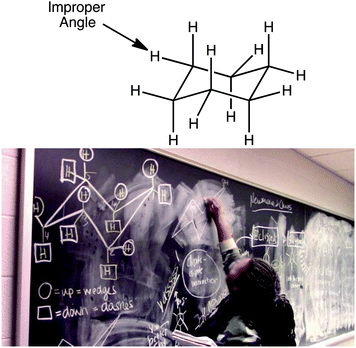
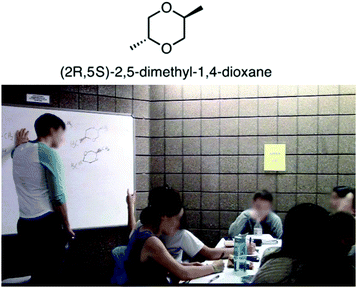
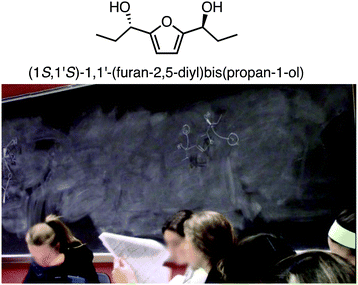
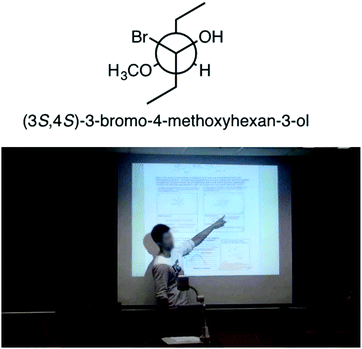
The relationship between subject matter knowledge and teaching effectiveness of undergraduate chemistry peer facilitators
J. R.
Boothe
 ,
R. A.
Barnard
,
R. A.
Barnard
 ,
L. J.
Peterson
,
L. J.
Peterson
 and
B. P.
Coppola
and
B. P.
Coppola
 *
*
Department of Chemistry, University of Michigan, 930 N. University Ave., Ann Arbor, MI 48109, USA. E-mail: bcoppola@umich.edu
First published on 13th November 2017
Abstract
Use of peer instruction and facilitation has surged in undergraduate education at large colleges and universities in recent years. Studies on peer instruction have been directed primarily at student learning gains and affective outcomes among the facilitators. For peer instructors, the relationship between their teaching effectiveness and their foundational content knowledge is assumed but understudied. In an effort to promote instructional coherence (i.e., instructional same-pageness) in the introductory organic chemistry program at the University of Michigan, we observed peer-led study group facilitators’ involvement in their study groups (as teachers of groups of 6–12 students) and in a companion course (as learners) designed to reinforce and enhance their content knowledge. Audiovisual recordings of the facilitators in both the companion course and, for ten of them, leading their study groups, were captured over each of the two week periods covering the topics of stereochemistry and also conformational analysis. Recordings were subsequently coded for topic and correctness in presentation of subject matter. Errors made in either study group or the companion course were investigated for error resolution (corrected or uncorrected), source of error, and propagation of corrected errors. Analysis of recordings revealed that facilitators who have their own errors corrected in the companion course, or observe their peers’ errors corrected in the companion course, correctly describe these concepts in study groups. On examining errors made by facilitators when they are leading study group sessions, a backwards analysis showed consistently that either the topics had not been addressed in the antecedent companion course, or the facilitator was not actively engaged with the discussion when the topics were being discussed. These findings have implications to inform not only our own implementation of peer-led study groups, but also those interested in designing subject matter companion courses for peer leaders in other instructional settings.
Introduction
The importance of subject matter knowledge (i.e., content knowledge) in teaching is a common sense idea supported by research (Shulman, 1986, 1987; Carlsen, 1992; Magnusson et al., 1999; Van Driel et al., 2002; Mavhunga and Rollnick, 2013; Aloisi et al., 2014; Gess-Newsome et al., 2017). At the post-secondary level, where the instructional workforce extends beyond the classroom school teacher, research into instructor content knowledge provides insight to practice in the diverse categories of instructional staff (van Dijk and Kattmann, 2007; Hill et al., 2008; Kind, 2009; Padilla and Van Driel, 2011; Mavhunga and Rollnick, 2013; Alvarado et al., 2015; Rollnick and Davidowitz, 2015; Mack and Towns, 2016), including the use of graduate student instructors or teaching assistants (GSIs) (Luft et al., 2004; Bond-Robinson and Rodriques, 2005; Hale et al., 2016), and, increasingly, using peer-to-peer methods (Jardine and Friedman, 2017). During the implementation of a course designed specifically to support the content knowledge of peer leaders in the peer led study group (PLSG) program at the University of Michigan (U-M), we were drawn to the question of how the content knowledge of these instructors shaped their teaching practices.The first term organic chemistry course, titled Structure and Reactivity I, is as a high-enrollment introductory course that, since 1989, has served as the gateway chemistry course at U-M (Coppola et al., 1997; Ege et al., 1997). The PLSG program at U-M is an important resource for undergraduate students, with approximately 60% of Structure and Reactivity I students electing to participate in PLSG (Coppola et al., 1997; Sandler and Salvatore, 2013). PLSGs typically comprise 6–12 students and are led by one peer facilitator, an upper level undergraduate who is interviewed, hired, and trained by the College's Science Learning Center (http://lsa.umich.edu/slc – referred to herein as the SLC). Students and facilitators are matched, by schedule, through an online sign-up system through the SLC web site. Facilitators usually only have one PLSG section per term, although some students will lead two if their schedule and interests permit. PLSGs meet for a single two hour session per week to work on various learning resources, usually problem sets that are sourced and reviewed by a GSI. Peer facilitators receive on-going pedagogical training organized by the SLC to encourage student discussion while avoiding a didactic “telling” role, but additional coverage of the subject matter is not included and the content expertise of the facilitators is presumed.
Based upon anecdotal reports – from students – of discrepancies in the subject matter between what the PLSG leaders were saying and what was being said in other parts of the course by GSIs and faculty instructors, we implemented a required subject matter course (CHEM 220: Teaching Experience for Undergraduates) for the PLSG facilitators (Barnard et al., 2017). Our goal was to make the instructional message more coherent across the different groups of instructors with whom our students interact.
We have reported previously, based on survey results, that participating in the CHEM 220 course was correlated with peer facilitators’ increased confidence in their subject matter knowledge, including an intriguing improvement in their self-awareness about weaknesses in their understanding (Barnard et al., 2017). To better understand the effects of this course, we undertook a more detailed observational study, described in this paper, to look at the content knowledge of our PLSG facilitators and how that may influence their interactions both in their study groups and in the CHEM 220 course itself. To these ends, we analysed recordings of the facilitators (as learners) in their CHEM 220 course during a short (ca. two week) period, covering a single general area within the lecture course (topics in three dimensional representation: stereochemical and conformational analysis). We also analysed recordings of these same facilitators (as teachers) during their PLSG sessions where this same subject matter was the topic of discussion. By studying the flow of information in our PLSG program, including the subject matter and its instruction, we hypothesized that we might provide insight into the effect of an instructor's content knowledge on peer instruction programs more generally.
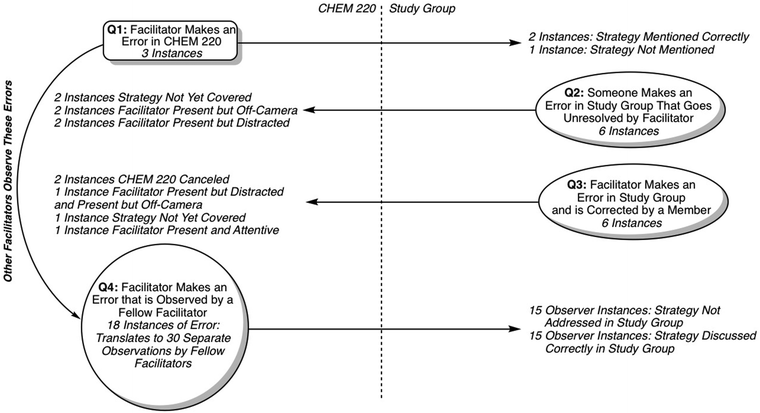
The aim of this study is to explore the relationship between subject matter knowledge of undergraduate chemistry peer facilitators and their teaching effectiveness in peer-led study groups. To probe this overarching aim, we developed four research questions about the content knowledge of the facilitators to drive our study. In two of these questions (1 and 4), we look for errors made and corrected in CHEM 220, and then look into the study groups to see if the error persisted. In the other two questions (2 and 3), we locate errors made during the study groups, and then look backwards into the antecedent CHEM 220 session:
(1) Do misunderstandings that the PLSG facilitators demonstrate during their weekly CHEM 220 sessions, which are addressed during CHEM 220, persist during the study group sessions; and if so, in what ways?
(2) When misunderstandings displayed by study group members are not corrected during study group, what was that facilitator doing during the relevant CHEM 220 discussion section?
(3) When facilitators make an error in study group that is corrected by a study group member, what was that facilitator doing during the relevant CHEM 220 discussion section?
(4) If a facilitator is present when another facilitator makes an error in CHEM 220 and sees it resolved, how does this observing facilitator handle that topic if/when it arises later in their own study group?
Background
Instructional context
Instructional coherence derives from presenting a unified message of correctness regarding content knowledge to students (Gess-Newsome et al., 2003). With a large teaching team serving a high-enrollment (ca. 1300–1600 students) introductory course such as Structure and Reactivity I, our primary goal in creating CHEM 220 as a required course was to improve instructional coherence, or consistency in message (including presentation, representation, and discussion of subject matter) across the entire instructional team (3–4 faculty instructors, 10–12 GSIs, and 70–80 study group peer facilitators). Our view of a coherent teaching team that includes these instructors is atypical, but not unlike the co-teaching models that are prevalent in K-12 teaching settings, where established principles include cooperation, communication, mutual planning, training, and agreement about subject matter (Walther-Thomas et al., 2000; Ploessl et al., 2010). We were particularly interested in the agreement in subject matter as a foundational aspect of teaching that would help improve our students’ learning by reducing any conflicting messages on subject matter from the instructional team. To assess our instructional coherence, we wanted to probe error propagation as a means of understanding the flow of information in this portion of the instructional structure (Friend and Cook, 2016).Several case studies have examined the importance of content knowledge when teaching in the sciences (Davis and Petish, 2005; Rollnick et al., 2008; Janssen et al., 2009; Adbo and Taber, 2014). One study conducted by Rollnick and coworkers investigated practices of secondary chemistry instructors with varying levels of subject matter knowledge, and found that instructors who have limited content knowledge “are constrained in their teaching by the limitations of their understanding of the concept” (Rollnick et al., 2008). Additional studies have found that in circumstances where secondary education teachers with limited content knowledge are exposed to new concepts (e.g., a study group member asks a facilitator a question about a problem solving strategy they are unfamiliar with), such instructors will fall back on rote teaching and learning due to their lack of confidence (Gess-Newsome and Lederman, 1999). Therefore, we established CHEM 220 as a required course to provide our facilitators with an opportunity to discuss content knowledge with an experienced GSI, providing a chance to catch teaching errors, and improve instructional coherence across the large instructional team. This experienced GSI acts as a liaison between the study group facilitators and the rest of the instructional team each term. The study groups (PLSG) described herein are two hour study blocks, attended once per week, during which introductory students solve chemistry problems in the presence of a more experienced peer (the PLSG facilitator), who serves as a discussion leader by asking leading questions and facilitating conversation and debate about content.
CHEM 220 is structured as a one credit course required for the experienced students who facilitate the PLSGs, with five to eight sections offered each term and enrolling an average of 8–12 facilitators per section. The CHEM 220 meeting consists of a mixture of lecture-style review of content (including instructional strategies tied to understanding the subject matter, which complements the more general pedagogical strategies provided in the training done by the SLC) and problem-solving time, all of which include liberal, freewheeling discussions led by both the liaison GSI and the students.
Content knowledge and instructional coherence
Pedagogical content knowledge (PCK) has been a topic of interest in educational circles for several decades, and represents the strategic instructional choices that can be made when a teacher has a deep understanding of both the subject matter and how to create effective learning environments. Seminal work from Shulman (1986) provides a background for many PCK studies, including those conducted with faculty members as well as graduate student instructors (Grossman, 1989; Bhattacharyya and Bodner, 2014). Most publications in the field of peer instruction in the past two decades have focused on the effectiveness of peer instruction as it pertains to students, and on the affective outcomes for the peer instructors, with less attention to the role that subject matter expertise plays (Ramaswamy et al., 2001; Tien et al., 2002; Lyle and Robinson, 2003; Micari et al., 2005; Gafney and Varma-Nelson, 2007; Quitadamo et al., 2009; Schray et al., 2009; Gosser et al., 2010; Lewis, 2011; Smith et al., 2014; Robert et al., 2016). Reports on peer instructors include, for example, their perceptions of subject matter orientation (i.e., disposition toward science) and leadership skills (Tenney and Houck, 2004; Tien et al., 2004; Gafney and Varma-Nelson, 2007; Amaral and Vala, 2009).An important consensus from the PCK literature is that the greater a teacher's subject matter knowledge, the more effective their instruction (Davis and Petish, 2005; Rollnick et al., 2008; Janssen et al., 2009; Adbo and Taber, 2014). One such study notes that an instructor with greater content knowledge “is able to display more powerful PCK where [his/her] nuanced [subject matter knowledge] allow [him/her] the flexibility to produce innovative approaches” (Rollnick et al., 2008). Others have also noted the importance of subject matter knowledge and PCK in the realm of effective instruction. McDiarmid summarizes the overall concept particularly well:
“To help learners develop integrated and meaningful understandings of subject matter, teachers need not only the substantive knowledge of their subject matter but understandings of what specialists in the field do, what constitutes knowledge in the discipline, how knowledge is generated and verified, and how knowledge is best taught and learned” (McDiarmid, 1990).
CHEM 220, and its concomitant integration into our instructional infrastructure for teaching organic chemistry, seeks to increase instructional coherence by (a) improving peer facilitators’ subject matter knowledge according to the ‘party line’ set by the faculty instructors, (b) allowing undergraduate peer facilitators to think explicitly about how chemistry knowledge is taught and learned, and (c) learning with an experienced GSI (the liaison GSI) who provides a direct link to the course, and a dedicated interest in the teaching effectiveness provided by this group of ca. 70–80 instructors (hereafter called “liaison GSI”).
Methods
Case study
The short term and highly focused inquiry described above is well suited to a case study analysis. Case studies provide depth of information, either longitudinally, or with rich data sources such as audio, visual, or interview data (Yin, 2014). Populations are generally smaller for case studies, but with a more limited population a researcher may gain greater insight into their subjects. By analogy, this process mirrors many aspects of a mechanistic study in chemistry: as an in-depth study of one or more compounds subjected to reaction conditions provides insight into mechanism of a larger suite of reactions, so the depth of a case study may provide insight to educational practice at a deeper level that may influence broader views of practice.Case studies are, by their nature, not generalizable. We cannot draw conclusions about what has happened, or what will happen, even in related situations, but we can draw inferences about where to look for effects from this type of intervention and with the teaching of this subject matter. Case studies are in-depth investigations into the educational practices or interventions in a specific location. However, the depth of analysis (including, but not limited to, collection and coding of audiovisual samples, as we have done) permits an increased understanding of a specific situation. While any claims made in this document represent a non-generalizable contribution thanks to the natural limits of case studies, they simultaneously represent a much greater understanding of how the PLSG program works at U-M, thereby allowing us to improve upon our existing frameworks with higher levels of confidence than before, potentially providing insight and direction to other PLSG, peer-led team learning, learning assistant programs, tutoring, or related programs where undergraduates take on teaching responsibilities.
In thinking about how to explore instructional coherence, we wanted to capture facilitator correctness and, more interestingly, episodes of incorrectness (hereafter referred to as “errors”) during their CHEM 220 experience (as learners) and in their own study groups (as teachers). This matching of learning and teaching around a specific subject area allows us to capture (1) the nature of facilitator error, (2) how these errors are addressed in CHEM 220, and (3) whether errors corrected in CHEM 220 are repeated in study groups, or are (putatively) resolved in CHEM 220.
According to Yin (2014), case studies traditionally fall into one of three different categories: descriptive, exploratory, and explanatory – much as with experimental methods, each type of study conducted provides insight through a different lens. Descriptive case studies set out to provide descriptions of phenomena, (i.e., how student dynamics function in the classroom), exploratory case studies set out to detail a new phenomenon or intervention (i.e., how does this new experiment help students learn?), and explanatory case studies set out to explain how a phenomenon occurs and indicate how such explanations may be applied to other situations. Yin details how case study research is chosen based on the nature of the research questions, and that each study must be built upon a particular case.
In this paper, we are reporting a case of correctness of study group facilitators and the connectedness between a learning setting and its corresponding facilitation setting. The type of case study we are reporting is exploratory, in that we are asking questions about whether the hypothesized connection, if any, exists between the correctness of a facilitator's subject matter knowledge as a learner and as a facilitator. We set out to explore (a) how often a PLSG facilitator correctly discusses a topic in stereochemistry or conformational analysis, (b) by connecting, in any instances of incorrect discussion, what they were doing in CHEM 220 while this topic was covered. This study will help us to (c) determine what we can do (if anything) to improve the design and implementation of CHEM 220, and (d) in a broader sense, understand how to guide other peer led instructional programs that may wish to introduce a content knowledge-based course or other related forms of subject matter instruction.
Data collection
The video data used for this analysis were collected during a two week period in the Fall 2015 semester, during which the topics of three-dimensional representations of molecules, conformational analysis (cycloalkane rings, cyclohexane chair conformations, Newman projections), and stereochemistry (sources of stereoisomerism, labelling stereocenters, identifying stereoisomeric relationships) were introduced, along with brief review of electrophilic addition, in the Structure and Reactivity I course. Stereochemistry and conformational analysis were chosen as convenient organic chemistry topics to study with respect to correctness, as much of the subject matter is made up of topological rules that define shape and spatial arrangement (Walba, 1985) and stereochemical assignments are binary and definite (Baker et al., 1998), that is, easily assigned as correct or incorrect. In contrast, using student-generated reaction mechanisms, or synthetic pathways, for example, would be more difficult. These latter topics are highly divergent, resulting in multiple possible solutions, requiring complex diagnostic heuristics, value judgments about how to weigh different factors (yield, purity, efficiency) and ultimately, experimental results, to determine a best answer.Researchers have convincingly demonstrated that audiovisual data can provide rich data about student conversations. Interactions, and even gestures may also be captured for further analysis (Stigler and Hiebert, 1999, p. 52; Stevens and Toro-Martell, 2003; Derry, 2007; Chen and Cowie, 2013; Kulatunga et al., 2013; Miles et al., 2014; Frøyland et al., 2015). To capture incidents of facilitator error, we postulated that audiovisual recordings of both CHEM 220 (during a given topic) and the study group facilitators’ associated study groups (around that given area) would be the most powerful way to sample facilitator conversations, to determine error frequency, and to pursue prospective errors from CHEM 220 into study groups, and to back-track errors from study groups to their antecedent CHEM 220 session.
Audiovisual recordings were conducted after consent was given by the members of CHEM 220 (the facilitators) and the members of their associated study groups. All participants in the study were given the option to opt-out of being recorded and participating in this study. Individuals who did not wish to be filmed would (a) be positioned off-camera, and/or (b) not have their conversations used in the analysis of recordings. In an abundance of caution, all faces were blurred in the images included in this work (see IRB-related note, below).
All 58 facilitators consented to be recorded during the two weeks of CHEM 220 where the topics covered were conformational analysis and stereochemistry. A total of 12 CHEM 220 sessions were recorded. Of the 58 facilitators, 12 consented to be recorded during their study groups. Because of scheduling, we were able to attend and record study groups for 10 of these 12 undergraduate facilitators. For this analysis, recordings from a total of 18 individual study group sessions were collected, providing over 40 hours of audiovisual data, including CHEM 220 recordings. Four of the 10 facilitators who were recorded were new facilitators who had not previously taken CHEM 220, while six were returning (see Table 1).
| Pseudonym | Study group facilitation experience level | Number of study group sessions recorded |
|---|---|---|
| Adam | Returning | 2 |
| Andy | Returning | 1 |
| Carrie | Returning | 2 |
| Daisy | Returning | 3 |
| Lupita | Returning | 1 |
| Max | Returning | 1 |
| Gwen | Novice | 2 |
| Harrison | Novice | 3 |
| Mark | Novice | 2 |
| Oscar | Novice | 3 |
Data were collected by one of the researchers using a digital camcorder and tripod during the relevant meetings of both CHEM 220 and the study groups. The camcorder was placed as far off to the side/back of the study group space as was possible while still being able to capture the white/black boards that facilitators and students worked on. During times of small group work the camcorder was panned to follow the liaison GSI (during CHEM 220), or study group facilitator (during the PLSG session), as he/she made his/her way around the room. This strategy allowed for the most optimal recording of facilitator/student interactions. Still images from recorded sessions are provided for instructional context; we have elected to blur the facial features of our students, even though our IRB-approved consent would have permitted us to use the unedited screen captures. By blurring, we did not wish to over-emphasize the identity of anyone while still purposely displaying to the reader a sense of the instructional environments. All transcriptions were performed by the researchers conducting the study, including JRB, who also served as the GSI for CHEM 220 sessions during the term under study.
Initial coding for data analysis
Coding of audiovisual data was performed using NVivo 11 software, in accordance with qualitative coding procedures in the literature (Stevens and Toro-Martell, 2003; Derry, 2007; Chen and Cowie, 2013; Frøyland et al., 2015). At least two researchers were always present for the coding of audiovisual data to ensure agreement and consistency in coding. Passages were simultaneously coded for:(1) one of six overarching topics (e.g., Stereoisomeric Relationships),
(2) problem solving strategies for each topic (e.g., use of the right-hand rule to determine R/S stereolabels for asymmetric carbons),
(3) the source of the strategy (e.g., study group member, facilitator, CHEM 220 liaison GSI), and
(4) the correctness of strategy implementation (i.e., was the strategy used correctly or not?).
The six broad topics (E/Z stereochemistry, electrophilic addition, Newman projections, R/S stereochemistry, rings, stereoisomer relationships) were known at the outset of the study, as both CHEM 220 sessions and study groups were purposefully sampled for the stereochemistry unit. These particular problem-solving strategies arose from the audiovisual data. While all strategy sources were coded, we focus here on the errors made by the facilitators in both CHEM 220 and study group. Additional detail about the source and use of each strategy across CHEM 220 and study group is provided in the tables in this article's appendices. The researchers watched and coded the videos of CHEM 220 prior to watching and coding the videos of study groups to capture the large number of strategies as presented by the liaison GSI.
Each individual strategy involved in solving a stereochemistry problem (e.g., how to assign a stereochemical label) was coded at each instance throughout all CHEM 220 and study group videos. Each strategy was coded for as long or as brief as the discussion was focused on the particular strategy. Data were not coded on simple utterances alone; rather, codes were applied when participants used strategies (a) in the context of solving practice problems, or (b) in small-group, theoretical discussions about the topic, but not resulting from solving a practice problem. Correctness codes were applied to all audiovisual clips of facilitators, when applicable.
For example, when reviewing CHEM 220 during a problem-solving session (e.g., Assignment of RR and SS stereoisomers as being enantiomers), the correct topic code (e.g., Stereoisomer relationships), strategy code (e.g., Assign enantiomer – change all R/S but not E/Z) as well as the correctness code (e.g., Strategy applied correctly vs. Strategy applied incorrectly) were applied. In any cases where facilitators or students were speaking about topics for which we could not assign correctness, the correctness code for these sections of video was omitted (e.g., a facilitator and study group peer member were discussing something written on the students’ paper that they had a question on from class). Additionally, in definitional conversations (e.g., didactic descriptions of how to assign stereoisomers as enantiomers) correctness codes were not applied. Subsequently, the Query feature of NVivo was used to interrogate all of the coded video clips; any videos containing the facilitator(s) in question could then be used to answer the given research question. Throughout the following sections of this report, each research question is framed with how the data provided insight as a collective whole, followed by individual descriptions of illustrative instances describing the origin of any applicable code along with transcripts and still images captured from video whenever possible.
Tracking of facilitator correctness and errors
After all video from both CHEM 220 and study groups were emergently coded for topic, strategy, source of strategy, and (where applicable) correctness, results of the Query feature of NVivo were used to cross-reference and track facilitator correctness (and, by extension, error) and strategy codes between CHEM 220 and study groups. Because we were exhaustively coding all instances, it was true that new codes continued to emerge until we had been through some fraction of the videos. By default, those new codes would not have appeared in earlier videos, meaning that those instances were simply not there. Thus, it was not necessary for the team to re-watch all audiovisual recordings once the full set of codes was created; by definition, and new codes had not previously appeared. A spot check of four early recordings (two from the CHEM 220 course and two from Structure and Reactivity I study groups) confirmed this conclusion. The code book presented in Appendix II of this article includes a collection of codes, including predominantly spoken words and some gestures captured in the recordings (Appendix II and Tables 6–11). Descriptions of codes are listed in the code book as topical, with a subset of strategy codes listed below, along with key concepts that tell how that code emerged from the data, the number of instances of that code, and correctness in use.Once generated, the code book, in conjunction with NVivo software, allowed us to search for any instances of a specific code (e.g., stereoisomeric relationships: Assign enantiomer – change all R/S but not E/Z), beginning with all instances of correctness and error on the part of the peer facilitator. This then allowed us to connect coded strategy errors back and forth between CHEM 220 and study groups (Fig. 1).
Our data allowed us to link episodes involving a facilitator error during the learning session (CHEM 220) by looking for the use of this topic during the facilitation sessions (study groups) via use of the code book, and vice versa (track any facilitator errors from study groups back to the antecedent CHEM 220 session).
Several errors made by facilitators during CHEM 220 were observed, allowing us to see whether errors on these topics were made again in study groups or were presumably resolved during the CHEM 220 session. In collecting errors made by PLSG facilitators in CHEM 220, we were able to follow these individuals into their own study group sessions, through the recordings of their teaching. We were also able to follow other facilitators, who had observed these errors during CHEM 220, to see how the topics were handled by them in their sessions.
Correspondingly, we probed instances during which a peer facilitator discussed a topic in study group incorrectly. For these errors, we tracked backwards to determine what facilitators were doing when these topics were covered in CHEM 220 (e.g., was the error made there and not corrected, was the topic not brought up, was the facilitator disengaged from the discussion), to see if we could observe any trends to help us better understand how to improve the implementation of CHEM 220.
Results
To best answer each question presented above, three different types of connections were interrogated (Fig. 1):(1) For facilitators who incorrectly answered a question in CHEM 220, we pursue these errors forward into study group to see if the errors appear (Q1),
(2) For facilitators who incorrectly discuss content in their study groups, we tracked these back to CHEM 220 to know what the facilitator was doing during the discussion of this topic (Q2 and Q3), and
(3) For facilitators who observe another facilitator's error being corrected in CHEM 220, we pursue these errors forward into the study groups of the observing facilitators to see if those topics are correctly discussed (Q4).
Throughout our analysis, we are linking together video data from two sources: (a) an origin video clip during which a facilitator makes an error using a coded strategy (referred to herein as an instance), and (b) any corresponding CHEM 220 or study group videos during which this facilitator is present for the use of this strategy.
In this paper, we report thirty-two instances (combined data from CHEM 220 meetings and study group sessions) that best highlight how facilitator correctness (or error) is carried between CHEM 220 and study group. As illustrated in Fig. 1, 21 of the errors were made in CHEM 220, while the remaining 11 were facilitator errors from study group. To put these 11 errors into context: of 395 coded instances of facilitators addressing content in their study groups, the clear majority (384/395) were coded as correct.
Each of the following sections informs a specific research question, with tabulated results of each instance of facilitator error from the entirety of our dataset, as well as an illustrative example of how this error was tracked back from study group to CHEM 220, or pursued from CHEM 220 into study group. Tabulated data sets are further sub-categorized by the nature of the error (or correctness) observed (e.g., based on a facilitator error made in study group, what the facilitator was recorded doing when this topic was discussed in CHEM 220). Finally, each instance has one or more corresponding strategy codes that provided a method of determining when a facilitator has described, asked questions about, or explained a stereochemical strategy for the purpose of connecting instances between CHEM 220 and study group.
Research Question 1: Do misunderstandings that the PLSG facilitators demonstrate during the CHEM 220 discussion sections, which are addressed during CHEM 220, persist during the study group; and if so, in what ways?
Throughout our analysis of CHEM 220 errors that were subsequently pursued to study groups, we found that these errors do not persist after they have been corrected in CHEM 220. We observed three instances of facilitators making errors in CHEM 220 that were corrected by the liaison GSI, and for whom we have corresponding audiovisual capture of their own study groups. After pursuing these errors into the study group sessions, in two of the three instances, the topic came up during the facilitator's study group, described correctly and free of error. In the third instance, the topic did not come up during that facilitator's corresponding study group (Table 2).
| Instance # | Pseudonym | Topic :: strategy | Error persists into study group? |
|---|---|---|---|
| 1 | Lupita | Rings :: preferences between chairs by substituent orientation. | No |
| Rings :: relative Keq | |||
| 2 | Adam | R/S Stereochemistry :: assign – right hand rule | No |
| R/S Stereochemistry :: assign – clockwise/counterclockwise method | |||
| 3 | Daisy | Stereoisomer relationships :: assign different molecule – new molecule is enantiomer or diastereomer of another | Strategies not used |
| Stereoisomer relationships :: assign different conformation – same molecule but sigma bond is rotated |
The following description of Instance 2 illustrates how we observed an error made by Adam in CHEM 220, and how we used the recordings of Adam's facilitation during study group to determine whether the error persisted. Instances 1 & 3 are summarized in Table 2 and descriptions can be found in Appendix I.
Observed during CHEM 220: During Instance 2, facilitators were presented the tetracycline shown in Fig. 2 as part of their practice problems for the week, and were asked to identify and label all asymmetric carbons (or “carbon stereocenters”) present in the compound. While facilitators are working on the practice problems for the day (a mix of individual work and group work), Adam can be seen working on a problem where he applies the “right hand rule” strategy and the “clockwise/counterclockwise” strategy to the asymmetric carbon bound to the dimethylamino group. Adam then turns to talk to the facilitators next to him to ask about the problem:
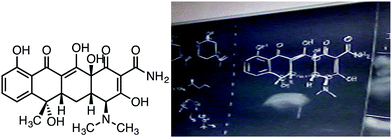 | ||
| Fig. 2 The tetracycline molecule used for study group facilitator R/S assignment practice (Instance 2). | ||
Other facilitator A: Did you get all S?
Adam: Wait, I got R.
Other facilitator B: For which one?
Adam: The bottom right one.
Other facilitator A: [inaudible]
Adam: Yeah.
Other facilitator C: I got S for that one.
Adam: (Talking about priorities) So I’ve got the N is one, then going to the right is two…
Other facilitator C: Yeah, it's S though.
Other facilitator A: Yeah it's S. so you did it right (talking about priorities) one, two, three, but…
Other facilitator B: Because in this case the hydrogen's in the back, so…
Adam: Oh, right! Whoops! Duh!
From the interaction between the facilitators during CHEM 220, it appears that Adam assigned his priorities correctly to the substituents, but he got mixed up with his R/S based on whether the priority 4 substituent was coming out of the plane or back behind the plane of the paper. [There are multiple other stereocenters in the molecule in question (tetracycline) that have the priority 4 substituent coming out of the plane of the paper (necessitating a reverse of the typical R/S assignment used for a strategy like “clockwise/counterclockwise”). Perhaps this caused Adam to be in the habit of assigning the opposite R/S than usual].
The other facilitators in the section are quick to point this out to him, and from his reaction it appears that once he was made aware of this he fully understood what his mistake was. Adam continues to discuss R/S assignments with the other facilitators, and he can be seen using the “right hand” rule along with the “clockface” rule in conjunction with “tripod arm” to visualize stereocenters. By discussing this strategy with his peers, Adam could go through stereochemical assignments using multiple strategies, and better understand how to use each one.
Observed during study group: There are four recordings of determining R/S assignments in Adam's study group. In all four instances, the clockwise/counterclockwise strategy is used. The strategy was observed being applied correctly three of the four times. For example, when Adam is helping walk his study group through the assignment of a stereocenter he was asked about, he first draws the compound on the board, then pulls out a model kit to emphasize which atoms are which, and finally assigns priorities to the groups. After assigning priorities with the lowest priority substituent oriented back into the plane of the board, the following conversation takes place:
Adam: So does everyone see kind of how I chose those?
Study Group Members: Yeah
Adam: Yeah? OK. So now we wanna read it from 1 to 2 to 3. So, 1 is blue, then white, then red. So that is going like that [draws a counterclockwise circular arrow on the molecule on the board in the proper direction of the priorities]. So is that like left or right?
Study Group Members: Left?
Adam: So like – left, or counterclockwise, so it's S.
During the fourth occurrence of this strategy, Adam was gesturing at one of his student's papers and we could not determine if he was applying the strategy correctly to the molecule in question. Overall, we did not observe Adam repeating the error that he originally made in CHEM 220 while he facilitated his study group.
Research Question 2: When misunderstandings displayed by study group members are not corrected during study group, what was that facilitator doing during the relevant CHEM 220 discussion section?
For six instances when errors made during the study group session went uncorrected, we could work backwards to the antecedent CHEM 220 meeting to see if the topic was being covered and/or what the facilitator was doing at the time. There was no single or general explanation for why the error was uncorrected. In two of these instances, the facilitator had not yet covered a strategy in CHEM 220 (Instances 4 & 5); in two instances, the facilitator was present in CHEM 220, but appears to be distracted, speaking with a colleague (Instances 8 & 9); and in the last two instances, the facilitator is off-screen (Instances 6 & 7). See Table 3.
| Instance | Pseudonym | Topic :: strategy | Facilitator behavior in CHEM 220 |
|---|---|---|---|
| 4 | Harrison | Rings :: axial or equatorial by parallels | Strategy not yet covered |
| 5 | Lupita | Rings :: chair flips by drawing both chairs | Strategy not yet covered |
| 6 | Oscar | Stereoisomer relationships :: assign meso – two opposite stereocenters with same substituents and internal molecular symmetry | Present but off-camera |
| 7 | Daisy | E/Z :: assign priority to substituents | Present but off-camera |
| 8 | Daisy | Stereoisomer relationships :: optically active – has R/S stereocenters but is not meso | Talking about problem over the instructor |
| 9 | Daisy | Stereoisomer relationships :: assign optically active – if compound is chiral // optically active – has R/S stereocenters but is not meso | Talking about problem over the instructor |
The following description of Instance 8 illustrates how we observed an unresolved error made by a member of Daisy's study group, and how we used the recordings from CHEM 220 to backtrack to what Daisy was doing when this topic was being discussed. Instances 4–7 & 9 are summarized in Table 3 and descriptions can be found in Appendix I.
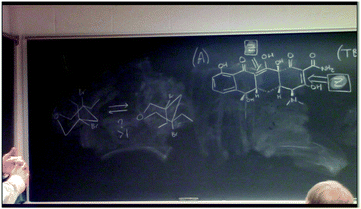
Observed during study group: Instance 8 occurred during a portion of Daisy's exam review session, during which she is leading students through a discussion about stereoisomers and their relationships, such as enantiomers and diastereomers. A diagram can be seen on the whiteboard that shows the relationships between all potential stereoisomers of a molecule containing two asymmetric carbon atoms (Fig. 3).
 | ||
| Fig. 3 A diagram used by Daisy to remember how changing stereocenters factors into stereoisomer relationships (Instance 8). | ||
This discussion leads to a question about meso compounds and how to identify them and their defining characteristics. The students and Daisy correctly state that if the compound in question was meso, then the S/R enantiomer would not exist (Daisy crosses this out on the diagram). Next, Daisy asks the students about how to identify meso compounds:
Daisy: How do you know if it's meso? What does meso mean?
Student A: It's…
Daisy: Yes?
Student A: Optically active but not chiral?
Daisy: (thinking) Um. Optically active… it itself can be optically active, but it does not have… yeah, that's right. But it's not chiral, so that's true.
Daisy then begins to talk about how to identify a meso compound based on if it has two similarly substituted stereocenters with internal symmetry. Her error of claiming that meso compounds are optically active goes either unnoticed or unchallenged by the students in her study group.
Observed during CHEM 220: the optical inactivity of meso compounds is addressed during the second week of CHEM 220 sessions on stereochemical topics. While no explicit description of optical activity is discussed in this context, several related concepts are. Several times, the liaison GSI includes the point that meso compounds are both achiral and optically inactive. In one such example, the liaison GSI says: “We can think about having two products that are optically active that are enantiomers to one another, and then the meso compound as well” – indicating the meso compound is separate from any optically active compounds. Additionally, another student had incorrectly identified a molecule in the worksheet as both chiral and meso on the board. Although the liaison GSI corrects this, Daisy and her classmate are observed having a sidebar conversation, and while it is on task (“Daisy: so, if it's meso, it does have an enantiomer but the stereocenters [inaudible]. So if the stereocenters are RS, the enantiomer would be SR, but it's the same thing, but it has a diastereomer [inaudible] it is SS, the enantiomer is RR, and the diastereomer is RS, so those are all there are”), she has also missed part of the discussion that might have caused her to question her understanding of optical activity with respect to meso compounds.
Research Question 3: When facilitators make an error in study group that is corrected by a study group member, what was that facilitator doing during the relevant CHEM 220 discussion section?
Analogous to what we found for Research Question 2, there is no single facilitator behaviour that is common to these uncorrected errors. In backtracking the coded strategies corresponding to the five instances of the facilitator making an error which is subsequently corrected by a study group member, we found distinct categories: two instances where the facilitator's CHEM 220 class on the topic was cancelled and a handout was emailed out (Instances 10 & 11), one where the facilitator was present in CHEM 220 but the strategy was not covered (Instance 13), one where the facilitator was (a) present but distracted during class or (b) off-screen (Instance 12), and one where the facilitator was present and paying attention in CHEM 220 (Instance 14).
The following description of Instance 14 illustrates how we observed an error made by Harrison during his study group that is corrected by a student, and how we used the recordings of CHEM 220 to backtrack to what Harrison was doing when this topic was being discussed. Instances 10–13 are summarized in Table 4 and comparable descriptions can be found in Appendix I.
| Instance # | Pseudonym | Topic :: strategy | Facilitator behavior in CHEM 220 |
|---|---|---|---|
| 10 | Andy | Newman projections :: assign or use gauche vs. periplanar | CHEM 220 canceled, handouts emailed out |
| 11 | Mark | Newman projections :: convert to Newman from dash and wedge | CHEM 220 canceled, handouts emailed out |
| 12 | Daisy | R/S stereochemistry :: assign chiral atom – requires 4 different substituents |
(a) present but distracted
(b) off screen |
| 13 | Daisy | R/S stereochemistry :: to get lowest priority in back – change perspective on the molecule | Strategy not covered |
| 14 | Harrison |
Stereoisomer relationships :: assign enantiomer – change of all R/S but not E/Z
Possibly: Stereoisomer relationships :: assign diastereomer – change of E/Z |
Present, looking silently at the board/GSI |
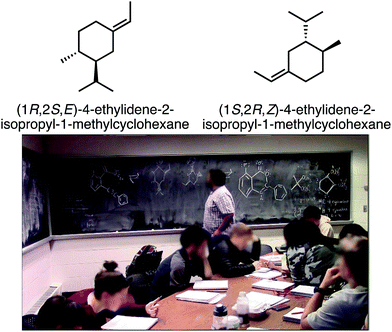
Observed during study group: In Harrison's study group, Instance 14 was observed during the discussion of the two molecules in Fig. 4. The problem asks students to agree or disagree with a series of statements about the relationship between the two molecular structures: they are enantiomers; they are diastereomers; they are different molecules; they are constitutional isomers; they are the same molecule.
In discussing this problem, Harrison first tells the study group members that the two molecules in question are not enantiomers or diastereomers, because they are “different molecules”:
Harrison: Oh wait, this just straight up isn’t the same molecule.
Student A: What?!
Student B: Yeah, they are different molecules.
Harrison: They’re just different molecules.
Student A: Wait, so they’re not enantiomers?
Harrison: No, they’re just different molecules. They’re connected wrong.
Student B: The connectivity is wrong.
Harrison: Count between the double bond and the two other substituents.
(Pause)
Harrison: (Pointing at molecule and counting carbons) So there's one carbon between this and this (pointing at alkene and isopropyl group). There are two carbons between this feature and this feature (pointing at alkene and methyl group).
Student A: Yeah, but what if you just flipped it?
Harrison: Then there's two carbons here (pointing at alkene and isopropyl group). This has three carbons here (pointing at alkene and methyl group).
Student A: But what if flipped all the wedges to dashes and all the dashes to wedges. Then you would have the same count.
Harrison: No. It's connected in a different spot. Right? Or am I going insane?
Student C: I’m counting it, and it seems like the connectivity is the same.
Student D: I think the connectivity's the same.
Student A: You got us all riled up.
Student D: [inaudibly speaking and pointing at his worksheet].
Harrison: Yeah, but those are different features. One is a methyl group and one is an isopropyl group.
[Lots of students talking at once]
Harrison: Oh no, yeah, you’re right. I’m just going insane. Oh my god. These are hard.
Harrison was convinced that the two molecules in question were structural isomers because he thought they contained different connectivity. He was confused in his counting of carbons, as he was not consistent in his counting from the alkene to the other substituents. However, after multiple students explained their thinking to him, including one student showing Harrison his worksheet to help explain his thinking, Harrison realized that the two molecules do indeed have the same connectivity.
Observed during CHEM 220: The relevant topic came up twice during the class Harrison attended. First, the liaison GSI gives definitional coverage of the strategies used to differentiate stereoisomers, though it is not done in the context of a problem. Harrison is present and appears to be looking at the board and liaison GSI during this time, but at no time speaks or interacts with others while this topic is discussed.
Later, the topic of differentiating stereoisomers came up again in the same CHEM 220 session, in the context of a specific problem. Harrison was off-screen during the discussion, and we cannot report direct observations of what he was doing. However, because we can hear clearly the audio of the entire classroom, we do have evidence that he did not speak up while this topic was being revisited; that is, we do not have evidence of hearing his voice asking or answering questions, or discussing anything out loud, during this time.
We are focussing on this instance because it is intriguing. As described above, Instances 10–13 are easy to explain: the topic was not covered or not covered yet in CHEM 220, and the facilitator's misunderstanding was not revealed. This is not true for Harrison. Even though he was present and attentive (i.e., not obviously participating in distracting behaviours such as chatting with a classmate) during the liaison GSI's discussion of how to distinguish stereochemical and other isomeric relationships, he was unable to employ these strategies himself. Alternately, Harrison could have been having an off day. However when you take his instance in combination with the others, it is also true that we have no example where a person who actively engages a topic in CHEM 220 ends up making an error in their PLSG session.
Although Harrison's is only a single instance from our recordings of study groups and CHEM 220 sections, it suggests that the facilitator can harbour misunderstandings when not actively confronted by the need to discuss or present their ideas. In situations where facilitators do not speak up about content, or do not participate in problem solving at the board, they are perhaps unaware of their own content limitations as opposed to uncertain (Egré and Bonnay, 2013). Providing an environment for undergraduate peer leaders to surface misunderstandings that they might not be aware they have is one of the major goals of CHEM 220. Although we cannot know the degree to which errors that would have otherwise been made have been intercepted by CHEM 220 (or not), these multiple and independent observations of corrections (and when errors persist) are consistent with a positive role for the CHEM 220 intervention. This is perhaps a critically significant aspect of the course – or more broadly, something to consider when using peer leaders in other instructional settings.
Research Question 4: If a facilitator is present when another facilitator makes an error in CHEM 220 and sees it resolved, how does this observing facilitator handle that topic if/when it arises later in their own study group?
In instances where we recorded a facilitator observing and being engaged with another facilitator making an error during CHEM 220, the observing facilitator does not make this error, either. We note emphatically that we cannot distinguish between a topic that the observing facilitator correctly understood anyway, versus a topic that the observing facilitator self-corrected because of observing the error. In this exploration, we reasoned that all errors were important to pursue for understanding the effects from CHEM 220 on the study group program and its facilitators, including when a facilitator was present when a peer was making an error, even if we could not know what the observing facilitator did or did not understand at the time.
Question 4 is also compelling because its effect is multiplicative: a facilitator is observed making an error, and it is corrected in front of a group of other facilitators, all of whom we can pursue into their study group sessions. Thus, compared with the other three research questions, we observed the greatest number of these types of instances (18) in CHEM 220, and we did not observe a single occurrence of the error in any of the subsequent study group sessions. These instances differ from Instance 14 (Harrison, see previous), in that Harrison was not observing an explicit correction of another facilitator's error.
In the 18 instances that fell under this question, we found two distinct outcomes that were equally likely: either (a) the topic was addressed by the observing facilitator correctly, or (b) the topic was not covered in the study group sessions for which we have audiovisual recordings. Inasmuch as some of these errors were made in the presence of multiple facilitators during the CHEM 220 session, there were actually 30 possible chances for the observing facilitator to make an error that we could follow forward into study group. Of these 30 possible chances for error, in 15 the observing facilitator used the strategy correctly, and in 15 the observing facilitator did not use that strategy. In no instance did we observe an observing facilitator making the error in their study group that they saw corrected in CHEM 220.
The following description of Instance 16 illustrates how we pursued the strategies employed in an error made by Adam during CHEM 220 (and observed by Daisy) into Daisy's study group. Instances 15 & 17–32 are summarized in Table 5 and detailed descriptions can be found in Appendix I.
| Instance # | Pseudonym of facilitator who saw another's error correction | Topic :: strategy | Correctness maintained in study group? |
|---|---|---|---|
| 15 | Mark | Rings :: determining the relative preferences of chair substituents and how that influences the relative Keq | Yes |
| 16 | Daisy | R/S stereochemistry :: (1) right hand rule; (2) assign – clockwise/counterclockwise method; and (3) assign – tripod arm method | Yes |
| 17 | Adam | R/S stereochemistry :: assign different molecule – new molecule is an enantiomer or diastereomer of another // assign different conformation – same molecule but sigma bond is rotated | Topic not addressed in study group |
| 18 | Oscar & Gwen | Electrophilic addition :: acid base rate is faster than EA | Yes |
| 19 | Mark & Lupita | R/S stereochemistry :: assign different molecule – new molecule is an enantiomer or diastereomer of another // assign different conformation – same molecule but sigma bond is rotated | Topic not addressed in study group |
| 20 | Mark & Lupita | R/S stereochemistry :: assign chiral Atom – requires 4 different substituents |
Mark: Yes
Lupita: Topic not addressed in study group |
| 21 | Harrison | R/S stereochemistry :: assign different molecule – new molecule is an enantiomer or diastereo of another // assign different conformation – same molecule but sigma bond is rotate | Yes |
| 22 | Andy & Carrie | Rings :: relative Keq | Topic not addressed in study group |
| 23 | Max |
Stereoisomer Relationships :: Assign meso –mirror image is same compound
Stereoisomer Relationships :: assign meso - two opposite stereocenters with same substituents and internal molecule symmetry |
Topics not addressed in study group |
| 24 | Max | Stereoisomer relationships :: assign enantiomer – change all R/S but not E/Z | Topic not addressed in study group |
| 25 | Max | Stereoisomer relationships :: assign diastereomer – change E/Z | Yes |
| 26 | Max | Stereoisomer relationships :: assign different molecule – new molecule is enantiomer or diastereomer of other | Topic not addressed in study group |
| 27 | Max | Stereoisomer relationships :: assign optical activity – based on enantiomeric or diastereomeric relationship to known compound | Topic not addressed in study group |
| 28 | Oscar & Gwen | Stereoisomer relationships :: assign different molecule – new molecule is enantiomer or diastereomer of other |
Gwen: Yes
Oscar: Not addressed |
| 29 |
Andy & Lupita
Andy & Mark Andy & Mark |
Stereoisomer relationships :: assign optical activity – based on enantiomeric or diastereomeric relationship to known compound
Stereoisomer relationships :: assign different molecule – new molecule is enantiomer or diastereomer of another Stereoisomer relationships :: assign different conformation – same molecule but sigma bond is rotated |
Andy: Correct definition of strategy
Lupita: Not addressed Mark: Correct definition of strategy Andy: Not addressed Mark: Correct definition of strategy Andy: Not addressed |
| 30 | Daisy & Adam |
Stereoisomer relationships :: assign meso – mirror image is the same compound
Stereoisomer relationships :: assign Chiral – has R/S stereocenters but isn’t meso Stereoisomer relationships :: assign Optically active – if compound is chiral |
Daisy: Yes
Adam: Not addressed Daisy: Yes Adam: Not addressed Daisy: Yes Adam: Not addressed |
| 31 | Harrison | Stereoisomer relationships :: assign different conformation – same molecule but sigma bond is rotated | Yes |
| 32 | Harrison | R/S stereochemistry :: priority of substituents – double bonds count as two identical branches | Yes |
Observed during CHEM 220: For Instance 16, we refer to the error previously described (Instance 2), where Adam struggled to assign R/S to a stereocenter in tetracycline during CHEM 220. However, in this section we turn our attention away from Adam and to a different facilitator that observed this error happen in CHEM 220: Daisy. To get a best answer to our research question, we pursued the strategies used by Adam into Daisy's study group to see if they are used correctly by Daisy when discussed with her study group members. As shown in Table 5, Adam employed three different strategies to assign stereolabels to asymmetric carbons. With these strategies in hand, we searched Daisy's study group recordings for her use of these three strategies.
Observed during study group: Throughout all videos of Daisy's study group, these strategies to assign stereolabels to an asymmetric carbon are used correctly. Daisy uses the “right hand rule” strategy five times throughout her study group videos, each time correctly arriving at the answer. In all fourteen uses of the “assign clockwise/counterclockwise” strategy in Daisy's three study group sessions, she correctly uses the strategy. The “tripod arm” strategy is not used in any of Daisy's study group sessions.
Daisy is an interesting individual for our case precisely because she was more inclined to make errors than some of the other leaders, and so the correlation of her correctness with CHEM 220, and the converse, if present, builds evidence for the intended effect of CHEM 220. An important distinction exists between Daisy's observation of the corrected strategy (Research Question 4) used to assign R/S stereolabels, and all of Daisy's other reported errors (Research Questions 1–3) from above (Tables 2–4). Of the six instances of error captured by Daisy while investigating Research Questions 1–3, Daisy makes errors on assigning E/Z stereolabels (Instance 7), identifying the relationship between a pair of stereoisomers (Instances 3, 8 & 9), and in identifying whether a carbon is asymmetric or not (Instance 12). When Daisy uses one of the three strategies for assigning R/S stereolabels that were explicitly engaged during CHEM 220, which she does seventeen out of seventeen times, she uses the strategy correctly. And while she does make an error in this specific topic (Instance 13), it is not because she gets one of the three strategies from CHEM 220 wrong, it is because she uses an unvetted strategy of her own devising.
Discussion
In examining the propagation of errors in understanding stereochemistry and conformational analysis by peer facilitators in our PLSG program, we found that facilitators whose errors are corrected, or who observe a peer being corrected, in CHEM 220 do not carry errors in these topics to their study groups. Overall, we observed that peer facilitators are overwhelmingly correct (384/395 instances) when discussing these topics with their students in their study groups. In the rare case (11/395) when they did make errors, either the specific topic did not come up, or come up yet, during CHEM 220, or the facilitator was disengaged from the discussion. Additionally, in five of 11 instances of facilitator errors, study group members corrected the error. Thus, we only observed six instances where an error made by a facilitator went uncorrected during a study group. We coded but did not analyse errors made by study group members for this study.While a gratifying endorsement of the PLSG program and its use of peer leaders (Coppola et al., 1997; Sandler and Salvatore, 2013), we hasten to remind readers that we intentionally selected stereochemistry and conformational analysis as topics because understanding these topics at the introductory level is neither highly conditional nor nuanced (Walba, 1985; Baker et al., 1998). We anticipated that these topics were going to be easy to study, and that errors would be infrequent and unambiguous. If a positive effect associated with CHEM 220 – emphasizing the importance of subject matter knowledge – could be detected in these topics, then it argues strongly for a potentially greater effect, one way or the other, when the subject matter topic is more conditional and/or nuanced.
From the inception of CHEM 220, we intended to provide an open environment to intercept and correct errors: for facilitators to ask questions, clarify misunderstandings, and refresh themselves on previously learned subject matter (Barnard et al., 2017). Additionally, this course provides exposure to multiple strategies for subject matter explanation and discussion led by both the liaison GSI and the other facilitators. As a matter of principle, we think it is better to have the entire instructional team, from the faculty members through the undergraduate peer leaders, as much on the same page as possible.
In trying to understand how any errors from CHEM 220 sessions may or may not persist into study groups, several distinct paths of facilitator error were made between CHEM 220 and study groups (Fig. 1). In general, our observations are consistent with a positive effect from having students participate in CHEM 220 as a required part of their role as study group facilitators.
Peer facilitators who made errors in stereochemistry and conformational analysis during CHEM 220 did not carry this misunderstanding into their study groups. We observed three specific instances of facilitators’ answering questions incorrectly in CHEM 220 that were clarified by the liaison GSI (Table 2). When probing whether these misunderstandings that were brought to light in CHEM 220 propagated to study group, we did not observe this error carried into the facilitator's own study group. In two of the three instances, the strategy was observed in their own study groups, and in all cases the strategy was applied correctly.
Peer facilitators who made uncorrected errors in stereochemistry and conformational analysis during study groups had various reasons why they did not capture this error, but in no instance was that topic one in which they engaged actively during the antecedent CHEM 220 meeting. We observed six specific instances of study group members making errors that went unresolved by the peer facilitator (Table 3). When back tracking these topics discussions in CHEM 220, we observed two possible sources of error: (1) in two instances, the peer facilitator covered material ahead of the pacing of CHEM 220 and (2) in two instances, the peer facilitator was present but distracted speaking with a colleague on camera. As described in Limitations below, the peer facilitator was off-camera during discussion of the topic in the final two instances, so no claim of behaviour may be made; however, the aforementioned clear audio evidence that the facilitator did not speak up while this topic was covered suggests the need for increased engagement from facilitators. The audiovisual data collected supports the ideas that (a) CHEM 220 should be more carefully paced ahead of study group topic coverage in order to allow for content review to take place, and (b) the CHEM 220 course should promote interactive learning and ensure all students are engaged throughout topical discussion.
Peer facilitators who made corrected errors in stereochemistry and conformational analysis during study groups, also had various reasons for not having these misunderstandings resolved in CHEM 220, but in no instance was that topic one in which they engaged actively during the antecedent CHEM 220 meeting. We observed five specific instances of facilitators making errors that were corrected by study group members (Table 4). When tracking these topical discussions back to CHEM 220, we observed three possible sources of error: (1) In two instances, handouts had been emailed in lieu of CHEM 220 being cancelled that week, (2) in one instance, the peer facilitator was present but distracted speaking with a colleague on camera, and (3) in one instance, the peer facilitator covered material ahead of the pacing of CHEM 220. Intriguingly, despite the peer facilitator being present on-camera and paying attention to the liaison GSI during CHEM 220, in the fifth instance (Harrison) the facilitator still made an error in study group. The audiovisual data collected reinforces the ideas (above) about pacing and interactive engagement, the latter being particularly important for cases such as Harrison, where enrollment and apparent attentiveness in class was not sufficient to surface a misunderstanding.
Peer facilitators who observed other facilitators make errors in stereochemistry and conformational analysis corrected by the GSI during CHEM 220 did not demonstrate this misunderstanding in their study groups. We observed eighteen specific instances of facilitators’ answering questions incorrectly in CHEM 220, and then these errors were subsequently clarified by the liaison GSI and observed by other facilitators, resulting in thirty instances to investigate for correctness (Table 5). In fifteen of the thirty instances, the instructional strategy was observed in the facilitator's study groups, and in all cases the strategy was applied correctly.
Limitations
While the audiovisual data we have collected and analysed points to CHEM 220 working as intended (i.e., correcting errors and improving subject matter knowledge), the limitations of our case study must be understood and considered. As a case study, we gained a deeper glimpse into how facilitators’ enrollment in CHEM 220 impacts their study group interactions, which can inform methods by which we may understand and improve instructional coherence through improving subject matter knowledge. For those involved with undergraduate peer instructors, the inference from our results is to highlight the role that attending to subject matter knowledge has on the question of their teaching effectiveness.Although the data we have collected for stereochemistry and conformational analysis provides a rich understanding of these specific topics, other topics may provide drastically different results. For example, studies on more complex topics (e.g., mechanism, synthetic pathways) could exhibit much greater error rates, much lower correction rates, and defy unambiguous analysis via analogous methods. In addition, the effect of CHEM 220 on complex topics might not be positive if the 50 minute time frame creates more uncertainty and tentative behaviour in the facilitators.
Additional variables unconsidered here are the identity and skill level of the liaison GSI, the primary instructor for CHEM 220, the GSI-led discussions, and even the faculty-led classroom sessions, which could provide increased variability in how topics are approached and discussed in any content review setting. The composition of the pool of facilitators is constantly shifting: they derive from different courses in different terms, they range in experience as facilitators – the dynamics inside the different CHEM 220 meetings will depend, in some part, on the constituent members. For this study, the 12 facilitators that volunteered to be videoed in their study sessions are, to some extent, self-selected – this may have represented the most motivated section of the entire cohort of 58.
Facilitators were neither screened nor assessed for their subject matter knowledge prior to CHEM 220 (other than hitting a B+ minimum grade requirement when hired by the Science Learning Center). The facilitators were not tested before or after their CHEM 220 session to try and establish a connection to what was happening during those 50 minute periods, nor were they interviewed or otherwise instructed to reflect on their CHEM 220 experience. Thus, we cannot make any claims regarding individual facilitator knowledge. Because we have no information about what the facilitators did between CHEM 220 and their PLSG session, we cannot exclude the effects from any other forms of reviewing and/or preparation that they might have done on their own and/or with others.
Additionally, and as mentioned previously, it is impossible to assess, experimentally, whether any given error made in the CHEM 220 setting would have propagated into the study group setting, or whether, in the case where the facilitator observed a corrected error, that the subject matter knowledge of the facilitator was different, empirically, because of the observation and concomitant discussion. However, the evidence is consistent for the positive effect of CHEM 220 on the subject matter correctness displayed by the facilitators: (a) we have no instance of a facilitator who actively engages their error in CHEM 220, or who engages the error made by others, who then makes the error in their session, (b) we have facilitators who, although they make errors in their PLSG sessions, can either be observed in CHEM 220 meetings where the topic is not discussed or where the facilitator was not actively engaged in the discussion.
Conclusions and implications for practice
In the context of a large instructional team, our case study results are consistent with positive effects from providing a forum – such as CHEM 220 – for developing, revisiting, and correcting subject matter knowledge of peer leaders. This intervention provides a potentially effective way to anticipate and correct leaders’ misunderstandings of course content.Observations derived from the four separate research questions build a single picture that is consistent with CHEM 220 improving and clarifying the subject matter knowledge of our facilitators in the topical area we examined, and on their subsequent instruction in those topical areas. We have reported instances where subject matter errors were explicitly corrected after being made or observed by peer facilitators in CHEM 220, which we then pursued into the teaching done in their study groups, and observed that these errors were not being propagated. We have also reported instances were errors were made by peer facilitators and back-tracked these topics into their antecedent CHEM 220 session, and observed that the topic, for various reasons, was not explicitly addressed by the facilitator. Although limited as previously outlined, the exploratory case we have presented provides compelling detail about the importance of ensuring correctness of subject matter knowledge when using peer-led instructional methods.
Our prior work on the introduction of CHEM 220 focused on the perceptions of the facilitators (Barnard et al., 2017), who reported that the utility of the course stemmed primarily from providing access to (a) physical resources (e.g., handouts and problem sets), (b) human resources (e.g., the liaison GSI and fellow peer facilitators), and (c) a means to refresh peer facilitators on their content knowledge. Our use of audiovisual data in this study has allowed us a much deeper perspective of the flow of subject matter knowledge from liaison GSI to facilitator to study group member in our PLSG program, as well as insight into the correctness of subject matter usage within both CHEM 220 and study groups.
In seeking to improve instructional coherence, our study reveals some outstanding challenges. First, some errors result from topics or strategies not yet being covered in CHEM 220; alternately, we may be able to increase instructional coherence by continuing to reinforce facilitators’ opportunities to ask the liaison GSI when confronted with topics from study group with which they are unfamiliar. Additionally, while some facilitators are present in CHEM 220 when topics are covered, additional effort must be placed on engaging all facilitators present. Given our findings, we contend that engagement is a key feature in allowing facilitator errors to come to light (Brown et al., 2014).
By thinking of our peer leaders as an integral part of the instructional team, we are elevating their status closer to that of faculty instructors. As peer-led methods continue to develop, diversify, and proliferate, running the gamut from formal to informal, face-to-face to cyber, from in-class to out-of-class, from using contemporary peers to senior students, we urge attention to the role that subject matter expertise plays for anyone who takes on this teaching role. This does not preclude inexperienced or naïve individuals from taking on a teaching role, but rather the need for explicit opportunities for teachers (and students) to confront their misunderstandings about content as a part of their learning. In principle, by increasing these opportunities for teacher-student interactions, regardless of who is taking on these roles, we increase the efficacy of our learning environments, i.e., improve learning.
Conflicts of interest
There are no conflicts to declare.Appendix I
Note from the authors
Throughout the following pages, research questions serve as headers for additional examples pulled from additional audiovisual data. As a reminder, in cases when facilitators or study group peer members are using a strategy to solve a problem on paper (i.e., we cannot see the problem they are working on) correctness codes have been omitted.Research Question 1: Do misunderstandings that the PLSG facilitators demonstrate during the CHEM 220 discussion sections, which are addressed during CHEM 220, persist during the study group; and if so, in what ways?
For Instance 1, facilitators in CHEM 220 are given a set of practice problems to work on. One of the problems involves a cyclohexane-like molecule shown below in Fig. 5. The facilitators must convert the molecule into its two chair forms, and then predict the equilibrium constant between the two forms based on what substituents are in the axial position vs. the equatorial position.
 | ||
| Fig. 5 Example Compound Given to Study Group Facilitators for a “Chair-type” Practice Problem in CHEM 220. | ||
Each chair form has one ethyl group in the axial position and one in the equatorial position (and the same for bromine substituents). This makes the chairs exactly equal in energy, and because of the nature of the molecule, the equilibrium constant should be 1 (as opposed to >1 or <1). When Lupita silently copies her answer to the problem onto the chalkboard, she correctly draws both chair forms. When she predicted the value of Keq (the equilibrium constant) between the two chair forms, what she wrote on the board is shown in Fig. 6.
As can be seen from Fig. 6, Lupita's answer is “?>1”, indicating that she thought the answer should be >1, but was not sure. Even if Lupita had given the correct answer, the presence of a “?” with her predicted value of >1 for the Keq is indicative of her lack of confidence with this problem. The liaison GSI proceeded to walk through the problem in front of the class in order to address the incorrect answer:
Liaison GSI: “So, for our first sample. As previously stated, there are a ton of R/S stereochemistry problem on this exam, as most second exams have. And so this was one of the products that was formed from the reaction shown on the page. As we’ll see next week, the rest of that problem discusses the stereoisomers of that and the different products that that has. And so, comparing these two different chair forms of the given product “A” I believe it is. What we see is that we have two carbons away from our oxygen we have a bromine and ethyl group. (pointing at the chair form to the left of the equilibrium arrows) and on the left hand side the bromine and ethyl group are axial and on the right hand side (pointing at chair to the right of the arrows) bromine and ethyl group are axial. So in this case, with this particular product, we actually see two products (chairs) that are in the same equilibrium ratio. Because we don’t gain anything energetically one over the other. So in this circumstance (erases wrong answer and writes “Keq= 1”) these are actually having a Keqequal to 1. And as a reminder on Keqstuff, because I think we’ve talked about it, but I’m not totally sure because it's something that took me a while to grasp, is that we want to determine where the equilibrium lies. If it's equivalent between the two, Keqis equal to one. If it favors the right hand side product (chair), then Keqwill be greater than 1, and if it favors the left then Keq will be less than 1. More on that problem next week…”
During the correction of her given answer Lupita did not ask any clarification questions. Lupita cannot be seen during the mini lecture on Keq by the liaison GSI, but he has corroborated that Lupita was paying attention while the error was corrected.
How this strategy was observed in study group: There is one instance of “preference between chairs by substituent orientation” in Lupita's study group. In response to an inaudible student question with a chair conformation on the board, she correctly explains that the “bulkier” substituent is more stable in the equatorial position in a chair conformation. This interaction in study group is also the only instance of the “Relative Keq” code that we have for Lupita. She correctly drew the two chair conformations to illustrate the effect of bulky substituent orientation on the relative Keq.
Instance 3 features Daisy working with other facilitators on a series of “box-check problems” in a semi-group/semi-individual manner at this point of the class (an illustrative example of a box-check problems is shown in Fig. 7 below; in this instance, Daisy is asking about problems similar to Part A). The facilitators are asking the CHEM 220 liaison GSI questions as they fill out the practice problems, and he is doing his best to answer the questions as the facilitators are working through the problems. Following a talk about optical activity, Daisy asks a question aloud to the group:
Daisy: Should you say “different molecules” if there's different stereocenters?
Other Facilitator A: That's what I was wondering too.
Liaison GSI: Yes.
Other Facilitator A: They’re different molecules if they have different stereocenters.
Other Facilitator B: The only time they’re the same is if they’re conformations.
Daisy: Like a chair flip?
Other Facilitator B: Exactly!
In this case, Daisy did not make an error that we can observe. However, the fact that she raises a question to the group while working on a box-check problem indicates that she did not have complete command of the topic in question. After she asks her question about whether or not two compounds are “different molecules” if they contain different stereocenters, another facilitator states that he had a similar question. This question is answered with a simple “yes” by the liaison GSI, but it is also answered in a more thorough manner by a fellow facilitator. The other facilitator expands upon the answer to the question by stating that two compounds would in fact be the same molecule if they are conformational isomers/conformers. Daisy then checks her understanding of conformational isomers by asking if a chair flip produces two images that are different conformations of the same cyclohexane ring.
How this strategy was observed in study group: There were no instances of the code “assign different molecule – new molecule is an enantiomer or diastereomer of another” being used in Daisy's study group sessions. There was one instance of the “assign different conformation if same molecule but sigma bond is rotated” strategy being used during Daisy's study group, but correctness could not be determined because Daisy was gesturing to work on a student's individual worksheet, and the answers on the worksheet could not be seen.
Research Question 2: When misunderstandings displayed by study group members are not corrected during study group, what was that facilitator doing during the relevant CHEM 220 discussion section?
In Instance 4, a student in Harrison's section had just finished explaining how she converts from the planar version of a 6-membered ring to the chair version by numbering her carbons on each structure drawing on the blackboard (Fig. 8). The molecule in question is a simple cyclohexane ring with only hydrogen substituents in all of the axial and equatorial positions. The conversation in study group went as follows:
Student A: (Discussing converting from planar version to chair version) Wedges will always be in the up position. And then whether you have this chair or the opposite chair will change whether it's equatorial or axial. Equatorial will always be going to the sides, while, wait, well yeah, and axial will always be going up or down. Let's see…
Student B: You should also remember that when you’re drawing, you know that equatorial H by 3? You would always want it to be parallel to 5 and 4.
Harrison: Yeah, the side lines, sort of, yeah.
Student B: That's just something to remember.
Harrison: Yeah.
Student A: This one? (pointing at her drawing).
Student B: Yeah. No! Go to your equatorial H, the one on 3. And you see how it's parallel to 5 and 4?
Student A: Oh! This! (pointing at a different spot).
Student B: Yeah!
Harrison: Yeah!
Student B: So you always want that.
Harrison: So your equatorial lines are always parallel to some other lines in your chair.
Student B: Yeah. It's just important…
Harrison: Yeah. You drew them right.
Student B: You drew them perfect.
Harrison: You drew a really good chair. If your chair looks like that on the exam you should be very happy.
Student A: Cool.
Even though both the facilitator and another student proclaim that the chair that was drawn is a perfect chair, the drawing in question did not have all equatorial H bonds drawn parallel to the corresponding bonds in the ring. This problem was coded as incorrect and unresolved because nobody pointed out the improper bond angle that exists for one of the equatorial hydrogens, and they in fact drew attention to the bond angles as a perfect example of a correct chair structure.
Facilitator behavior during CHEM 220 when this strategy was covered: While this is a rather small point in proper representation of chair conformations, Harrison is emphasizing that this is a perfect drawing – for completeness of presenting our case study, we have included this error in our analysis. In CHEM 220 the liaison GSI said
“I am operating under the assumption that the majority of you are very familiar with chairs, drawing chairs, drawing substituents. If you have questions on that, feel free to let me know. I am mostly going to focus on the idea of chair flips ‘cause that is something that students can get very confused on very quickly, and there are multiple methods for doing that.”
In Instance 5, Lupita has just finished talking through how to accomplish a chair flip by drawing both chairs, and she asks the study group if anybody knew of a different way to do a chair flip (looking for a study group member to explain “chair flips by rotating substituents around ring”). Nobody says anything for a few moments, then a student begins describing something that her discussion GSI showed her:
Lupita: What's the other way that we can chair flip? [silence] I’ll give you a hint. I can draw the exact same chair (as before) and still chair flip this guy with the same shape. But what would happen to my substituents? [silence]
Student: Is it like a mirror?
Lupita: Explain.
Student: I remember from discussion my [GSI] drew, like, two exactly the same just mirrored. And he said they’re different because, it's like the hand. It's never going to be, like, exactly on top of one another. I don’t know…
Lupita: Okay.
Student: It's difficult to understand.
Lupita: No. Yeah. Maybe. I don’t want to say I should know about, like, draw it up there and see [inaudible]. Anybody else?
From the video, it appears that Lupita does not know if the strategy presented by the student is a valid way to accomplish a chair flip. From the description given by the student, the strategy would not accomplish a chair flip, and would instead give you the enantiomer of the original chair structure that was given. In fact, the strategy suggested by the study group member to draw the two chairs “exactly the same just mirrored” fits perfectly with our code “draw enantiomer – mirror image is new compound”. For this reason, the strategy described by the student was coded as “incorrect”, and because Lupita's answer was essentially “maybe, draw it and see”, we coded this instance as “unresolved”.
Facilitator behaviour during CHEM 220 when this strategy was covered: When this study group had occurred, Lupita had not yet been “refreshed” about enantiomers and diastereomers in CHEM 220, causing us to list this as “strategy not yet covered” under the “Facilitator Behaviour in CHEM 220” heading. When the topic was addressed in CHEM 220 it was not done in the context of chair flips making this student's “strategy” especially challenging to troubleshoot.
Instance 6 occurred during Oscar's exam review session, in which the students are working on “box check problems.” In this instance, Oscar's group is working on a problem like the Part B examples from Fig. 7. For one of the problems, the molecule shown in Fig. 9 is in question:
The students are talking amongst themselves about which descriptions (as boxes they have checked) are true for this molecule:
Student A: I have ‘has at least one chiral diastereomer’, ‘a meso compound’, and ‘optically inactive’.
Student B: What?
Student C: Yeah, that's what I got.
Student B: I just got (boxes) 1 and… It's not meso.
Student A: It is meso.
Oscar: Hey, which one are we on?
Student D: Don’t both have to be wedged or both have to be dashed?
Other students: No.
Student E: One has to be R and one has to be S.
Oscar: Okay, so let's… So what do we think about the first one?
Student A: We said the left one was R and the right one was S. Then I said it has at least one chiral diastereomer, it's a meso compound, and optically inactive.
Oscar: Why do we think it's meso?
Student B: It's not meso.
Student F: There's no line of symmetry.
Oscar: I’m going to draw something…
Student E: There is a line of symmetry.
Student B: Where?
Student E: There is. Right here (points towards her worksheet).
The students continue to talk amongst themselves as Oscar draws the molecule in question on the board and then redraws it with the wedge and dash representations inverted to show (incorrectly) that you cannot superimpose the two molecules:
Oscar: (gesturing towards whiteboard) So I’ve switched R and S here and then I flipped it over. Are these things the same?
Students: No.
Oscar: Apparently not.
Student E: Wait, wait, wait. That's the problem, right? (pointing at original molecule).
Oscar: This is the problem. I switched R and S, and then I flipped this thing over (gesturing to show a rotation of the whole molecule, flipped like a pancake). Notice that this ‘O’ is now shifted over one.
Student B: So they’re not meso. We were right.
Oscar: They are not meso.
Oscar failed to execute the proper rotations that would have shown that the molecule is indeed meso. However, the answer key he was provided for the practice exam that he was going through with his students incorrectly claimed that this molecule is not meso. Oscar was, for the most part, just sticking with the answer that the key to the exam provided for him. For this reason, he did not exhaust the possibilities for rotating the molecule to see if it was actually meso or not. If he had not already been convinced by the answer key that the molecule in question was not meso, perhaps Oscar would have spent more time manipulating the molecule and discovered that it is indeed meso.
Facilitator behavior during CHEM 220 when this strategy was covered: The “Assign Meso – two opposite stereocenters with same substituents and internal molecular symmetry” strategy is touched on at the end of the second week of CHEM 220 along with several other features of meso compound determination. Oscar was present during this discussion of determining if a compound is meso, and received the complementary handout mentioned via email after discussion.
Liaison GSI: Meso compounds have internal symmetry, which cancels out any optical activity. We did not discuss this in detail today, I have a blurb that Professor X sent me last term, about uhm, optical – excuse me, meso compound identification. The easiest way to identify a meso compound that I usually think of is if I have a molecule [draws molecule] I use the traditional version of taking the enantiomer, which is draw this with a mirror plane [draws], and I notice that I have the same starting material. You can also swap all sources of R/S stereochemistry, and if you still have the same molecule, the meso compound is the same molecule if you rotate about this axis [draws] but theres more detail in the email than that.
Interestingly enough, the sample used during discussion is the same molecule from Oscar's study group problem. All facilitators were off camera during this concept review, so additional insight into Oscar's attentiveness during this segment is not available.
In Instance 7, Daisy presented the structure shown in Fig. 10 to help students practice assigning R/S stereochemistry, and the study group member that presented the molecule correctly assigned both stereocenters as “S”. However, after Daisy affirms the “S” assignments, a student asks about also labelling the alkene stereochemistry:
Student: Oh wait! Aren’t you also supposed to label the alkenes?
Student A (that wrote down answer on board): Oh yeah, I didn’t write that down. Sorry.
Daisy: Oh yeah! Yes, but in this case since it's a small ring they’re always going to be “E”.
Student A: What?
Daisy: So you only label E or Z in a ring if it's greater than eight carbons. So this ring, it has five. So I guess if you did it on an exam you wouldn’t be wrong, but in small rings they’re always going to be E. Because in small rings they’re flexible enough to be Z, because then you would have to break the bond, like, break the ring.
Student B: Do we have to label them in the ring?
Daisy: No. If it's greater than eight carbons.
Student B: Yeah. [inaudible]. So we’re just not dealing with the ones in the rings now?
Daisy: I think for now, for the exam coming up, for tomorrow, I think that they’re not going to give you any double bonds in rings. But you can do them in rings, but you assume the small rings are always going to be E.
Student A: But isn’t that top one Z, though?
Daisy: Yeah. But you’re going to assume it's going to be E. So if it's in a small ring it's so strained that you’re not going to have, like, a Z conformation in the ring. So that's why they don’t ask you to label them. But if it's greater than eight carbons, then you would label it like you normally would. So if this was greater than eight carbons you guys could do it because you can label priories. I don’t know if they are going to ask you that, but in this case you don’t have to label them because it's less than eight.
When Daisy is asked the question about labelling alkene stereochemistry in the ring of the compound, she mistakenly tells the students that alkenes in small rings can only ever have E stereochemistry. Daisy did not explain her reasoning for this statement, so we cannot make a guess as to her thought process while discussing this problem. Even after she is confronted by a student about the alkenes in the example problem being Z, she still sticks with her answer that “you’re going to assume it's going to be E”. Because Daisy never deviates from her original, incorrect answer, this instance of incorrectness was never resolved in the study group.
Facilitator behavior during CHEM 220 when this strategy was covered: E/Z assignment within rings were discussed in CHEM 220 during the first week on stereochemical topics. Daisy was present, but off camera, as the liaison GSI discussed two examples of assigning Z stereochemistry to double bonds in the six membered rings of tetracycline.
Liaison GSI: For E/Z stereochemistry there are two possibilities. In terms of priorities the first thing is atomic number followed by atomic mass. Then, what we look at if this – so in the case of this first double bond [gestures to the left-hand alkene from Fig. 6, part A] on the right-hand side, comparing an oxygen to a carbon, oxygen has a higher atomic number and atomic mass, but, higher atomic number gives our oxygen priority. On the left-hand side, it's a carbon and a carbon, so what do you do when you have the same stuff? You compare what's bound to that stuff. On the one hand, we have a carbon double bound to an oxygen [gesturing at the carbonyl] here, on the otherside we have two carbon–carbon bonds and a hydrogen. That means the carbonyl is our highest priority [writes Z next to the alkene].
Therefore, examples of assigning E/Z to double bonds present in rings smaller than eight carbons were indeed covered by the liaison GSI in CHEM 220.
Instance 9's unresolved error stems from the same larger discussion that the previous error (Instance 8) came from. Daisy is still leading a discussion about meso compounds, diastereomers, and enantiomers with the study group members. During the talk about meso compounds the topic of optical activity is touched on once again:
Daisy: So in this case, this structure right here (pointing at board), since it is the meso compound it has chirality. No, it doesn’t have, it's achiral but it is optically active. Because optical activity means that it has, like, a stereocenter, basically. So because it has two stereocenters, it is optically active. Or I mean… (thinking) it is optically active, but it is achiral. Okay?
Student A: So something can be optically active but be achiral?
Daisy: Only if it's meso.
Student B: But otherwise being chiral means…
Daisy: Being optically active (nodding).
Student B: So it's like the one exception.
Daisy: Yes, exactly.
Once again this error goes unnoticed/unchallenged by the students in Daisy's study group. Similar to Instance 8, Daisy seems to be confused about the nature of meso compounds. In this example, she explicitly tells the members of her study group that meso compounds are achiral (which is correct) and optically active (which is incorrect). Being chiral and being optically active are mutually necessary, and it does not appear that Daisy is aware of this fact.
Facilitator behavior during CHEM 220 when this strategy was covered: See Instance 8 for details.
Research Question 3: When facilitators make an error in study group that is corrected by a study group member, what was that facilitator doing during the relevant CHEM 220 discussion section?
During Instance 10, Andy's study group is going through the answers to the practice exam together. The facilitator is presenting the exam answer key projected on a screen at the front of the classroom. The problem in question involves identifying the destabilizing forces present in a Newman Projection (Fig. 11). During the discussion, a student openly wonders why “gauche interactions between –OCH3 and the ethyl group” is not on the answer key. Instead of taking the study group member's answer into full consideration, the facilitator dismisses it:
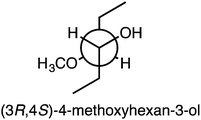 | ||
| Fig. 11 Newman Projection used in Andy's study group for a practice problem concerning destabilizing forces. | ||
Andy: If it's not on the answer key it's not a right answer.
Student A: Yeah, I’m trying to understand why.
Andy: If there are more answers, then they will say there are opportunities for more answers.
(Pause)
Andy: So the question is, “why can’t gauche interactions between –OCH3and the ethyl group be an answer?”
Student B: Is it because they’re really far away?
Andy: Literally, that's it.
Student A: What did he say?
Andy: It's because they’re on different molecules, and they’re pretty far away.
Student C: One's in the front and one's in the back.
Andy: They’re on different carbons.
Student A: But on the first problem that's an answer.
Student A proceeds to show/explain to Andy how the preceding question that involved an extremely similar Newman Projection listed “gauche interactions between –OCH3 and the ethyl group” as a destabilizing force. The preceding Newman Projection can be seen in Fig. 12.
Andy: Interesting… Good point.
After the students made their case, Andy acknowledged that their original answer is an acceptable answer for this question. Andy was too “married” to the answer key at first, and instead of thinking through the possible answers put forth by the students he immediately assumed any answer not listed on the key was incorrect. However, after some explanation by the study group members he realized he was incorrect, and the error was resolved.
Facilitator behavior during CHEM 220 when this strategy was covered: We do not have audiovisual data for Newman Projections from CHEM 220, as they were covered the week before we started recording. Andy's section of CHEM 220 was canceled that week and handouts were emailed instead (noted in Table 4.)
During Instance 11, Mark mistakenly perpetuated an error made by a study group member when they were asked to take a dash/wedge drawing in Fig. 13 and convert it to a Newman Projection. Mark correctly identified the fact that the member made an error, and even erased the error. However, when he went to draw in the correct Newman he accidentally re-drew the incorrect Newman. After his error was pointed out to him by the members of the study group, he subsequently corrected his error.
 | ||
| Fig. 13 Practice problem as well as incorrect Newman Projection (left) and correct Newman Projection (right) from Mark's study group. | ||
Facilitator behavior during CHEM 220 when this strategy was covered: as with Instance 10, we do not have audiovisual data for these topics from CHEM 220; Newman Projections were covered the week before we started recording. Mark's section of CHEM 220 was canceled and handouts were emailed instead (noted in Table 4 of the manuscript).
Instance 12 was part of Daisy's exam review session. The group is working on some box-check problems (Fig. 14). The student standing up at the board asks the facilitator, Daisy, to help her get started on this problem. Daisy gives the member the advice to label the stereocenters first:
Daisy: Let's start at the basics, and label each stereocenter. Have you done that? Or no?
Student A: No, but it's kind of hard because if you have something like this (pointing at molecule) and they’re the same (pointing at the internal symmetry of the molecule in question).
Daisy: Yeah, that's true. So there are no stereocenters, right?
Student A: Right.
Daisy: So there you go.
Student A: So it's not optically active?
Daisy: Exactly! So in this case, you don’t have any stereocenters, because it's symmetrical around the whole way, right? There's not four different groups on a carbon, so it's not optically active…
(Pause)
Student B: Can’t you have a stereocenter right here (pointing at molecule on board). Aren’t these two, can’t you make these two stereocenters? You have this hydrogen, four, one, two, three (counting out priorities).
Daisy: Oh yeah, technically, oh you’re right.
Student A: So you have two.
Student B: Yeah, two (pointing at lower two stereocenters on molecule).
Daisy: And the top ones!
Student B: Yeah.
Daisy: You’re right. She's right. So this one isn’t a stereocenter (pointing at only carbon on ring that is not a stereocenter) because it's symmetrical. But these two and these two are. So you’re actually right.
Daisy failed to recognize that the internal molecular symmetry only prevents one carbon on the ring from being chiral (the carbon in the ring opposite from the oxygen). She did not realize her mistake on her own, and it was only after some argument/explanation from a study group member that Daisy saw her error.
Facilitator behavior during CHEM 220 when this strategy was covered: Daisy was present in CHEM 220 the week this topic was covered. She was having a conversation with her peer when the liaison GSI went over an example where dashes and wedges do NOT indicate chiral atoms. Although Daisy's conversation is inaudible, but the liaison GSI's description follows:
Liaison GSI: One more thing I’ve noticed before is that there are exam questions and coursepack questions where you have wedges and dashes on things that don’t need wedges and dashes. For example, this compound here [draws a molecule on the board but off-camera] has 1,2,3 propane, a propyl group, a hydrogen, and two equivalent methyls. There's no need for a wedge or dash, there's no chiral carbon, there's no stereocenters there. However, sometimes problems will be drawn that have those in coursepack questions and stuff. So always make sure that students know just because dashes and wedges are shown does not mean that's a stereocenters.
The second time the topic is mentioned, Daisy is present but off screen.
During Instance 13, Daisy and her students are looking at the molecule shown below (Fig. 15) moments after Instance 12. They are trying to determine the R/S assignments for the two stereocenters present in the molecule.
Daisy: (pointing at the stereocenter on the right) Yes, that one is S I think. No, yes, that one is S. This one is S. Is that the one you were looking at?
Student A: Yes. It is S though.
Student B: Wait, how is that one S?
Daisy: Okay, so let's say you want the hydrogen in the back, right? So you’re looking at it again from this way (draws line of perspective from top down). So you have oxygen, and you’re going like this (motioning in a circle). Pretend like you’re on the other side of the carbon, and you’re going from one to this way (pauses to think).
Student B: Because the hydrogen is down, so if it's straight down and you stick your thumb towards it…
Daisy: No, you’re right, it's R. It's R.
This seems to be a case of Daisy getting mixed up when she tried to change her perspective of the molecule in order to use the “assign – clockwise/counterclockwise method” code to assign R/S stereocenters. Visualizing the molecule in 3D proves to be a challenge for her, and she is unable to properly apply the “assign – clockwise/counterclockwise method” strategy because of this. After another student questions her assignment and makes a comment about using the right hand rule, Daisy realizes her mistake.
Facilitator behavior during CHEM 220 when this strategy was covered: The exact situation that Daisy is trying to cope with (mental visualization for the “R” and “S” assignment when the lowest priority group is in the plane of the writing surface) was not explicitly addressed during her CHEM 220 session, although several strategies for getting the proper perspective for the lowest priority group were in evidence (such as the use of Newman Projections to re-orient the molecule). We infer that Daisy had a challenge with this particular type of visualization, and because it was not addressed explicitly during CHEM 220, she persisted with an unaddressed weakness into her own group session.
Research Question 4: If a facilitator is present when another facilitator makes an error in CHEM 220 and sees it resolved, how does this observing facilitator handle that topic if/when it arises later in their own study group?
In Instance 15, Lupita was incorrect about determining the relative preferences of chair substitutes and how that influences the relative Keq. Mark was present during CHEM 220 when this happened, and we have video records of two sessions of Mark's study group. The concepts preference between chairs by substituent orientation and relative Keq comes up three times during the first study group session and not at all during the second study group.
Mark's study group engages in a conversation concluding that bigger groups are more stable in equatorial positions. This concept undergirds the assigning of the Keq as greater than, less than, or equal to one. Later, they discuss the application of Keq to a pair chair conformations and correctly determine the relative Keq for the pair, and how the sign would change if they were written in a different arrangement (left/right). A study group member correctly determines the relative Keq for the pair of chair conformations that was drawn on the board. Mark affirms the member's answer and explanation. [Author Note: This is the EXACT same pair of chairs as was used in CHEM 220 – showcasing facilitator use of the problems in study groups that were covered during their CHEM 220 class!] Later in the same study group, Mark re-draws one of the stereocenters on the chair so that one chair has both bromines equatorial and both ethyl groups axial and they correctly discuss how they would assign the relative Keq for these new compounds. A study group member correctly assigns the relative Keq, which Mark affirms as correct.
In Instance 17, Daisy asked questions in CHEM 220 about checking the “different molecule” of something else is an enantiomer or diastereomer during a ‘box-check’ problem, and if you should check “different conformation” if the molecules are only different by the rotation of a sigma bond (this is described in Instance 3). Adam was present in CHEM 220 during this conversation.
We have video from one of Adam's study group sessions. In this study group, neither “Assign different molecule – new molecule is an enantiomer or diastereomer of another” nor “Assign different conformation – same molecule but sigma bond is rotated” are used. Thus we cannot determine if Adam learned from the conversation that Daisy started during CHEM 220.
In Instance 18, a facilitator who did not consent for us to come to her study group answers a problem on the board about a molecule with a double bond and basic amine and incorrectly did an electrophilic addition reaction instead of the acid/basic reaction that would take place. The liaison GSI redirects the question to the facilitators with the information that “acid/base reactions are the fastest kind of reactions” and asks if that changes how they would answer the question. The facilitators nod their head and the liaison GSI proceeds to correct the problem on the board. Both Oscar and Gwen are present when this happens.
Oscar had one instance of this strategy in his study groups. He used the same problem from CHEM 220 and handled it correctly when a study group member asked how two similar reaction conditions could yield two different products.
Gwen had one instance of this strategy in her study group. She used the same problem from CHEM 220 and handled it correctly when a student struggles to answer the question on the board.
In Instance 19, Mark and Lupita are present when the same error as in Instance 17 occurs by another facilitator in CHEM 220. The liaison GSI again gives the hint about relative reaction rates and corrects the problem at the board. As the hint is given, facilitators appear to quickly understand how this would impact the product of the reaction in question.
We do not have any instances of this strategy being used in either Mark or Lupita's study groups.
In Instance 20, an anonymous facilitator misidentifies an atom as chiral when it is not due to internal molecular symmetry. The liaison GSI verbally corrects this error on the board when going over the practice problems. Both Mark and Lupita are present when this error is corrected.
This strategy comes up in Mark's study group five times and is handled correctly in all five occurrences.
This strategy does not occur in any of the study group audiovisual data that we have for Lupita's study group.
In Instance 21, an anonymous facilitator made the same mistake as in Instance 20 at the board. The liaison GSI again corrects facilitator error when going over the practice problems. Harrison is present when this error is corrected. Two of the three times this strategy is used in Harrison's study group, it is used correctly. The third time it was used correctly in a conversation, but was not applied to a specific problem.
In Instance 22, a facilitator drew both chair conformations but left blank the relative Keq between them. The liaison GSI affirms the chair conformations and explains that the relative Keq is one and why. Both Andy and Carrie are present when this occurs. This strategy is used in neither Carrie nor Andy's study groups.
In Instance 23, a facilitator asks for clarification about a specific compound, and whether it is meso or not. The liaison GSI explains that the general ways he identifies a molecule as meso. Meso assignment is not discussed in the week of Max's study group that we were able to record.
In Instance 24, a facilitator asks for clarification from her peers about the stereochemical relationship between two compounds that are E/R and E/S. Her peers respond that the correct relationship is enantiomer. Instance 24 is immediately followed by Instance 25 where a different facilitator inquires about the stereochemical relationship between two molecules which are E/R and Z/S. Facilitators immediately respond that the correct relationship is that of diastereomers. The liaison GSI was not a part of either conversation. Max was present during this back and forth between his peers. The “Assign diasteromer – change of E/Z” strategy does come up three times in Max's study group and is handled correctly in all instances. There were no instances of “Assign enantiomer – change all RS but not EZ” in Max's study group session.
In Instance 26, a facilitator asks the liaison GSI if stereoisomers are classified as different molecules. The liaison GSI affirms that they are. Max is present and attentive during this conversation, but this strategy does not come up in his study group.
In Instance 27, a facilitator asks her peer about the how to determine experimental values of optical activity from known stereochemical relationships. Her peers answer her correctly that enantiomers have the same value but opposite sign for the optical activity and that you cannot determine the optical activity value for a molecule that is the diastereomer of a molecules whose optical activity you do know. The liaison GSI was not a part of the conversation. Max was present and appeared to be engaged during this conversation, but this strategy was not observed to occur in his study group.
In Instance 28, the liaison GSI is going over a ‘box-check’ problem where a facilitator correctly indicated that two molecules are diastereomers, but did not also indicate that they are different molecules. Oscar and Gwen were present (but off screen) when this happened. The “Assign different molecule if new molecule is enant or diast of other” strategy is used once in Gwen's study group when determining how E and Z alkenes are diastereomers that are also distinct products, different molecules. This strategy is not used in Oscar's study group.
In Instance 29, Lupita and Mark have been working together. Mark asks how to determine the optical activity between two molecules which are enantiomers or diastereomers. The liaison GSI tells them that you cannot determine the optical activity for the diastereomer of a compound, and enantiomers have optical activity of equal magnitude and opposite sign. Lupita asks the liaison GSI if stereoisomers are different molecules and if different conformations are the same molecule. The liaison GSI affirms both as true.
Andy is present but only paying attention during the discussion of different conformations being the same molecule with the sigma bond rotate. This strategy is used in Andy's study group, and while used correctly, it is discussed as a definitional statement.
The strategy about assigning optical activity values from enantiomer/diastereomer relationship(s) is not used in the study groups that we have recorded for Lupita.
The strategy about assign different molecule if new molecule is enantiomer or diastereomer of other is used once in Mark's study group, but only as a conversation and not in the course of problem solving – once again, the discussion is correct. The strategy to assign different conformation if same molecule but sigma bond is rotated strategy comes up in the same conversation. It is also a correct statement.
In Instance 30, a facilitator incorrectly labeled a structure as meso when it was not. Because of this error, the facilitator also misses that the compound is chiral, has an enantiomer, is optically active, and has a diastereomer. Both Daisy and Adam are present.
The “assign meso – mirror image is same compound” strategy is used once in Daisy's study group. The strategy is used correctly by the facilitator. The strategy to “assign chiral – Has R/S stereocenters but is not meso” is used four times in Daisy's study group. It is applied by the facilitator and is used correctly in all instances. The “assign optically active – compound is chiral” strategy is used once in Daisy's study group. The strategy is applied by Daisy and is used correctly.
The “assign meso – mirror image is same compound” strategy is not used in Adam's study group. The strategy to “assign ‘chiral’ if the compound has R/S stereocenter but is not meso” is not used in Adam's study group. The “assign optically active if compound is chiral” strategy is not used in Adam's study group.
In Instance 31, a facilitator incorrectly indicated that two compounds were different conformations. The liaison GSI corrected this at the board during the problem review. Harrison was present (but off camera) during this correction
The “assign different conformation – same molecule be sigma bond is rotated” strategy was used three times in Harrison's study groups. In all three instances, it was used correctly by Harrison in response to a student question regarding a hypothetical scenario the student proposes.
In Instance 32, a facilitator asks a clarification question about how to use “ghost atoms” as place holders when determining R/S priority around stereocenter. The liaison GSI reviews again how to use “ghost atoms” as place holders. Harrison is present and appears to pay attention.
The strategy that when assigning R/S to a stereocenter to count a double bond as two identical “branched atoms” was used three times in Harrison's study group. It was used by a study group member once, and the other two usages were by Harrison. In all instances the strategy was applied correctly.
Appendix II
Note from the authors
The code book used for analysis is presented in the following pages (Tables 6–11) – complete with code, description of code, number of times mentioned, and frequency of correctness vs. incorrectness in code usage. As a reminder, in cases when facilitators or study group peer members are using a strategy to solve a problem on paper (i.e., we cannot see the problem they are working on) correctness codes have been omitted.| Strategy | Description | Total use | Facilitator use | Member use | Correct | Incorrect |
|---|---|---|---|---|---|---|
| Assign – E if alkene shaped like a Z | Method of alkene stereochemistry assignment denoted with phrasing specifically or analogous to “alkene shaped like a Z” | 2 | 0 | 1 | 0 | 0 |
| Assign E/Z – requires 2 unique substituents per carbon | To have E/Z assignments, the alkene must have two unique substituents on each carbon | 2 | 2 | 1 | 1 | 0 |
| Assign Z if on same side | If both high priority substituents are on the same side, alkene is Z | 9 | 6 | 8 | 7 | 0 |
| Assign priority to substituents | As with R/S stereochemistry, Cahn-Ingold-Prelog rules are used to assign substituent priorities for assigning alkene stereochemistry | 26 | 16 | 19 | 20 | 2 |
| Consider branching when assigning priority to substituents | Cahn-Ingold-Prelog: involving branching to determine points of divergence | 6 | 3 | 5 | 6 | 0 |
| Assign E/Z by entgegen vs. zusammen | Derivations of the German Entgegen for apart and Zusammen for together | 0 | 0 | 0 | 0 | 0 |
| Assign E for “epposite” and Z for “zame” meaning | If both highest priority substituents are on the “Zame” side of the alkene = Z; if both are on “Epposite” sides of the alkene = E | 17 | 11 | 8 | 12 | 0 |
| Strategy | Description | Total use | Facilitator use | Member use | Correct | Incorrect |
|---|---|---|---|---|---|---|
| Acid base rate is faster than EA | Acid–base reactions will be faster than electrophilic addition | 3 | 3 | 1 | 3 | 0 |
| Carbocation stability – substitution | The more substituted carbocations will be more stable (3° > 2° ≫ 1°) | 19 | 14 | 11 | 15 | 1 |
| Resonance contributes to carbocation stability | Resonance delocalization of positive charge confers a greater stability to carbocations | 15 | 14 | 6 | 11 | 1 |
| Resonance overrides degree of substitution for C+ | When confronted with an electrophilic addition reaction wherein the one potential carbocation has a greater substitution, but another has the ability for resonance, the resonance-stabilized carbocation is more stable | 3 | 3 | 2 | 2 | 0 |
| Strong versus weak acid | Understanding of the active acid in solution for alkene addition reactions | 11 | 8 | 7 | 9 | 1 |
| Strategy | Description | Total use | Facilitator use | Member use | Correct | Incorrect |
|---|---|---|---|---|---|---|
| Assign or draw eclipsed | Correct assignment or representation of eclipsed conformations | 10 | 9 | 5 | 4 | 0 |
| Assign or draw staggered | Correct assignment or representation of staggered conformations | 8 | 6 | 5 | 5 | 0 |
| Assign or use anti vs. syn arrangement | Correct assignment or representation of substituents as syn or anti | 4 | 3 | 2 | 3 | 0 |
| Assign or use gauche vs. periplanar | Correct assignment or representation of substituents as gauche or periplanar | 12 | 8 | 9 | 4 | 1 |
| Conf. pref. – charge to charge interaction | Understanding whether a conformation that has a charge–charge interaction is greater or less stable due to this interaction | 7 | 3 | 5 | 4 | 1 |
| Conf pref – based on sterics | Understanding that steric interactions are generally minimized in Newman Projections | 26 | 17 | 17 | 9 | 0 |
| Conf pref – H-bonding | Understanding cases when hydrogen-bonding impacts stability of Newman projections | 16 | 10 | 10 | 6 | 1 |
| Conf pref for staggered | Understanding that staggered conformations are lower energy than eclipsed conformations | 10 | 7 | 5 | 2 | 0 |
| Convert to Newman from chair VIA PLANAR | Using Newman projections to visualize chair conformations, by first drawing the planar cyclohexane ring, then drawing the Newman projection | 1 | 0 | 1 | 1 | 0 |
| Convert to Newman from dash and wedge | Correct generation of a Newman projection from a standard dash-and-wedge molecular representation | 33 | 20 | 18 | 16 | 2 |
| Convert to Newman projections from chairs | In contrast to VIA PLANAR, this is a direct conversion to a Newman projection from a chair conformation | 5 | 2 | 4 | 4 | 0 |
| Draw different conformations | Correct rotation about central carbons to represent different Newman projections of the same molecule | 15 | 15 | 5 | 5 | 0 |
| Energy levels of Newman conformations | Understanding the comparative (not specific) energy levels of various Newman projections | 12 | 13 | 7 | 7 | 0 |
| Relative conf. – A values | Rationalizing relative conformational energy levels based on substituent A-values | 1 | 1 | 1 | 1 | 0 |
| Strategy | Description | Total use | Facilitator use | Member use | Correct | Incorrect |
|---|---|---|---|---|---|---|
| [OLD] Assign – swap atoms and assign opposite RS | An archaic code recently replaced and broken down into two codes: to get lowest priority in back – swap back atom with lowest priority, assign – opposite R–S if atoms were swapped and any other assign codes used (while archaic, included for completeness) | 0 | 0 | 0 | 0 | 0 |
| Assign – clockwise/counterclockwise method | Method of assigning stereochemistry using a clockface description (clockwise is R, counterclockwise is S) | 51 | 27 | 32 | 37 | 8 |
| Assign – opposite R/S if atoms were swapped | Used with to get lowest priority in back – swap back atom with lowest priority | 5 | 3 | 3 | 3 | 0 |
| Assign – right hand rule | The right-hand rule method of assignment (point thumb in the direction of priority 4; if fingers curl 1, 2, 3 then assignment is R, if not assignment is S) | 39 | 33 | 12 | 26 | 1 |
| Assign – steering wheel method | Assigning stereochemistry as if driving a car (turn left for S, turn right for R) | 3 | 0 | 2 | 1 | 0 |
| Assign chiral atom – requires 4 different substituents | In order to be considered chiral, an atom must be bound to four unique substituents | 47 | 30 | 26 | 32 | 4 |
| Consider branching when assigning priority to substituents | When determining priorities, if two substituents are the same (i.e., carbon), then all substituents attached to each carbon must be assigned priority, and so on, until a point of divergence is identified | 39 | 29 | 23 | 29 | 5 |
| Priority of substituents – double bonds count as two identical branches | Following the Cahn–Ingold–Prelog rules for assigning priority in the case of all double (and triple) bonds | 17 | 11 | 11 | 14 | 2 |
| Assign priority to substituents | Cahn–Ingold–Prelog substituent rules properly used | 75 | 48 | 45 | 61 | 8 |
| To get lowest priority in back – change perspective on the molecule | Used in cases when a student is presenting a problem and describes mentally placing themselves elsewhere in space around the molecule without employing any other strategy, such as a Newman Projection, etc. | 5 | 4 | 3 | 4 | 1 |
| To get lowest priority in back – use a Newman projection | Using a Newman Projection of a bond to perform a rotation and place the lowest priority substituent in back | 7 | 4 | 3 | 4 | 0 |
| To get lowest priority in back – swap back substituent with lowest priority substituent | Used in conjunction with assign – opposite R–S if atoms were swapped; describes when a student swaps the back atom with the lowest priority, assigns stereochemistry (as if normal), and then “swaps” back their assignment | 5 | 3 | 3 | 3 | 0 |
| To get lowest priority in back – tripod arm method | Using the thumb, index, and middle finger as a tripod of substituents, and the arm as a fourth substituent to visualize bond rotation | 1 | 1 | 0 | 1 | 0 |
| To get lowest priority in back – umbrella method | Visualization of chiral atom as an umbrella to perform a bond rotation | 2 | 0 | 2 | 1 | 0 |
| To get lowest priority in back – propeller method | Visualization of chiral atom as a propeller to perform a bond rotation | 3 | 3 | 1 | 0 | 0 |
| To get lowest priority in back – use a model kit | Visualization of chiral atom with a molecular model kit in order to rotate and depict chiral atom with lowest priority in back for stereochemical assignment | 2 | 0 | 2 | 2 | 0 |
| Strategy | Description | Total use | Facilitator use | Member use | Correct | Incorrect | |
|---|---|---|---|---|---|---|---|
| Assign cis or trans by moving your point of reference | Using Newman projections, new chair orientations, or other frame-of-reference switching tools to re-orient one's point of reference in order to visualize how to assign cis or trans | 1 | 1 | 0 | 0 | 1 | |
| Assign cis or trans from wedges and dashes | If both substituents are dashes or wedges, cis; if one is dashed, one wedged; trans | 5 | 4 | 2 | 4 | 0 | |
| Assign cis or trans if on same/different face of ring | When dealing with a planar ring, substituents that are both dashes or both wedges are cis, while mixed dash/wedge substituents are trans | 4 | 4 | 3 | 2 | 0 | |
| Assigning equatorial or axial from bulkiness of subs’t | Comparative use of substituent sterics to understand whether a substituent prefers equatorial or axial conformation (e.g., “tert-butyl is bigger than chloride, so if I need on to be equatorial, I'll choose the biggest: tert-butyl”) | 6 | 2 | 5 | 2 | 0 | |
| Axial or equatorial by parallels | When drawing a chair, understanding the sets of parallels needed to correctly draw the chair's substituent orientations | 4 | 1 | 3 | 2 | 1 | |
| Chair flips – axial and equatorial orientations change | When performing a chair flip (i.e., interconverting between two chair conformations), understanding that all axial substituents become equatorial, and all equatorial substituents become axial | 18 | 9 | 11 | 10 | 4 | |
| Chair flips – up stays up, down stays down | When performing a chair flip, knowing that all substituents that were pointed ‘up’ above the plane of the ring will still be pointed ‘up’; same for ‘down’ | 9 | 5 | 4 | 6 | 0 | |
| Chair flips by drawing both chairs | Drawing both chairs (left-leaning and right-leaning) and visualizing substituent orientation change in this manner | 13 | 5 | 8 | 8 | 4 | |
| Chair flips by rotating around the ring | Drawing the same chair (e.g., just the right-leaning chair) and rotating the substituents around the ring to perform a chair-flip | 8 | 8 | 2 | 4 | 1 | |
| Check chair flips by drawing planar version | Verifying an accurate chair flip was performed by drawing the dash-wedge planar version of the chair | 2 | 1 | 1 | 0 | 0 | |
| [Gesture] visualization of substituents during flip with fingers | A gesture made in some study group(s) involving pointer and middle finger maintaining the same orientations, but by flicking the wrist, a student watches substituents maintain orientations while still ‘flipping’ | 1 | 0 | 1 | 1 | 0 | |
| ID Conformers by difference in axial and equatorial for a subs’t | Determining that two molecules are conformers based on the idea that a chair flip (change in conformation) will result in two conformers, and extrapolating that to changes in axial/equatorial substituents | 1 | 1 | 0 | 0 | 0 | |
| Preference between chairs – H Bond | Understanding how hydrogen-bond ability may impact which is the preferred chair conformation | 3 | 2 | 3 | 3 | 0 | |
| Preference between chairs – Newman | Using Newman projections to visualize the preferred chair conformation | 2 | 1 | 1 | 0 | 0 | |
| Pref. between chairs by substituent orientation | Understanding how relative substituent orientation affects which chair is the preferred conformation | 13 | 8 | 8 | 11 | 1 | |
| Preference between chairs by A-values | Understanding how substituent A-values help determine which chair is the preferred conformation | 6 | 6 | 0 | 0 | 0 | |
| Relative Keq | Knowing which direction the equilibrium lies in equilibrium between two chairs | 15 | 9 | 11 | 12 | 2 | |
| To draw – number the carbons | Numbers carbons when converting to or from a chair in order to preserve consistency and minimize mistakes (book-keeping) | 13 | 8 | 8 | 7 | 0 | |
| To draw planar from chair, up wedges down dashes | When drawing a planar (dash-and-wedge) cyclohexane from a chair and visualizing from the top looking down on the chair, remembering that all ‘up’ substitutents are wedges, all ‘down’ substitutents are dashes | 7 | 5 | 5 | 6 | 1 | |
| To draw ring from planar, wedges up dashes down | When drawing a chair from a (dash-and-wedge) cyclohexane and visualizing from the top looking down on the chair, remembering that all ‘up’ substitutents were wedges, all ‘down’ substituents were dashes | 17 | 9 | 9 | 11 | 2 | |
| Strategy | Description | Total use | Facilitator use | Member use | Correct | Incorrect |
|---|---|---|---|---|---|---|
| Assign achiral – E/Z or cis/trans only source of stereochemistry | Understanding that unsymmetrical alkenes without a chiral atom present are achiral molecules that still possess stereochemical labels | 11 | 8 | 8 | 3 | 0 |
| Assign achiral diastereomer – no R/S stereocenters | When determining whether a molecule has an achiral diastereomer: cases when there are no chiral atoms present in the molecule | 14 | 11 | 7 | 6 | 0 |
| Assign chiral – has R/S stereocenters but isn’t meso | Molecules that contain chiral atoms that are not meso compounds are themselves chiral molecules | 30 | 26 | 14 | 16 | 2 |
| Assign constitutional (structural) isomer – same formula but different connectivity | Assigning a molecule as a constitutional (structural) isomer if the same atoms are present, but in a different arrangement | 12 | 8 | 7 | 7 | 1 |
| Assign diastereomer – change of E/Z | Whenever changing alkene stereochemistry, labeling the new stereoisomer as a diastereomer of the original | 25 | 18 | 13 | 13 | 0 |
| Assign diastereomer – change of less than all R/S stereocenters | In cases when any R/S stereocenters are changed (but not all of them); assigning the new stereoisomer as a diastereomer | 49 | 40 | 23 | 29 | 1 |
| Assign diastereomer – ring cis vs. trans | Assigning ring cis/trans partners as diastereomers of one another | 8 | 6 | 2 | 4 | 0 |
| Assign different conformation – same molecule but sigma bond rotated | In cases when the relationship between two molecules is assigned, understanding a conformational shift as opposed to two different molecules | 14 | 12 | 5 | 3 | 0 |
| Assign different molecule – new molecule is enantiomer or diastereomer of another | Understanding that stereoisomers are different molecules, including enantiomers and diastereomers | 7 | 8 | 3 | 2 | 0 |
| Assign enantiomer – change all of R/S but not E/Z | Understanding that enantiomers have all R/S stereocenters changed and none of the E/Z stereocenters changed | 49 | 35 | 25 | 32 | 5 |
| Assign enantiomer – mirror image is new compound | When taking the mirror image of a compound, understanding that the original and mirror image are enantiomers | 14 | 8 | 10 | 5 | 0 |
| Assign meso – mirror image is same compound | If, when taking the mirror image of a compound: the original and mirror image are the same: correctly assigning the molecule as meso | 12 | 11 | 4 | 7 | 1 |
| Assign meso – two opposite stereocenters with the same substituents and internal molecular symmetry | When a compound contains two stereocenters with identical substituents (i.e., there is internal molecular symmetry) and the stereocenters have opposite assignment, assigning the compound as meso | 47 | 36 | 19 | 35 | 3 |
| Assign optically active – if compound is chiral | Properly demonstrating understanding that all chiral compounds are optically active | 17 | 13 | 7 | 7 | 1 |
| Assign optical activity – based on enantiomeric or diastereomeric relationship to known compound | When presented a known optical activity value for a compound, understanding the ability to (a) assign optical activity to the enantiomer, (b) know that the optical activity cannot be assigned to diastereomers from an experimental value, and (c) assign optical activity if the same molecule | 4 | 4 | 1 | 0 | 0 |
| Assign same molecule – only perspective changes | In cases when two depictions of the same molecule have been used, and students are asked to compare them, assigning that they are the same molecule (i.e., stereoisomerism is preserved) | 2 | 1 | 1 | 1 | 0 |
| Chart visualization technique to show relationship between stereoisomers | A chart used to show the relationships between stereoisomers, from lecture | 3 | 0 | 3 | 1 | 0 |
| Draw diastereomer – change E/Z or cis/trans | When asked to draw a diastereomer of a compound, changing E/Z or cis/trans | 4 | 3 | 1 | 2 | 0 |
| Draw diastereomer – flip two substituents of less than all chiral C | When asked to draw a diastereomer of a compound, changing any number of R/S stereocenters except for all R/S stereocenters | 3 | 1 | 2 | 2 | 0 |
| Draw enantiomer – draw mirror image of original compound | In order to produce the enantiomer of a compound, draw the mirror image | 4 | 4 | 1 | 2 | 0 |
| Draw enantiomer – change all R/S stereocenters but not E/Z | In order to produce the enantiomer of a compound, change all R/S stereocenters but not E/Z alkene isomerism | 7 | 6 | 1 | 8 | 2 |
| Draw meso – correct internal molecular symmetry needs to be present | When asked to draw a meso compound, correctly showing the internal molecular symmetry present | 1 | 1 | 1 | 0 | 0 |
| Meso – convert all dashes and wedges to get the same molecule you started with | When determining whether a compound is meso, if you convert all dashes to wedges (and wedges to dashes), thereby changing all R/S stereocenters, you end up with the same compound (due to the compound being meso) | 9 | 6 | 5 | 8 | 1 |
| No. of stereoisomers possible is 2n stereocenters (unless meso) | The classic 2n rule from lecture regarding the number of stereoisomers represented by a given connectivity | 15 | 9 | 9 | 9 | 1 |
| Optically active – has R/S stereocenters but is not meso | Assigning any chiral compounds as optically active from the presence of R/S stereocenters (no meso compounds) | 39 | 25 | 20 | 23 | 2 |
| Without stereocenters – cannot be meso, cannot have an enantiomer, and is not optically active | A collective code where a compound without stereocenters cannot possess any of the qualities listed in the code | 2 | 2 | 1 | 1 | 0 |
Acknowledgements
The authors would like to thank our participants for letting us into their CHEM 220 sessions and study groups. JRB, RAB, LJP, and BPC would like to thank Prof. Leah Bricker, University of Michigan School of Education, for her insightful feedback and editorial assistance. We would also like to thank the members of the Science Learning Center at the University of Michigan, in particular Joe Salvatore and Kelley Emerson. We also thank Professors William Gehring, Pratibha Varma-Nelson, John Wolfe, and Drs Solaire Finkenstaedt-Quinn and Alena Moon for their insightful comments on earlier versions of our manuscript. JRB and LJP would like to thank Professor John Wolfe in the University of Michigan Chemistry Department, and Rackham Graduate School for financial support. JRB, RAB, and LJP would like to thank CSIE|UM, and thank Prof. Anne McNeil for the idea for research question 4.References
- Adbo K. and Taber K. S., (2014), Developing an Understanding of Chemistry: a case study of one Swedish student's rich conceptualisation for making sense of upper secondary school chemistry, Int. J. Sci. Educ., 36(7), 1107–1136, DOI:10.1080/09500693.2013.844869.
- Aloisi C., Major L. E., Coe R. and Higgins S., (2014), What makes great teaching? Review of the underpinning research, The Sutton Trust, retrieved from http://https://www.suttontrust.com/research-paper/great-teaching.
- Alvarado C., Canada F., Garritz A. and Mellado V., (2015), Canonical pedagogical content knowledge by CoRes for teaching acid–base chemistry at high school, Chem. Educ. Res. Pract., 16(3), 603–618, 10.1039/C4RP00125G.
- Amaral K. E. and Vala M., (2009). What Teaching Teaches: Mentoring and the Performance Gains of Mentors, J. Chem. Educ., 86(5), 630, DOI:10.1021/ed086p630.
- Baker R. W., George A. V. and Harding M. M., (1998), Models and Molecules – A Workshop on Stereoisomers, J. Chem. Educ., 75(7), 853, DOI:10.1021/ed075p853.
- Barnard R. A., Boothe J. R., Salvatore J., Emerson K., Boone A., Sandler C. and Coppola B. P., (2017), Course-based Support for Peer-Led Study Group Facilitators in a Large Instructional Team, J. Coll. Sci. Teach., in press.
- Bhattacharyya G. and Bodner G. M., (2014), Culturing reality: how organic chemistry graduate students develop into practitioners, J. Res. Sci. Teach., 51(6), 694–713, DOI:10.1002/tea.21157.
- Bond-Robinson J. and Rodriques R. A. B., (2005), Instruments to Drive Effective Constructivist Laboratory Teaching, Chem. Educ., 10(2), 154–162.
- Brown P. C., Roediger H. L., McDaniel M. A., and Ebrary I., (2014), Make it stick: the science of successful learning, Cambridge, Massachusetts: The Belknap Press of Harvard University Press.
- Carlsen W. S, (1992), Closing down the conversation: discouraging student talk on unfamiliar science content, Journal of Classroom Interaction, 27(2), 15–22.
- Chen J. and Cowie B., (2013), Developing ‘Butterfly Warriors’: a Case Study of Science for Citizenship, Res. Sci. Educ., 43(6), 2153–2177, DOI:10.1007/s11165-013-9349-y.
- Coppola B. P., Ege S. N. and Lawton R. G., (1997), The University of Michigan Undergraduate Chemistry Curriculum 2. Instructional Strategies and Assessment, J. Chem. Educ., 74(1), 84, DOI:10.1021/ed074p84.
- Davis E. A. and Petish D., (2005), Real-World Applications and Instructional Representations Among Prospective Elementary Science Teachers, J. Sci. Teach. Educ., 16(4), 263–286, DOI:10.1007/s10972-005-8892-4.
- Derry S. J., (2007), Guidelines for Video Research in Education, retrieved from http://drdc.uchicago.edu/what/video-research-guidelines.pdf.
- Ege S. N., Coppola B. P. and Lawton R. G., (1997), The University of Michigan Undergraduate Chemistry Curriculum 1. Philosophy, Curriculum, and the Nature of Change, J. Chem. Educ., 74(1), 74, DOI:10.1021/ed074p74.
- Egré P. and Bonnay D., (2013), Metacognitive perspectives on unawareness and uncertainty, in Foundations of Metacognition, Oxford Scholarship Online, retrieved from http://www.oxfordscholarship.com/view/10.1093/acprof:oso/9780199646739.001.0001/acprof-9780199646739-chapter-020.
- Friend M. and Cook L., (2016), Interactions: collaboration skills for school professionals, New York: Pearson.
- Frøyland M., Remmen K. B., Mork S. M., Ødegaard M. and Christiansen T., (2015), Researching science learning from students’ view – the potential of headcam, Nordic Stud. Sci. Educ., 11(3), 249–267.
- Gafney L. and Varma-Nelson P., (2007), Evaluating Peer-Led Team Learning: A Study of Long-Term Effects on Former Workshop Peer Leaders, J. Chem. Educ., 84(3), 535, DOI:10.1021/ed084p535.
- Gess-Newsome J. and Lederman N. G. (ed.), (1999), Examining Pedagogical Content Knowledge, Springer, retrieved from http://www.springer.com/us/book/9780792359036.
- Gess-Newsome J., Southerland S. A., Johnston A. and Woodbury S., (2003), Educational Reform, Personal Practical Theories, and Dissatisfaction: the Anatomy of Change in College Science Teaching, Am. Educ. Res. J., 40(3), 731–767.
- Gess-Newsome J., Taylor J. A., Carlson J., Gardner A. L., Wilson C. D. and Stuhlsatz M. A. M., (2017), Teacher pedagogical content knowledge, practice, and student achievement, Int. J. Sci. Educ., 1–20, DOI:10.1080/09500693.2016.1265158.
- Gosser D. K., Kampmeier J. A. and Varma-Nelson P., (2010), Peer-Led Team Learning: 2008 James Flack Norris Award Address, J. Chem. Educ., 87(4), 374–380, DOI:10.1021/ed800132w.
- Grossman P. L., (1989), Learning to teach without teacher education, Teach. Coll. Rec., 91, 191–208.
- Hale L. V. A., Lutter J. C. and Shultz G. V., (2016), The development of a tool for measuring graduate students’ topic specific pedagogical content knowledge of thin layer chromatography, Chem. Educ. Res. Pract., 17(4), 700–710, 10.1039/C5RP00190K.
- Hill H. C., Ball D. L. and Schilling S. G., (2008), Unpacking Pedagogical Content Knowledge: Conceptualizing and Measuring Teachers’ Topic-Specific Knowledge of Students, J. Res. Math. Educ., 39(4), 372–400.
- Janssen F. J. J. M., Tigelaar D. E. H. and Verloop N., (2009), Developing Biology Lessons Aimed at Teaching for Understanding: A Domain-specific Heuristic for Student Teachers, J. Sci. Teach. Educ., 20(1), 1–20, DOI:10.1007/s10972-008-9118-3.
- Jardine H. E. and Friedman L. A., (2017), Using Undergraduate Facilitators for Active Learning in Organic Chemistry: A Preparation Course and Outcomes of the Experience, J. Chem. Educ., 94(6), 703–709, DOI:10.1021/acs.jchemed.6b00636.
- Kind V., (2009), Pedagogical content knowledge in science education: perspectives and potential for progress, Stud. Sci. Educ., 45(2), 169–204, DOI:10.1080/03057260903142285.
- Kulatunga U., Moog R. S. and Lewis J. E., (2013), Argumentation and participation patterns in general chemistry peer-led sessions, J. Res. Sci. Teach., 50, 1207–1231, DOI:10.1002/tea.21107.
- Lewis S. E., (2011), Retention and Reform: An Evaluation of Peer-Led Team Learning, J. Chem. Educ., 88(6), 703–707, DOI:10.1021/ed100689m.
- Luft J. A., Kurdziel J. P., Roehrig G. H. and Turner J., (2004), Growing a Garden Without Water: Graduate Teaching Assistants in Introductory Science Laboratories at a Doctoral/Research University, J. Res. Sci. Teach., 41(3), 211–233.
- Lyle K. S. and Robinson W. R., (2003), A Statistical Evaluation: Peer-led Team Learning in an Organic Chemistry Course, J. Chem. Educ., 80(2), 132, DOI:10.1021/ed080p132.
- Mack M. R. and Towns M. H., (2016), Faculty beliefs about the purposes for teaching undergraduate physical chemistry courses, Chem. Educ. Res. Pract., 17(1), 80–99, 10.1039/C5RP00148J.
- Magnusson S., Krajcik J. and Borko H., (1999), Nature, sources, and development of pedagogical conent knowledge for science teaching, in Examining Pedagogical Content Knowledge: The Construct and its Implications for Science Education, pp. 95–135.
- Mavhunga E. and Rollnick M., (2013), Improving PCK of Chemical Equilibrium in Pre-service Teachers, Afr. J. Res. Math., Sci. Technol. Educ., 17(1–2), 113–125, DOI:10.1080/10288457.2013.828406.
- McDiarmid G. W., (1990), The liberal arts: Will more result in better subject matter understanding? Theory Pract., 29(1), 21–29, DOI:10.1080/00405849009543426.
- Micari M., Streitwieser B. and Light G., (2005), Undergraduates Leading Undergraduates: Peer Facilitation in a Science Workshop Program, Innov. High. Educ., 30(4), 269, DOI:10.1007/s10755-005-8348-y.
- Miles M. B., Huberman A. M. and Saldaña J., (2014), Fundamentals of Qualitative Data Analysis, in Qualitative Data Analysis: A Methods Sourcebook, 3rd edn, Los Angeles, CA: Sage Publications, pp. 69–104.
- Padilla K. and Van Driel J., (2011), The relationships between PCK components: the case of quantum chemistry professors, Chem. Educ. Res. Pract., 12(3), 367–378, 10.1039/C1RP90043A.
- Ploessl D., Rock M. L., Schoenfeld N. A., and Blanks B., (2010), On the same page: practical techniques for enhancing co-teaching interactions, Interv. Sch. Clin., 45 (3), 158–168.
- Quitadamo I., Brahler C. J. and Crouch G. J., (2009), Peer-Led Team Learning: A Prospective Method for Increasing Critical Thinking in Undergraduate Science Courses, Sci. Educ., 18(1), 29–39.
- Ramaswamy S., Harris I. and Tschirner U., (2001), Student Peer Teaching: An Innovative Approach to Instruction in Science and Engineering Education, J. Sci. Educ. Technol., 10(2), 165–171, DOI:10.1023/A:1009421231056.
- Robert J., Lewis S. E., Oueini R. and Mapugay A., (2016), Coordinated Implementation and Evaluation of Flipped Classes and Peer-Led Team Learning in General Chemistry, J. Chem. Educ., 93(12), 1993–1998, DOI:10.1021/acs.jchemed.6b00395.
- Rollnick M. and Davidowitz B., (2015), Topic Specific PCK of Subject Matter Specialists in Grade 12 Organic Chemistry, in Proceedings of the 23rd Annual Meeting of the Southern African Association for Research in Mathematics, Science and Technology Education, Eduardo Mondlane University, Maputo: SAARMSTE, pp. 243–250.
- Rollnick M., Bennett J., Rhemtula M., Dharsey N. and Ndlovu T., (2008), The Place of Subject Matter Knowledge in Pedagogical Content Knowledge: a case study of South African teachers teaching the amount of substance and chemical equilibrium, Int. J. Sci. Educ., 30(10), 1365–1387, DOI:10.1080/09500690802187025.
- Sandler C. and Salvatore J., (2013), Peer-Led Study Groups as Learning Communities in the Natural Sciences, in Exemplary College Science Teaching, NSTA Press, pp. 47–60.
- Schray K., Russo M. J., Egolf R., Lademan W. and Gelormo D., (2009), Are In-Class Peer Leaders Effective in the Peer-Led Team-Learning Approach? J. Coll. Sci. Teach., 38(4), 62–67.
- Shulman L. S., (1986), Those Who Understand: Knowledge Growth in Teaching, Educ. Res., 15(2), 4–14, DOI:10.2307/1175860.
- Shulman L. S., (1987), Knowledge and Teaching: Foundations of the New Reform, Harvard Educ. Rev., 57(1), 1–22.
- Smith J., Wilson S. B., Banks J., Zhu L. and Varma-Nelson P., (2014), Replicating Peer-Led Team Learning in cyberspace: Research, opportunities, and challenges, J. Res. Sci. Teach., 51(6), 714–740, DOI:10.1002/tea.21163.
- Stevens R. and Toro-Martell S., (2003), Leaving a Trace, J. Mus. Educ., 28(2), 25–31, DOI:10.1080/10598650.2003.11510479.
- Stigler J. and Hiebert J., (1999), Understanding and Improving Classroom Mathematics Instruction: An Overview of the TIMMSS Video Study, in Australian Council for Educational Research (ACER). (1999). Raising Australian Standards in Mathematics and Science: Insights from TIMSS (Conference Proceedings). 1997 – Raising Australian Standards in Mathematics and Science: Insight from TIMSS, pp. 52–65.
- Tenney A. and Houck B., (2004), Learning about Leadership, J. Coll. Sci. Teach., 33(6), 25.
- Tien L. T., Roth V. and Kampmeier J. A., (2002), Implementation of a Peer-Led Team Learning Instructional Approach in an Undergraduate Organic Chemistry Course, J. Res. Sci. Teach., 39(7), 606–632.
- Tien L. T., Roth V. and Kampmeier J. A., (2004), A Course To Prepare Peer Leaders To Implement a Student-Assisted Learning Method, J. Chem. Educ., 81(9), 1313, DOI:10.1021/ed081p1313.
- van Dijk E. M. and Kattmann U., (2007), A research model for the study of science teachers’ PCK and improving teacher education, Teach. Teach. Educ., 23(6), 885–897, DOI:10.1016/j.tate.2006.05.002.
- Van Driel J. H., Jong O. D. and Verloop N., (2002), The development of preservice chemistry teachers' pedagogical content knowledge, Sci. Educ., 86(4), 572–590, DOI:10.1002/sce.10010.
- Walba D. M., (1985), Topological stereochemistry, Tetrahedron, 41(16), 3161–3212, DOI:10.1016/S0040-4020(01)96671-2.
- Walther-Thomas C., Korinek L., McLaughlin V. L. and Williams B. T. (2000), Collaboration for inclusive education: developing successful programs, Boston: Allyn and Bacon.
- Yin R. K., (2014), Case Study Research, 5th edn, Sage Publications.
| This journal is © The Royal Society of Chemistry 2018 |