bridging course  .
.
As new chemistry concepts were presented in the second half of the chemistry component of Health Science I, the outcome depended on whether the student remained complacent or was motivated to engage with the new material to improve their academic performance.
Becky: My anxiety spiked and I questioned myself a bit the first time we hit something we hadn’t looked at  .
.
Brett: At the beginning of the semester I didn’t really need to study, as I knew how to use the formulas. And then when I started learning something new towards the end of the semester, I needed to knuckle down  .
.
Brett and Becky were a little complacent to start with because of their background knowledge (Reflexivity) but then knew they had to focus on study if they were to learn new material (Reductivity). So new material in the curriculum can lead to an increase or decrease in motivational level,  , which will impact study habits,
, which will impact study habits,  , and academic outcomes,
, and academic outcomes,  , as illustrated in Fig. 10.
, as illustrated in Fig. 10.
Students with a poor chemistry background (PC students) consistently mentioned that they needed more time during lectures to digest new chemistry material and felt confident that they could achieve a higher outcome if this had been the case.
Paris: I still don’t think I can do it. I just find that each time I look at it (a chemistry concept), it's not there, like it just doesn’t stick, and I just have to keep going back and back and back until I find it somewhere  .
.
Paula: I could see it (chemistry) was out of my depth as I didn’t have any previous knowledge. A bit scary. In the middle of the semester I just felt so overwhelmed with more anxiety  .
.
Pam: If it was longer for what we had throughout the year, I reckon we could have got it more. So, instead of doing two or three units at a time in one lecture, spread it out more, to make it longer, so people can actually understand it more and have more time to ask questions. I reckon people would get better marks  .
.
Paula: Because I didn’t have the background, I needed more time  .
.
According to Pam and Paula, more time would have increased their engagement with the material and allowed space for asking questions. In terms of the SCT model, increasing the time of exposure of a motivated student to the curriculum materials enhances the chance that mastery can be achieved along with improved academic performance. The statements made by Pam and Paula suggest the existence of a circular bidirectional relationship. Relatively poor grades (low academic performance L, <45%) compared to that achieved by other students prompted the statements by Pam and Paula. They thought more time would have given them more control over their learning, and also would have given them opportunity to connect with the content by asking more questions,  , the answers to which would have increased their understanding,
, the answers to which would have increased their understanding,  . The previously stated benefits of tutorial group work indicate that this sequence could be reversed. If a student still didn’t understand a chemistry idea, they could easily connect with the lecturer, tutor, or fellow student,
. The previously stated benefits of tutorial group work indicate that this sequence could be reversed. If a student still didn’t understand a chemistry idea, they could easily connect with the lecturer, tutor, or fellow student,  , who had proved themselves well capable of explaining ideas,
, who had proved themselves well capable of explaining ideas,  , which the students believed would inevitably improve their understanding and their test scores,
, which the students believed would inevitably improve their understanding and their test scores,  (Fig. 9). This circular bidirectional relationship, (
(Fig. 9). This circular bidirectional relationship, ( and
and 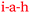 ) is visualised in Fig. 9 and 10 taken in combination.
) is visualised in Fig. 9 and 10 taken in combination.
Another comparison to be made if one considers the information in Fig. 9 and 10 in combination is the bidirectional relationship between confidence and learning outcomes, 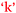 and
and 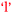 . Villafane et al. (2016) demonstrated such reciprocal causation between self-efficacy and academic performance in a quantitative study of 173 first-semester organic chemistry students from a research-intensive university. A combination of Fig. 9 and 10 also reveals bidirectional or reciprocal causation behaviour between confidence and social interaction (
. Villafane et al. (2016) demonstrated such reciprocal causation between self-efficacy and academic performance in a quantitative study of 173 first-semester organic chemistry students from a research-intensive university. A combination of Fig. 9 and 10 also reveals bidirectional or reciprocal causation behaviour between confidence and social interaction (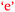 and
and  ), confidence and exposition (
), confidence and exposition ( and
and  ), confidence and control of learning (
), confidence and control of learning ( and
and 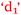 ), anxiety and control of learning (
), anxiety and control of learning (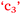 and
and 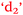 ), and learning outcomes and control of learning (
), and learning outcomes and control of learning ( and
and 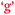 ).
).
Conversations related to chemistry and its application to nursing
There was some variety in the way PC students spoke about the relevance of chemistry to nursing. Compare the following statements by Paul, Phebe, and Paula, noting the importance score (Table 3) given at the beginning of the semester and end of semester respectively in brackets after each statement. Academic Performance (Table 3) is also given after each statement. The red letters refer to the relationships shown in Fig. 11.
Paul: I’m happy that we’re studying chemistry because I think it is relevant to nursing  . (4, 4) (H)
. (4, 4) (H)
Phebe: I know it (chemistry) is background knowledge but when are we ever going to use this on the ward and how much chemistry are we going to remember once we start working as nurses  . (1, 1) (L)
. (1, 1) (L)
Paula: I think some of the basic ideas of chemistry are important but I don’t know if the level of chemistry taught to us is really necessary  . (4, 1) (L)
. (4, 1) (L)
In Phebe's case applicability of chemistry to nursing was rated at a level of only ‘1’ at the beginning and end of the semester (Table 3). This belief would not have helped her academic performance,  . In Paula's case there was a high expectation of relevance at the beginning of the semester (level ‘4’) which decreased significantly to level ‘1’ as a result of chemistry study (Table 3). Low academic performance may have led Paula to question the relevance of the depth of chemistry study for nursing,
. In Paula's case there was a high expectation of relevance at the beginning of the semester (level ‘4’) which decreased significantly to level ‘1’ as a result of chemistry study (Table 3). Low academic performance may have led Paula to question the relevance of the depth of chemistry study for nursing, 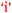 . Paul had a different perspective on chemistry's relevance to nursing and one must allow for the possibility of a bidirectional relationship between Curriculum-Application and Academic Performance as shown in Fig. 11. It is interesting to note that, at the end of semester, low achievers rated the ‘importance of chemistry to nursing’ at a significantly lower level than both average (p = 0.023, d = 0.53) and high (p < 0.001, d = 1.15) achievers when ANOVA followed by Scheffe tests was applied to the data from all the research participants (N = 101). There was no significant difference between the average and high performers (p = 0.120). Since PC students have the largest percentage of low achievers (see Table 3), it is no surprise that PC students in the focus groups showed the largest decrease in the score for ‘importance of chemistry to nursing’ in Table 4 given the ANOVA results previously mentioned.
. Paul had a different perspective on chemistry's relevance to nursing and one must allow for the possibility of a bidirectional relationship between Curriculum-Application and Academic Performance as shown in Fig. 11. It is interesting to note that, at the end of semester, low achievers rated the ‘importance of chemistry to nursing’ at a significantly lower level than both average (p = 0.023, d = 0.53) and high (p < 0.001, d = 1.15) achievers when ANOVA followed by Scheffe tests was applied to the data from all the research participants (N = 101). There was no significant difference between the average and high performers (p = 0.120). Since PC students have the largest percentage of low achievers (see Table 3), it is no surprise that PC students in the focus groups showed the largest decrease in the score for ‘importance of chemistry to nursing’ in Table 4 given the ANOVA results previously mentioned.
As previously seen, Pippa and Bronte made reference to specific chemistry topics which they thought were important for nursing and these comments have been classified as follows according to Fig. 11.
Pippa: I was really confident when I saw topics like osmosis and oedema that applied to nursing 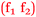 .
.
Bronte: Since we’ve been doing Anatomy & Physiology more…I understand how important it is to have chemistry with nursing. The buffers, the pH levels, now it's all starting to fit into place 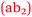 .
.
A curriculum that contains applications of chemistry to nursing will impact the learning strategies of students and the nature of chemistry will determine the kinds of applications that will be relevant to both nursing and chemistry.
As far as student orientations to chemistry itself were concerned, bidirectional relationships were a direct feature of some of the student comments. For example, the relationship between enjoyment of chemistry and confidence was described in alternate directions by Beth and Sofia. The comparison of enjoyment scores (Table 3) from the beginning of the semester to its conclusion, is given after each statement as is the level of academic performance (H, Av, or L).
Beth: I enjoyed chemistry. It was fun, therefore it gave me confidence 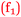 . (4 to 3), (H)
. (4 to 3), (H)
Sofia: With the confidence came the enjoyment 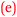 . (0 to 3), (H)
. (0 to 3), (H)
For Beth, being strongly engaged or connected with chemistry was enjoyable and this resulted in the growth of her confidence, 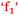 . That is, confidence was an outcome of her enjoyment. For Sofia, enjoyment was an outcome of confidence,
. That is, confidence was an outcome of her enjoyment. For Sofia, enjoyment was an outcome of confidence, 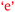 . This bidirectional feature is shown in Fig. 11. The initial enjoyment score of zero for Sofia reflects her unfavourable high school chemistry experience as previously explained by her under Theme 3-Reflexivity.
. This bidirectional feature is shown in Fig. 11. The initial enjoyment score of zero for Sofia reflects her unfavourable high school chemistry experience as previously explained by her under Theme 3-Reflexivity.
While over 50% of focus group students rated enjoyment of chemistry as better than average (Table 5), not all students enjoyed chemistry. Pam, Paula, and Phebe gave enjoyment score changes across the semester as (3 to 0), (1 to 1), and (0 to 1) respectively and all three were low achievers. All high achievers in the focus groups registered enjoyment scores ≥3 at the end of the semester but not all low achievers in the focus groups registered enjoyment scores <3. Scores of 3 and 4 also appeared amongst the low achievers.
Students from across the prior chemistry experience spectrum spoke freely about how difficult it was to achieve high scores in chemistry because of the difficulty of the subject.
Sofia: My anxiety levels were higher, definitely higher, for chemistry than psychology and sociology. Chemistry has always been a hard subject in my mind 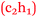 .
.
Beth: When I first went to the chemistry course, it was like stepping into another world. It (chemistry) was absolute Greek  .
.
Pam: So you have to imagine what atoms are like in your head without physically touching them  .
.
Paula: Once the chemical equations started to roll in, I lost it  .
.
Pam observes that to achieve some level of understanding in chemistry one has to imagine what atoms are like in order to learn the subject because one cannot physically manipulate atoms. This suggests an internal relationship between nature of chemistry and control of learning. The use of the imagination was, for Pam, an important learning strategy for understanding the properties of the basic objects of chemistry like the atom.
These examples are only a sampling of the kinds of relationships that can be deduced from student conversations and the relationships depend very much on the topic of conversation. The images in Fig. 9–11 are not meant to stipulate all possible relationships but they do give a visual snapshot of what students in conversation were thinking about the topics in question. The relationships in Fig. 9 for tutorial sessions focus on social interaction and exposition because tutorial sessions were where students adopted a pedagogical role. The relationships in Fig. 10 related to prior knowledge focus on curriculum and control of learning because foundation knowledge very much determines how a student will relate to new knowledge. The relationships in Fig. 11 related to chemistry in the nursing curriculum focus on curriculum-application and nature of chemistry because the fundamentals of chemistry as a discipline very much determines what the authentic applications to nursing might be. The combined relationships between themes and between themes and learning outcomes for the three topics discussed here and visualised in Fig. 9–11 are shown in Fig. 12. Students were asked to reflect on the relationships shown in Fig. 12 in terms of their overall experience of chemistry in Health Science I. Some of the outcomes of this reflection are discussed in the next section.
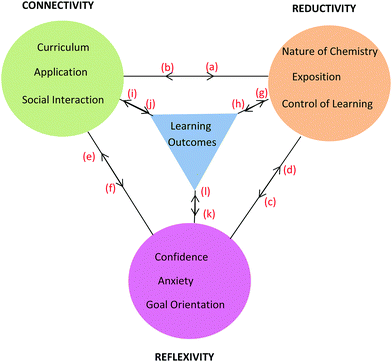 | ||
Fig. 12 Framework showing relationships  to to  between the three themes and between the three themes and learning outcomes depicting possible overall experiences of chemistry in Health Science I. between the three themes and between the three themes and learning outcomes depicting possible overall experiences of chemistry in Health Science I. | ||
Individual student comments on the framework and relationships in Fig. 12
While the framework in Fig. 8 was developed from a focus group study of student conversations, a student from each focus group was asked to assess the strength of the relationships embedded in the framework in Fig. 12 from their individual point of view. This was one way of testing the validity of the framework as far as students were concerned. The individual interviews were conducted after completing Health Science I. When the student was shown the framework and relationships in Fig. 12, the following questions were asked:(a) What role did each of these categories play in your confidence and anxiety levels over the semester?
(b) How did each affect your academic performance?
(c) If you had to indicate the strengths of the interactions or relationships [ to
to  ], which ones could be categorized as ‘strong’ for you?
], which ones could be categorized as ‘strong’ for you?
For questions (a) and (b), the students agreed with the depictions visualised in Fig. 9–11 and in answer to question (c), the strong relationships nominated by each student from Fig. 12 are shown in Table 7.
| Individual | Relationship | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | g | h | i | j | k | l | |
| Beth | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Brett | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Paula | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Pippa | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Samuel | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Sofia | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
The data in Table 7 demonstrates how diverse the human experience of a discipline like chemistry can be given the fact that no two students had exactly the same set of strong experiences. This is the case even within the same prior chemistry experience group. Sofia was the only student who nominated all relationships [ to
to  ] as ‘strong’ and was the only student who nominated relationship
] as ‘strong’ and was the only student who nominated relationship  as strong. That is, most students focussed on academic performance as an outcome of strong connections with the staff, the discipline of chemistry and its applications to nursing
as strong. That is, most students focussed on academic performance as an outcome of strong connections with the staff, the discipline of chemistry and its applications to nursing  rather than these connections being an outcome of academic performance
rather than these connections being an outcome of academic performance  . The most prevalent bidirectional relationship was between learning/academic performance and reflexivity,
. The most prevalent bidirectional relationship was between learning/academic performance and reflexivity,  and
and  . This is particularly seen in the relationship between confidence and academic performance: increasing confidence leads to improved academic performance and increasing academic performance leads to enhanced confidence. In this regard the quantitative findings on reciprocal causation by Villafane et al. (2016) complement the qualitative findings in this study. The relationship between anxiety and academic performance is also bidirectional: increased anxiety lowers academic performance and poor academic performance increases anxiety.
. This is particularly seen in the relationship between confidence and academic performance: increasing confidence leads to improved academic performance and increasing academic performance leads to enhanced confidence. In this regard the quantitative findings on reciprocal causation by Villafane et al. (2016) complement the qualitative findings in this study. The relationship between anxiety and academic performance is also bidirectional: increased anxiety lowers academic performance and poor academic performance increases anxiety.
Limitations of the study
The fact that the focus groups contained students across the spread of gender, academic performance, and enjoyment levels did help to reduce bias in the responses but one must be aware that even these precautions cannot eliminate potential bias entirely.Individual interviewees were asked to comment on the degree to which their participation in the focus group may have been influenced by other members of the group. All enjoyed the experience and felt the group environment was “more stimulating” (Pippa) because it provided opportunity to “bounce off each other's experiences” (Paula). Only Pippa suggested that some of her comments may have been a little inhibited because at that stage of the course she did not know Pierce very well. Of course, individual interviewees provide only one perspective on the framework model and this itself is a limitation. This bias, however, is somewhat mitigated by the fact that the individual interviewees covered the full range of academic achievement levels (Low, Average, and High), levels 1 to 4 in ‘importance of chemistry to nursing’, levels 1 to 4 in ‘enjoyment of chemistry’, and levels 2 to 4 in the ‘expected chemistry grade’.
When students are conversing about their experiences in chemistry they do not typically divide their experience into sequences so that when a student engages with the chemistry curriculum and experiences an increased understanding as a result,  , the student may not tell the interviewer what their learning strategies were in the process. So the path from ‘Connectivity’ to ‘Learning’ may have passed via
, the student may not tell the interviewer what their learning strategies were in the process. So the path from ‘Connectivity’ to ‘Learning’ may have passed via to ‘Reductivity’ and then
to ‘Reductivity’ and then  to ‘Learning’ even though the student may not have mentioned this in their conversation. This is one of the difficulties in ‘student conversation’ research. However the use of the framework in modelling student conversations on a particular topic did help to locate which part of the framework was prominent in student thinking.
to ‘Learning’ even though the student may not have mentioned this in their conversation. This is one of the difficulties in ‘student conversation’ research. However the use of the framework in modelling student conversations on a particular topic did help to locate which part of the framework was prominent in student thinking.
This research has been restricted to nursing chemistry. The extent to which the framework applies more broadly to introductory chemistry for other professions needs further research. The teacher/researcher nexus and the extent to which this impacted student conversation overtly or subtly is difficult to measure but remains an inherent limitation of which to be cognisant.
Conclusion and recommendations
While much research into chemistry education provides statistics relating to chemistry self-efficacy, anxiety, prior chemistry experience, motivational factors and academic performance, this paper presents the voices of first-year nursing students, representing their stories and experiences and uncovering a nexus of factors that impact chemistry learning at the tertiary level, particularly for novices. As such, it serves to start the discourse on developing meaningful qualitative models to encompass more than just a few constructs and provides insights and some explanations for the numerous quantitative findings. We have seen how important a qualitative analysis of student conversations has been in complementing our knowledge of what statistical data analysis can achieve. For example, the qualitative data presented here supports the relationships found in Fig. 1 between chemistry achievement and prior chemistry knowledge, between autonomy support and motivation in Fig. 2, and between chemistry anxiety and chemistry self-efficacy in Fig. 3. The emergent themes of ‘connectivity’, ‘reductivity’, and ‘reflexivity’ give rise to a model which demonstrates the dynamic and interactive effect operating within and across the themes and with ‘learning outcomes’, extending previous research in this area and representing a novel way of thinking about the chemistry learning process. Individual student experiences may reflect all of the relationships or a subset of the relationships in the framework which illustrates how diverse student experiences can be. Thus the answer to Research Questions 1 and 2 must be in the affirmative.Each of the categories identified in this research provides chemistry educators with insight into how units can be organised and delivered more effectively and a number of recommendations can be made, particularly when teaching chemistry to novices. For example, the research highlights the importance of foundation knowledge when studying chemistry, showing how crucial it is for curriculum developers to be cognizant of the difficulties associated with learning chemistry, particularly cognitive overload. Providing considerable scaffolding in the form of well-structured activities and mastery experiences contributes to self-efficacy, anxiety reduction and academic performance by providing additional layers of support for novice students. Social interaction plays an important role in the development of cognition (Vygotsky, 1978) so providing opportunities for students to work in groups during tutorials facilitates co-construction of knowledge as students attempt to explain concepts to each other. Student comments also showed that peers act as a potent force in the development of confidence due to exposure to modelling academic and domain-specific skills. Bridging course attendance, even if only for three days, increases confidence and fosters peer connections.
The framework in Fig. 8–12 reminds us that the learning strategies our students adopt can be impacted by non-cognitive variables (Bandura, 1986) summarised under Reflexivity and the level of connectedness our students experience with the curriculum and its personnel. This remains a challenge in chemistry education at all levels.
Conflicts of interest
There are no conflicts to declare.References
- Aydin, Y. C., Uzuntiryaki, E. and Demirdogen, B., (2011), Interplay of motivational and cognitive strategies in predicting self-efficacy and anxiety, Educ. Psychol., 31(1), 55–66.
- Bandura, A., (1986), Social foundations of thought and action: A social cognitive theory, Englewood Cliffs, NJ: Prentice Hall.
- Bandura, A., (1994), Self-efficacy, in Ramachaudran, V. S. (ed.), Encyclopedia of Human Behaviour, vol. 4, pp. 71–81.
- Bandura, A., (1997), Self-efficacy: The exercise of control, New York: W.H. Freeman & Company.
- Barbour, R., (2008), Introducing qualitative research: A student guide to the craft of doing qualitative research, London: Sage Publications.
- Bauer, C. F., (2005), Beyond “student attitudes”: Chemistry self-concept inventory for assessment of the affective component of student learning, J. Chem. Educ., 82(12), 1864–1870.
- Bell, P. and Volckmann, D., (2011), Knowledge surveys in general chemistry: Confidence, overconfidence, and performance, J. Chem. Educ., 88(11), 1469–1476.
- Billington, S., Smith, R. B., Karousos, N. G., Cowham, E. and Davis, J., (2008), Covert approaches to countering adult chemophobia, J. Chem. Educ., 85(3), 379–380.
- Boddey, K. and de Berg, K., (2015), The impact of nursing students’ prior chemistry experience on academic performance and perception of relevance in a health science course, Chem. Educ. Res. Pract., 16, 212–227.
- Brooks, B. J. and Koretsky, M. D., (2011), The influence of group discussion on students’ responses and confidence during peer instruction, J. Chem. Educ., 88, 1477–1484.
- Brown, C. E., Henry, M. L. M., Barbera, J. and Hyslop, R. M., (2012), A bridge between two cultures: Uncovering the chemistry concepts relevant to the nursing clinical practice, J. Chem. Educ., 89, 1114–1121.
- Charmaz, K., (2006), Constructing grounded theory: A practical guide through qualitative analysis, Thousand Oaks, California: Sage Publications.
- Cheung, D., (2015), The combined effects of classroom teaching and learning strategy use on students’ chemistry self-efficacy, Res. Sci. Educ., 45, 101–116.
- Cochrane-Smith, M. and Lytle, S. L., (1999), The teacher research movement: A decade later, Educ. Res., 28(7), 15–25.
- Creswell, J. W., (2008), Educational research: Planning, conducting and evaluating quantitative and qualitative research, 3rd edn, Upper Saddle River, NJ: Pearson/Merrill Prentice Hall.
- Dalgety, J., Coll, R. and Jones, A., (2003), Development of chemistry attitudes and experiences questionnaire (CAEQ), J. Res. Sci. Teach., 40(7), 649–668.
- Dalgety, J. and Coll, R., (2006), Exploring first-year science students' chemistry self-efficacy, Int. J. Sci. Math. Educ., 4(1), 97–116.
- El-Farargy, N., (2009), Chemistry for student nurses: applications-based learning, Chem. Educ. Res. Pract., 10, 250–260.
- Ferrell, B. and Barbera, J., (2015), Analysis of students’ self-efficacy, interest, and effort belief in general chemistry, Chem. Educ. Res. Pract., 16, 318–337.
- Ferrell, B., Phillips, M. M. and Barbera, J., (2016), Connecting achievement motivation to performance in general chemistry, Chem. Educ. Res. Pract., 17, 1054–1066.
- Glaser, B., (1992), Basics of grounded theory analysis, Mill Valley, CA: Sociology Press.
- Gonzalez, A. and Paolini, P., (2015), Perceived autonomy-support, expectancy, value, metacognitive strategies and performance in chemistry: A structural equation model in undergraduates, Chem. Educ. Res. Pract., 16, 640–653.
- Johnstone, A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist. Learn., 7, 75–83.
- Johnstone, A. H., (1997), Chemistry teaching: science or alchemy? J. Chem. Educ., 74(3), 262–268.
- Johnstone, A. H., (2000), Teaching of chemistry – logical or psychological? Chem. Educ. Res. Pract., 1(1), 9–15.
- Johnstone, A. H., (2006), Chemical education research in Glasgow in perspective, Chem. Educ. Res. Pract., 7(2), 49–63.
- Lewis, S. E., Shaw, J. L. and Heitz, J. O., (2009), Attitude counts: Self-concept and success in, general chemistry, J. Chem. Educ., 86(6), 744–749.
- Lincoln, Y. S. and Guba, E. G., (2007), But is it rigorous? Trustworthiness and authenticity in naturalistic evaluation, New Dir. Eval., 114, 15–25.
- Lodico, M. G., Spaulding, D. T. and Voegtle, K. H., (2006), Methods in educational research: From theory to practice, San Francisco, CA: Jossey-Bass.
- Marton, F., (1981), Phenomenography: Describing conceptions of the world around us, Instruct. Sci., 10, 177–200.
- Merchant, Z., Goetz, E. T., Keeney-Kennicutt, W., Kwok, O., Cifuentes, L. and Davis, T. J., (2012), The learner characteristics, features of desktop 3D virtual reality environments, and college chemistry instruction: A structural equation modeling analysis, Comput. Educ., 59, 551–568.
- Mertens, D., (2010), Research and evaluation in education and psychology: Integrating diversity with quantitative, qualitative and mixed methods, 3rd edn, Thousand Oaks, California: Sage Publications.
- Pajares, F., (1996), Self-efficacy beliefs in academic settings, Rev. Educ. Res., 66(4), 543–578.
- Pajares, F., (2002), Overview of Social Cognitive Theory and of self-efficacy, retrieved from http://www.emory.edu/EDUCATION/mfp/eff.html.
- Patton, M. Q., (2002), Qualitative research and evaluation methods, 3rd edn, Thousand Oaks, California: Sage Publications.
- Richardson, J. T. E., (1999), The concepts and methods of phenomenographic research, Rev. Educ. Res., 69(1), 53–82.
- Salomon, G., (1984), Television is ‘easy’ and print is ‘tough’: The differential investment of mental effort in learning as a function of perceptions and attributions. J. Educ. Psychol., 76(4), 647–658.
- Schmid, S., Youl, D. J., George, A. V. and Read, J. R., (2012), Effectiveness of a short, intense bridging course for scaffolding students commencing university-level study of chemistry, Int. J. Sci. Educ., 34(8), 1211–1234.
- Seery, M., (2009), The role of prior knowledge and student aptitude in undergraduate performance in chemistry: a correlation-prediction study, Chem. Educ. Res. Pract., 10(3), 227–232.
- Sinapuelas, M. L. S. and Stacy, A. M., (2015), The relationship between student success in introductory university chemistry and approaches to learning outside of the classroom, J. Res. Sci. Teach., 52(6), 790–815.
- Taasoobshirazi, G. and Glynn, S., (2009), College students solving chemistry problems: A theoretical model of expertise, J. Res. Sci. Teach., 46(10), 1070–1089.
- Taber, K., (2013), Revisiting the chemistry triplet: Drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14, 156–168.
- Turner, R. C. and Lindsay, H. A., (2003), Gender differences in cognitive and noncognitive factors related to achievement in organic chemistry, J. Chem. Educ., 80(5), 563–568.
- Uzuntiryaki, E. and Aydin, Y. C., (2009), Development and validation of Chemistry Self-Efficacy Scale for College Students, Res. Sci. Educ., 39(4), 539–551.
- Villafane, S. M., Garcia, C. A. and Lewis, J. E., (2014), Exploring diverse students’ trends in chemistry self-efficacy throughout a semester of college-level preparatory chemistry, Chem. Educ. Res. Pract., 15, 114–127.
- Villafane, S. M., Xu, X. and Raker, J. R., (2016), Self-efficacy and academic performance in first-semester organic chemistry: testing a model of reciprocal causation, Chem. Educ. Res. Pract., 17, 973–984.
- Vygotsky, L. S., (1978), Mind in society: The development of higher psychological processes, Cambridge, MA: Harvard University Press.
- Williams, A. and Katz, L., (2001), The use of focus group methodology in education: Some theoretical and practical considerations, Int. Elec. J. Lead. Learn., 5(3), 1–10, retrieved from http://people.ucalgary.ca/~huartson/iejll/.
- Xu, X., Villafane, S. M. and Lewis, J. E., (2013), College students’ attitudes toward chemistry, conceptual knowledge and achievement: Structural equation model analysis, Chem. Educ. Res. Pract., 14, 188–200.
- Zusho, A., Pintrich, P. R. and Coppola, B., (2003), Skill and will: The role of motivation and cognition in the learning of college chemistry, Int. J. Sci. Educ., 25(9), 1081–1094.
| This journal is © The Royal Society of Chemistry 2018 |