Transition-metal-free, visible-light-induced oxidative cross-coupling for constructing β-acetylamino acrylosulfones from sodium sulfinates and enamides†
Deli
Sun
a and
Ronghua
Zhang
 *ab
*ab
aSchool of Chemical Science and Engineering, Tongji University, 1239 Siping Road, Shanghai 200092, China. E-mail: rhzhang@tongji.edu.cn
bShanghai Key Lab of Chemical Assessment and Sustainability, Tongji University, 1239 Siping Road, Shanghai 200092, China
First published on 22nd September 2017
Abstract
A visible-light-induced, Rose Bengal catalyzed photoredox process for synthesizing β-acetylamino acrylosulfones has been discovered. This transformation represents an efficient and attractive method for synthesizing β-acetylamino acrylosulfones from sodium sulfinates and enamides under transition-metal-free conditions in moderate to good yields. In addition, it exhibits a good substrate scope and functional group tolerance. The use of inexpensive organic dye Rose Bengal as the photocatalyst with easy operation at room temperature makes this protocol very practical.
Introduction
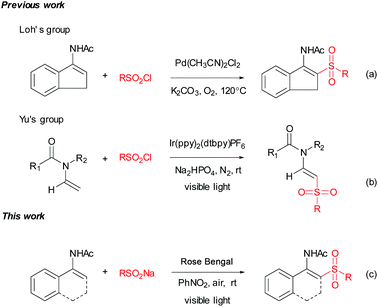
With substantial progress in medicinal and organic chemistry, sulfone-containing skeletons have gained increasing attention,1 especially over the past few years, mainly due to their attractive bioactivity2 and broad applications in synthetic methods.3 Therefore, the synthesis of sulfones has gained much attention; one of the most common methods for this transformation is the addition of sulfonyl radicals to olefins.4 A lot of arenesulphonyl halides, alkane-sulphonyl chlorides and sulfinates were added to olefins under the corresponding conditions.5 The use of sodium sulfinates as sulfonylation reagents has undergone much development in recent years.6 Sodium sulfinates are stable, easy to handle and readily available from their corresponding materials.7 Although some progress has been made in sulfonylation with sodium sulfinates, there still remains a great challenge in developing green, efficient and practical strategies to synthesize sulfones.Enamides are powerful building blocks for the synthesis of valuable synthetic intermediates as well as various bioactive molecules,8 especially for the synthesis of small but complex nitrogen-containing compounds.9 Enamides characteristically exhibit a fine balance of stability and reactivity, which has currently led to their wide applications in organic transformation.10 In the past decade, transition metal-catalyzed direct C–H bond functionalization of enamides has undergone substantial development.11 Among these methods, a Pd-catalyzed C–H bond direct sulfonylation of enamides with arylsulfonyl chlorides was reported by Loh's group.12 The products β-acetylamino acrylosulfones are valuable intermediates which could be applied to synthesize biologically active β-amido sulfones.13 Since enamides could be conveniently synthesized by a variety of methods,14 the direct sulfonation of enamides represents a flexible approach to this kind of motif.
Visible-light-initiated photoredox catalysis has been established as a uniquely powerful tool for synthesizing new chemical bonds under very mild conditions in the presence of photoredox catalysts.15 Although ruthenium, iridium or copper complexes as photoredox catalysts have been well demonstrated for carbon–carbon and carbon–heteroatom bond coupling under visible-light irradiation,16 organic dyes such as Eosin Y, Fluorescein, and Rose Bengal (RB) have proven to be efficient in some visible-light-promoted organic transformations and show the advantages of efficiency, cheapness and non-toxicity compared with metal-based photoredox catalysts.17 Recently, visible-light-induced generation of sulfonyl radicals was developed.18 It is noteworthy that König's group reported the photoredox catalysis for the synthesis of vinyl sulfones from sulfinates and alkenes.19 However, to the best of our knowledge, the generation of in situ of sulfonyl radicals from sulfinates for the synthesis of β-acetylamino acrylosulfones by visible-light photoredox catalysis has not been reported. As a part of studies focusing on the synthesis of sulfone-containing compounds, we wish to report a transition-metal-free, visible-light-induced synthesis of β-acetylamino acrylosulfones from sulfinates and enamides using the organic dye RB as the photocatalyst, and nitrobenzene and air as the oxidants (Scheme 1c).
Results and discussion
We initially started the investigation of the visible-light-induced reaction using N-(3,4-dihydronaphthalen-1-yl)acetamide (1a) and sodium benzenesulfinate (2a) as the model substrates, in the presence of nitrobenzene. When the model reaction was carried out in DMF/H2O (v/v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 mL) as the solvent, under irradiation with 5 W green LEDs, the desired product 3a was obtained in 12% yield after 17 h (Table 1, entry 1). To our delight, when Eosin Y was added as the photocatalyst to the reaction mixture, the yield of product 3a was up to 80% yield and the structure of 3a was further confirmed by X-ray crystal analysis (CCDC 1548828†). During the subsequent optimization, common photocatalysts Eosin B, RB, Fluorescein, Methylene Blue and Ru(bpy)3Cl2·6H2O were screened (Table 1, entries 3–7). RB was found to be the most efficient photocatalyst and afforded the product 3a in 83% yield (Table 1, entry 4). Further elaboration of the solvents proved that DMF/H2O (v/v = 3
1, 2 mL) as the solvent, under irradiation with 5 W green LEDs, the desired product 3a was obtained in 12% yield after 17 h (Table 1, entry 1). To our delight, when Eosin Y was added as the photocatalyst to the reaction mixture, the yield of product 3a was up to 80% yield and the structure of 3a was further confirmed by X-ray crystal analysis (CCDC 1548828†). During the subsequent optimization, common photocatalysts Eosin B, RB, Fluorescein, Methylene Blue and Ru(bpy)3Cl2·6H2O were screened (Table 1, entries 3–7). RB was found to be the most efficient photocatalyst and afforded the product 3a in 83% yield (Table 1, entry 4). Further elaboration of the solvents proved that DMF/H2O (v/v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
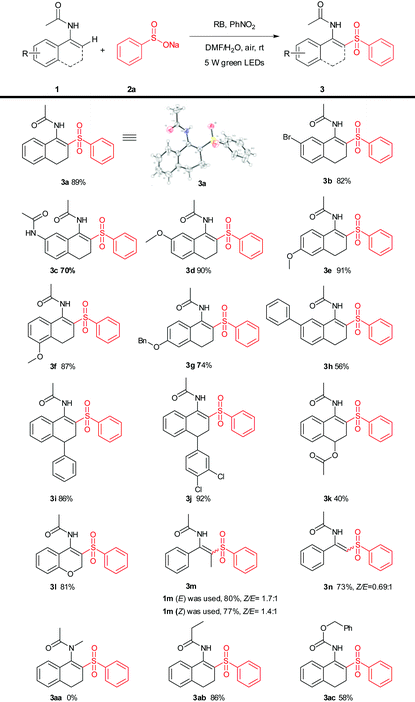
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 mL) was the best choice (Table 1, entries 4 and 8–13). Among the oxidants screened (Table 1, entries 14–21), it was revealed that the optimal reaction oxidant was nitrobenzene (Table 1, entries 14). Additionally, there was no conversion in the absence of an oxidant under a N2 atmosphere (Table 1, entry 22). With the optimal reaction conditions in hand, the scope of this photoredox process was applied to a variety of substituted enamides and the results are summarized in Table 2. The substituents on different positions of the enamides with benzenesulfinate (2a) were first tested. It was found that various functional groups were tolerated well under the present oxidative conditions affording the products in fair to good yields (Table 2, 3a–3n). Whereas in the case of employing tertiary enamides (without N-H), no desired product (Table 2, 3aa) was isolated. Furthermore, another enamide and enecarbamate were used to give the desired product (Table 2, 3ab–3ac).
1, 2 mL) was the best choice (Table 1, entries 4 and 8–13). Among the oxidants screened (Table 1, entries 14–21), it was revealed that the optimal reaction oxidant was nitrobenzene (Table 1, entries 14). Additionally, there was no conversion in the absence of an oxidant under a N2 atmosphere (Table 1, entry 22). With the optimal reaction conditions in hand, the scope of this photoredox process was applied to a variety of substituted enamides and the results are summarized in Table 2. The substituents on different positions of the enamides with benzenesulfinate (2a) were first tested. It was found that various functional groups were tolerated well under the present oxidative conditions affording the products in fair to good yields (Table 2, 3a–3n). Whereas in the case of employing tertiary enamides (without N-H), no desired product (Table 2, 3aa) was isolated. Furthermore, another enamide and enecarbamate were used to give the desired product (Table 2, 3ab–3ac).
| Entry | Photocatalyst | Oxidant (equiv.) | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.6 mmol), photocatalyst (2.5 mol%), oxidant, solvent (2 mL), 5 W green LEDs, rt, air, 17 h. b Isolated yield. c Without light irradiation. d Under N2. e Under air. f Without oxidant under N2. | ||||
| 1 | None | PhNO2 (1.0) | DMF/H2O | 12 |
| 2 | Eosin Y | PhNO2 (1.0) | DMF/H2O | 80 |
| 3 | Eosin B | PhNO2 (1.0) | DMF/H2O | 78 |
| 4 | RB | PhNO2 (1.0) | DMF/H2O | 83 |
| 5 | Fluorescein | PhNO2 (1.0) | DMF/H2O | 75 |
| 6 | Methylene Blue | PhNO2 (1.0) | DMF/H2O | 76 |
| 7 | Ru(bpy)3Cl2·6H2O | PhNO2 (1.0) | DMF/H2O | 81 |
| 8 | RB | PhNO2 (1.0) | DMF | 60 |
| 9 | RB | PhNO2 (1.0) | DMSO | 78 |
| 10 | RB | PhNO2 (1.0) | DMSO/H2O | 65 |
| 11 | RB | PhNO2 (1.0) | EtOH | 76 |
| 12 | RB | PhNO2 (1.0) | THF | 40 |
| 13 | RB | PhNO2 (1.0) | MeCN | 32 |
| 14 | RB | PhNO 2 (2.0) | DMF/H 2 O | 89 |
| 15 | RB | p-BrPhNO2 (2.0) | DMF/H2O | 89 |
| 16 | RB | MeNO2 (2.0) | DMF/H2O | 35 |
| 17 | RB | Na2S2O8 (2.0) | DMF/H2O | Trace |
| 18 | RB | O2 | DMF/H2O | 18 |
| 19c | RB | PhNO2 | DMF/H2O | Trace |
| 20d | RB | PhNO2 | DMF/H2O | 87 |
| 21e | RB | Air | DMF/H2O | 61 |
| 22f | RB | N2 | DMF/H2O | — |
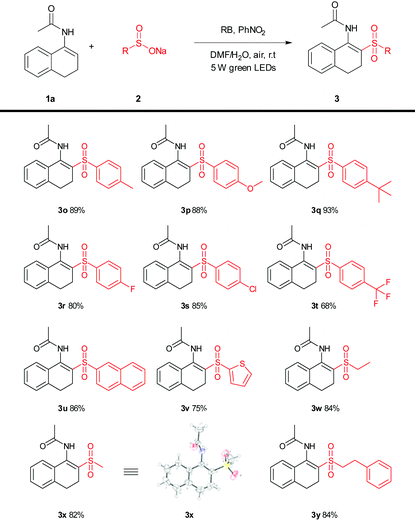
Next, the enamide (1a) was also successfully applied to a variety of sulfonates to broaden the scope. As shown in Table 3, the sodium salts of alkyl, aryl and heteroaryl sulfinates reacted smoothly with enamides (1a) to give the sulfonylation products (Table 3, 3o–3y), in good to excellent yields of 68–93%. The structure of 3x was further confirmed by X-ray crystal analysis (CCDC 1568727†).
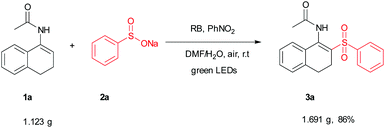
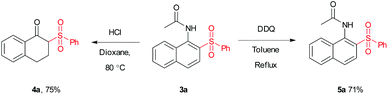
To test the scalability and practicality of the newly established method, a gram-scale experiment was conducted under the standard conditions (Scheme 2). Remarkably, an excellent yield of 86% was achieved, which paves the way for its further application in organic transformation. To demonstrate the synthetic utility of the method, β-acetylamino acrylosulfone (3a) was subjected to subsequent transformations. It was hydrolyzed in the next step by using hydrochloric acid to provide the corresponding ketone (4a) in 75% yield (see the ESI†). Furthermore, the oxidation of 3a with DDQ led to N-(2-(phenylsulfonyl)naphthalen-1-yl)acet-amide (5a) in the yield of 71% (Scheme 3). Some of the β-acetylamino acrylosulfones could also be applied to synthesize biologically active β-amido sulfones.13
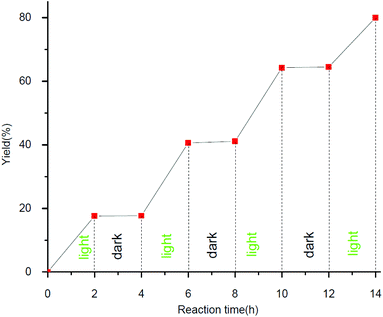
In order to understand the mechanism of this visible-light-induced photoredox reaction, several control experiments were carried out (Table 4). When the reaction was performed under N2, a mixture of nitrobenzene and azoxybenzene was obtained. However, when the reaction was performed under air, a trace byproduct was found and 91% of nitrobenzene was recovered. A trace desired product was found when TEMPO (2,2,6,6-tetra-methyl-1-piperidinyloxy, a common radical scavenger) was added. These results indicated that the reaction might involve a radical pathway and the radical could be intercepted by TEMPO. However, we cannot exclude the radical-chain propagation pathway, an on/off visible light irradiation experiment was performed (Fig. 1). It can be concluded from the graph that the RB catalyzed transformation has been verified to require continuous photo-irradiation which indicates that chain propagation is not the significant pathway for the transformation.18
| Entry | Conditions | Resultsb |
|---|---|---|
a Reaction conditions: 1a (0.2 mmol), 2a (0.6 mmol), RB (2.5 mol%), DMF/H2O (2 mL, v/v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 5 W green LEDs, rt.
b Isolated yield. 1), 5 W green LEDs, rt.
b Isolated yield.
|
||
| 1 | PhNO2 (2 equiv.), N2 | 3a (87%), PhNO2 (38%), azoxybenzene (28%) |
| 2 | PhNO2 (2 equiv.), air | 3a (89%), PhNO2 (91%) |
| 3 | PhNO2 (2 equiv.), TEMPO (2 equiv.), air | 3a (trace), 1a (87%) |
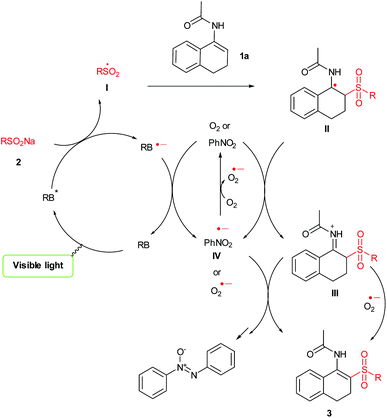
Although the mechanism of this visible-light-induced photoredox reaction is not yet completely clear, a plausible catalytic mechanism also can be proposed on the basis of the above observations and previous studies11e,19 (Scheme 4). First, irradiation with visible light excites photocatalyst RB into the excited state RB* which is reductively quenched by the sulfinate 2 giving a corresponding radical I. Subsequently radical I attacks the double bond of 1a to form intermediate II which could be further oxidized by nitrobenzene or O2 to give the iminium ion III. Finally, the abstraction of the H+ from III will lead to the formation of the desired product 3.
Conclusions
In conclusion, we report a protocol for direct synthesis of β-acetylamino acrylosulfones via a visible-light-induced oxidetive cross-coupling with enamides and sodium sulfinates under transition-metal-free conditions. This transformation was performed effectively and attractively at room temperature which involved cheap, readily available photocatalyst RB, sodium sulfinates and low Watt visible-light. Moreover, this strategy afforded desired β-acetylamino acrylosulfones with fine functional group tolerance and practically scalable synthesis. In addition, the product β-acetylamino acrylosulfone was transformed into the corresponding β-ketosulfone after hydrolysis, or into the N-(2-(phenylsulfonyl)-naphthalen-1-yl)-acetamide in a single oxidative step. Ongoing research including more exquisite substrates and expanding the methodology for the preparation of potentially bioactive molecules are currently underway in our laboratory and will be reported in due course.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank the National Natural Science Foundation of China (20972113/B020502). We thank Dr Wenyan Dan (Tongji University) for analytical support.Notes and references
- (a) B. A. Frankel, M. Bentley, R. G. Kruger and D. G. McCafferty, J. Am. Chem. Soc., 2004, 126, 3404 CrossRef CAS PubMed; (b) A. F. Kisselev, W. A. van der Linden and H. S. Overkleeft, Chem. Biol., 2012, 19, 99 CrossRef CAS PubMed; (c) L. Ni, X. S. Zheng, P. K. Somers, L. K. Hoong, R. R. Hill, E. M. Marino, K.-L. Suen, U. Saxena and C. Q. Meng, Bioorg. Med. Chem. Lett., 2003, 13, 745 CrossRef CAS PubMed; (d) Y. Fang, Z. Luo and X. Xu, RSC Adv., 2016, 6, 59661 RSC; (e) R. Mao, Z. Yuan, R. Zhang, Y. Ding, X. Fan and J. Wu, Org. Chem. Front., 2016, 3, 1498 RSC.
- (a) R. Ettari, E. Nizi, M. E. Di Francesco, M.-A. Dude, G. Pradel, R. Vičík, T. Schirmeister, N. Micale, S. Grasso and M. Zappalà, J. Med. Chem., 2008, 51, 988 CrossRef CAS PubMed; (b) J. M. Sanfrutos, A. M. Fernandez, F. H. Mateo, D. G. Gonzalez, R. S. Gonzalez and F. S. Gonzalez, Org. Biomol. Chem., 2011, 9, 851 RSC; (c) V. P. Sandanayaka, A. S. Prashad, Y. Yang, R. T. Williamson, Y. I. Lin and T. S. Mansour, J. Med. Chem., 2003, 46, 2569 CrossRef CAS PubMed.
- (a) Q. Zhu and Y. Lu, Org. Lett., 2009, 11, 1721 CrossRef CAS PubMed; (b) S. Mao, Y. R. Gao, X. Q. Zhu, D. D. Guo and Y. Q. Wang, Org. Lett., 2015, 17, 1692 CrossRef CAS PubMed; (c) W. Wei, J. Li, D. Yang, J. Wen, Y. Jiao, J. You and H. Wang, Org. Biomol. Chem., 2014, 12, 1861 RSC.
- (a) I. C. Popoff, J. L. Dever and G. R. Leader, J. Org. Chem., 1969, 34, 1128 CrossRef CAS; (b) Q. Q. Lu, J. Zhang, G. L. Zhao, Y. Qi, H. M. Wang and A. W. Lei, J. Am. Chem. Soc., 2013, 135, 11481 CrossRef CAS PubMed; (c) W. Wei, C. L. Liu, D. S. Yang, J. W. Wen, J. M. You, Y. R. Suo and H. Wang, Chem. Commun., 2013, 49, 10239 RSC; (d) Y. J. Jiang and T. P. Loh, Chem. Sci., 2014, 5, 4939 RSC; (e) X. Li, Y. Xu, W. Wu, C. Jiang, C. Qi and H. Jiang, Chem. – Eur. J., 2014, 20, 7911 CrossRef CAS PubMed; (f) K. D. Zhou, H. G. Xia and J. Wu, Org. Chem. Front., 2017, 4, 1121 RSC.
- (a) A. K. Singh, R. Chawla and L. D. S. Yadav, Tetrahedron Lett., 2014, 55, 2845 CrossRef CAS; (b) N. Taniguchi, Synlett, 2011, 1308 CrossRef CAS; (c) T. Liu, D. Q. Zheng and J. Wu, Org. Chem. Front., 2017, 4, 1079 RSC; (d) Q. Jiang, B. Xu, J. Jia, A. Zhao, Y. R. Zhao, Y. Y. Li, N. N. He and C. C. Guo, J. Org. Chem., 2014, 79, 7372 CrossRef CAS PubMed; (e) N. Kamigata, H. Sawada, N. Suzuki and M. Kobayashi, J. Org. Chem., 1983, 48, 3793 CrossRef CAS.
- (a) R. Chawla, A. K. Singh and L. D. S. Yadav, Eur. J. Org. Chem., 2014, 2032 CrossRef CAS; (b) B. V. Rokade and K. R. Prabhu, J. Org. Chem., 2014, 79, 8110 CrossRef CAS PubMed; (c) F. Xiao, H. Chen, H. Xie, S. Chen, L. Yang and G. J. Deng, Org. Lett., 2014, 16, 50 CrossRef CAS PubMed; (d) Y. Xu, X. Tang, W. Hu, W. Wu and H. Jiang, Green Chem., 2014, 16, 3720 RSC.
- (a) L. K. Liu, Y. Chi and K. Y. Jen, J. Org. Chem., 1980, 45, 406 CrossRef CAS; (b) J. Liu, X. Zhou, H. Rao, F. Xiao, C. J. Li and G. J. Deng, Chem. – Eur. J., 2011, 17, 7996 CrossRef CAS PubMed.
- (a) J. H. Xie, S. F. Zhu and Q. L. Zhou, Chem. Rev., 2011, 111, 1713 CrossRef CAS PubMed; (b) A. Kumar and M. K. Muthyala, Tetrahedron Lett., 2011, 22, 1287 Search PubMed; (c) C. Curti, M. Laget, A. O. Carle, A. Gellis and P. Vanelle, Eur. J. Med. Chem., 2007, 42, 880 CrossRef CAS PubMed; (d) H. Yang, R. G. Carter and L. N. Zakharov, J. Am. Chem. Soc., 2008, 130, 9238 CrossRef CAS PubMed.
- (a) N. Hu, G. Zhao, Y. Zhang, X. Liu, G. Li and W. Tang, J. Am. Chem. Soc., 2015, 137, 6746 CrossRef CAS PubMed; (b) G. Liu, X. Liu, Z. Cai, G. Jiao, G. Xu and W. Tang, Angew. Chem., Int. Ed., 2013, 52, 4235 CrossRef CAS PubMed; (c) M. N. Zhao, Z. H. Ren, Y. Y. Wang and Z. H. Guan, Org. Lett., 2014, 16, 608 CrossRef CAS PubMed; (d) M. N. Zhao, Z. H. Ren, Y. Y. Wang and Z. H. Guan, Chem. Commun., 2012, 48, 8105 RSC; (e) C. H. Lei, L. Zhao, D. X. Wang, J. Zhu and M. X. Wang, Org. Chem. Front., 2014, 1, 909 RSC.
- (a) S. Tong, D. X. Wang, L. Zhao, J. Zhu and M. X. Wang, Angew. Chem., Int. Ed., 2012, 51, 4417 CrossRef CAS PubMed; (b) C. H. Lei, D. X. Wang, L. Zhao, J. Zhu and M. X. Wang, J. Am. Chem. Soc., 2013, 135, 4708 CrossRef CAS PubMed.
- (a) H. Zhou, W. J. Chung, Y. H. Xu and T. P. Loh, Chem. Commun., 2009, 23, 3472 RSC; (b) M. Prakash, S. Muthusamy and V. Kesavan, J. Org. Chem., 2014, 79, 7836 CrossRef CAS PubMed; (c) Q. G. Lu, C. Liu, Z. Y. Huang, Y. Y. Ma, J. Zhang and A. W. Lei, Chem. Commun., 2014, 50, 14101 RSC; (d) Y. C. Tang, Y. Zhang, K. F. Wang, X. Q. Li, X. S. Xu and X. H. Du, Org. Biomol. Chem., 2015, 13, 7084 RSC; (e) R. Ding, Q. C. Zhang, Y. H. Xu and T. P. Loh, Chem. Commun., 2014, 50, 11661 RSC.
- (a) Y. H. Xu, M. Wang, P. Lu and T. P. Loh, Tetrahedron, 2013, 69, 4403 CrossRef CAS.
- J. Jiang, Y. Wang and X. M. Zhang, ACS Catal., 2014, 4, 1570 CrossRef CAS.
- (a) W. Tang, A. Capacci, M. Sarvestani, X. Wei, N. K. Yee and C. H. Senanayake, J. Org. Chem., 2009, 74, 9528 CrossRef CAS PubMed; (b) Z. H. Guan, K. X. Huang, S. C. Yu and X. M. Zhang, Org. Lett., 2009, 11, 481 CrossRef CAS PubMed; (c) Z. H. Guan, Z. Y. Zhang, Z. H. Ren, Y. Y. Wang and X. M. Zhang, J. Org. Chem., 2011, 76, 339 CrossRef CAS PubMed; (d) H. Zhao, C. P. Vandenbossche, S. G. Koenig, S. P. Singh and R. P. Bakale, Org. Lett., 2008, 10, 505 CrossRef CAS PubMed; (e) J. T. Reeves, Z. Tan, Z. S. Han, G. Li, Y. Zhang, Y. Xu, D. C. Reeves, N. C. Gonnella, S. Ma, H. Lee, B. Z. Lu and C. H. Senanayake, Angew. Chem., Int. Ed., 2012, 51, 1400 CrossRef CAS PubMed; (f) J. Han, M. Jeon, H. K. Pak, Y. H. Rhee and J. Park, Adv. Synth. Catal., 2014, 356, 2769 CrossRef CAS.
- (a) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed; (b) J. Xuan and W. J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828 CrossRef CAS PubMed; (c) D. P. Hari and B. König, Chem. Commun., 2014, 50, 6688 RSC; (d) D. Ravelli, S. Protti and M. Fagnoni, Chem. Rev., 2016, 116, 9850 CrossRef CAS PubMed; (e) G. Y. S. Qiu, Y. W. Li and J. Wu, Org. Chem. Front., 2016, 3, 1011 RSC.
- (a) Y. M. Xi, H. Yi and A. W. Lei, Org. Biomol. Chem., 2013, 11, 2387 RSC; (b) J. R. Chen, X. Q. Hu, L. Q. Lu and W. J. Xiao, Acc. Chem. Res., 2016, 49, 1911 CrossRef CAS PubMed; (c) K. L. Skubi, T. R. Blum and T. P. Yoon, Chem. Rev., 2016, 116, 10035 CrossRef CAS PubMed; (d) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102 RSC.
- (a) M. Majek, F. Filace and A. J. von Wangelin, Beilstein J. Org. Chem., 2014, 10, 981 CrossRef CAS PubMed; (b) I. Ghosh and B. König, Angew. Chem., Int. Ed., 2016, 55, 7676 CrossRef CAS PubMed; (c) Y. H. Pan, C. W. Kee, L. Chen and C. H. Tan, Green Chem., 2011, 13, 2682 RSC; (d) Q. Y. Meng, J. J. Zhong, Q. Liu, X. W. Gao, H. H. Zhang, T. Lei, Z. J. Li, K. Feng, B. Chen, C. H. Tung and L. Z. Wu, J. Am. Chem. Soc., 2013, 135, 19052 CrossRef CAS PubMed; (e) D. Ravelli, M. Fagnoni and A. Albini, Chem. Soc. Rev., 2013, 42, 97 RSC; (f) T. Hering, A. U. Meyer and B. König, J. Org. Chem., 2016, 81, 6927 CrossRef CAS PubMed; (g) A. K. Yadav and L. D. S. Yadav, Green Chem., 2015, 17, 3515 RSC.
- (a) H. Jiang, Y. Z. Cheng, Y. Zhang and S. Y. Yu, Eur. J. Org. Chem., 2013, 5485 CrossRef CAS; (b) W. Yang, S. Yang, P. Li and L. Wang, Chem. Commun., 2015, 51, 7520 RSC; (c) H. Wang, Q. Lu, C. W. Chiang, Y. Luo, J. Zhou, G. Wang and A. Lei, Angew. Chem., Int. Ed., 2017, 56, 595 CrossRef CAS PubMed; (d) M. H. Huang, Y. L. Zhu, W. J. Hao, A. F. Wang, D. C. Wang, F. Liu, P. Wei, S. J. Tu and B. Jiang, Adv. Synth. Catal., 2017, 359, 2229 CrossRef CAS; (e) G. Zhang, L. Zhang, H. Yi, Y. Luo, X. Qi, C.-H. Tung, L.-Z. Wu and A. Lei, Chem. Commun., 2016, 52, 10407 RSC; (f) D. Yang, B. Huang, W. Wei, J. Li, G. Lin, Y. Liu, J. Ding, P. Sun and H. Wang, Green Chem., 2016, 18, 5630 RSC.
- (a) A. U. Meyer, S. Jäger, D. P. Hari and B. König, Adv. Synth. Catal., 2015, 357, 2050 CrossRef CAS; (b) A. U. Meyer, K. Straková, T. Slanina and B. König, Chem. – Eur. J., 2016, 22, 8694 CrossRef CAS PubMed; (c) A. U. Meyer, V. W.-H. Lau, B. König and B. V. Lotsch, Eur. J. Org. Chem., 2017, 2179 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1548828 and 1568727. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7qo00729a |
| This journal is © the Partner Organisations 2018 |