Solid-state fluorescent materials based on coumarin derivatives: polymorphism, stimuli-responsive emission, self-assembly and optical waveguides†
Rong Rong
Cui
a,
Yuan Chao
Lv
b,
Yong Sheng
Zhao
 b,
Na
Zhao
b,
Na
Zhao
 *a and
Nan
Li
*a and
Nan
Li
 *a
*a
aKey Laboratory of Macromolecular Science of Shaanxi Province, Key Laboratory of Applied Surface and Colloid Chemistry of Ministry of Education, School of Chemistry & Chemical Engineering, Shaanxi Normal University, Xi’an, 710119, China. E-mail: nzhao@snnu.edu.cn; nli@snnu.edu.cn
bKey Laboratory of Photochemistry, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
First published on 6th February 2018
Abstract
Solid-state fluorescent materials have attracted a surge of interest in recent years due to their wide applications in the fields of photoelectric devices, memory storage and fluorescent probes. Compared to the synthesis of new molecules, exploring new properties in known molecules is a facile approach to obtain functionalized fluorescent materials. In this report, we systematically explored the solid-state photoluminescence properties and applications of 7-(diethylamino)coumarin-3-aldehyde (DCA) and 7-(diethylamino)coumarin-3-carboxylic acid (DCCA). Both fluorophores exhibited a special concentration-dependent emission effect. They displayed polymorphism dependent solid-state emission and single crystal analysis revealed that enhanced overlap between neighbouring molecules resulted in a red-shifted emission. Crystal-to-crystal transformation has also been achieved for both DCA and DCCA by employing an external thermal treatment. In addition, the solid powder of DCA and DCCA displayed fluorescence response to HCl and NH3 gas with high sensitivity. Furthermore, 1D micromaterials were assembled for both fluorophores and DCA exhibited outstanding optical waveguide behavior.
Introduction
Organic solid-state fluorescent materials have received considerable attention due to their broad range of applications in the fabrication of organic light-emitting diodes (OLEDs), laser dyes, memory storage materials and fluorescent probes.1–4 Appropriate solid-state fluorescent materials could provide an optimal choice and could be employed to increase the precision, sensitivity and spatial resolution in diverse research areas.5 Since the photoluminescence properties of many solid-state fluorophores are significantly influenced by their molecular arrangements, there is an increasing interest in the development of functional materials whose emission color can be efficiently tuned by external stimuli through rearrangement of the molecular packing.6Synthesis of fluorescent molecules with structure diversity is the most creative activity in the field of fluorescent materials. However, creativity in functionalized fluorescent materials has often arisen from novel conceptual interpretations of well-established molecules.7 Compared to the development of new molecules that may suffer from synthesis complexity and property uncertainty, investigating known fluorophores to explore their superior properties and versatile applications is undoubtedly a shortcut strategy to obtain functionalized fluorescent materials. Indeed, many outstanding properties of star fluorescent molecules were dug out after the first report.8
Coumarin, one of the most noted organic fluorophores, has been widely and extensively used in both material and biological sciences due to its inherent physicochemical and photophysical characteristics, such as reasonable stability and relative ease of synthesis.9 However, in comparison with abundant reports about the photoluminescence research of coumarins in solution, investigations of fluorescence properties as well as potential utilization in the solid state are still rare.10
Herein, we report on the exploration of the solid-state optical properties and applications of two fluorophores based on an aminocoumarin scaffold, 7-(diethylamino)coumarin-3-aldehyde (DCA) and 7-(diethylamino)coumarin-3-carboxylic acid (DCCA) (Scheme 1).11 Both fluorophores displayed special concentration-dependent emission behaviour. Importantly, two types of crystal with varied emission color were obtained for DCA and DCCA. Subsequent single crystal analysis revealed the origin of this polymorphism phenomenon. Upon thermal treatment, crystal-to-crystal transformations were achieved for both fluorophores. Meanwhile, the solid state of DCA and DCCA exhibited sensitive fluorescence response to HCl and NH3 gas with different modes. Moreover, DCA and DCCA were able to assemble into 1D micromaterials, while DCA possessed remarkable optical waveguide behaviour.
Results and discussion
Concentration-dependent emission
The photophysical properties of DCA and DCCA were studied in different solvents (Table S1 and Fig. S1, S2, ESI†). The results confirmed their ICT character12 and DCCA exhibited a more pronounced solvatochromic effect due to the stronger electron withdrawing ability of carboxylic acid than aldehyde in the 3-position of coumarin. Interestingly, both fluorophores presented a special concentration-dependent emission effect. As displayed in Fig. 1A, with increasing concentration of DCA in THF solution from 1 × 10−6 M to 5 × 10−2 M, a significant bathochromic shift of emission peak (from 470 nm to 520 nm) was observed, while the emission color was changed from light blue to green under 365 nm UV irradiation. Similarly, about 40 nm red-shifted emission was achieved for DCCA when the concentration was gradually enhanced from 1 × 10−6 M to 1 × 10−2 M (Fig. 1B). Considering the π-conjugated structure of the coumarin core, the degree of π-overlap could be enhanced by increasing their concentration, which led to relatively larger electronic delocalization and resulted in its red-shifted emission color.13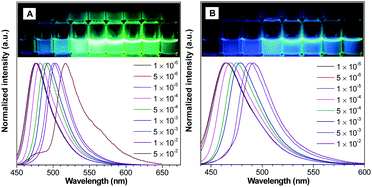 | ||
| Fig. 1 Normalized PL spectra of DCA (A) and DCCA (B) in THF at different concentrations. Inset: Photograph of DCA (A) and DCCA (B) in THF with varied concentrations under 365 nm UV irradiation. | ||
Polymorphism-dependent emission
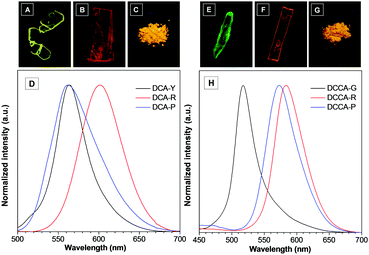
Since both fluorophores were easy to crystallize, the crystals of DCA and DCCA were then grown in different solvents. It is worth noting that two plate-like crystals of DCA with distinct emission colors were successfully obtained by slowly evaporating their diethyl ether/ethyl acetate and hexane/acetone solution (termed DCA-Y and DCA-R), respectively. As shown in Fig. 2A–D and Table 1, DCA-Y displayed yellow emission at 564 nm with a ΦF of 7.5% and an average fluorescence lifetime (τavg) of 8.25 ns, whereas DCA-R exhibited red-shifted emission at 602 nm (ΦF = 7.2%, τavg = 16.77 ns). It was noted that the corresponding as-prepared powder sample of DCA (termed DCA-P) gave an orange emission color and the emission spectrum almost overlapped with that of DCA-Y, while the ΦF and τavg of DCA-P were determined to be 7.9% and 5.73 ns, respectively.| Compd | λ em [nm] | Φ F (%) | τ avg [ns] | k f [109 s−1] | k nr [109 s−1] |
|---|---|---|---|---|---|
| a Absolute fluorescence quantum yield was measured by using the calibrated integrating sphere system. b Average fluorescence lifetime. | |||||
| DCA-Y | 564 | 7.5 | 8.25 | 0.009 | 0.112 |
| DCA-R | 601 | 7.2 | 16.77 | 0.004 | 0.055 |
| DCA-P | 562 | 7.9 | 5.73 | 0.014 | 0.160 |
| DCCA-G | 518 | 9.6 | 3.77 | 0.025 | 0.240 |
| DCCA-R | 584 | 16.5 | 45.13 | 0.004 | 0.018 |
| DCCA-P | 573 | 8.5 | 19.53 | 0.004 | 0.047 |
Interestingly, two forms of crystals with a nod and plate shape (called DCCA-G and DCCA-R) were obtained simultaneously by slowly evaporating the dichloromethane/methanol solution of DCCA (Fig. 2E–H). A green emission at 518 nm was observed for DCCA-G with ΦF of 9.6% and τavg of 3.77 ns. In contrast, DCCA-R emitted red light at 584 nm (ΦF = 16.5%, τavg = 45.13 ns). Different from DCCA-G and DCCA-R, the as-prepared powder sample of DCCA (named DCCA-P) displayed the orange emission color (λem = 573 nm, ΦF = 8.5%, τavg = 19.53 ns). It's obvious that both DCA and DCCA presented significantly polymorphism-dependent emission.14 Their solid-state emission could be easily tuned by altering the preparation precedures.
PXRD of DCA in different states was performed and the results showed that the diffraction profile of DCA-P was almost the same as that of DCA-Y (Fig. S3A, ESI†), suggesting their similar packing arrangement and almost identical emission. Additionally, the different diffraction pattern of DCCA in three states indicated its distinct packing assignment (Fig. S3B, ESI†), which resulted in the distinguishing emission.
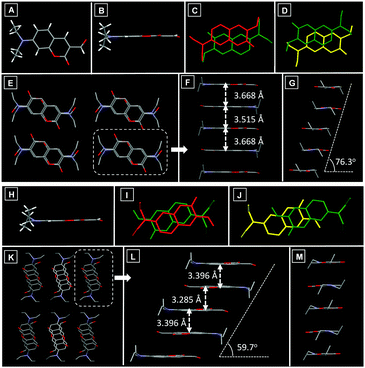
To gain further insight into the mechanism of the above pronounced polymorphism properties, single crystal X-ray structural analysis was carried out and the corresponding crystal data are summarized in Table S2 (ESI†). X-ray diffraction analysis revealed that the molecules in DCA-Y adopted a coplanar conformation (Fig. 3A and B) and exhibited a head-to-tail antiparallel packing mode with two types of overlap arrangement (Fig. 3C and D), which resulted in the formation of polymer columns along the b-axis direction (Fig. 3E). Within each column, the distances between adjacent molecules were 3.668 Å and 3.515 Å, respectively, suggestive of the strong intermolecular π–π stacking (Fig. 3F). A modest molecular overlap led to a negligible slip angle along the long molecular axis, whereas the slip angle along the short molecular axis was 76.3° (Fig. 3G). Meanwhile, multiple C–H⋯O (2.524 Å, 2.613 Å and 2.633 Å) interactions existed within the crystal (Fig. S4A, ESI†), which linked columns to form a 3D structure in the crystal.
Although the conformation of the DCA-R crystal was similar to that of DCA-Y (Fig. 3H), the packing mode was entirely different. In the DCA-R, molecules also adopted a head-to-tail antiparallel packing mode (Fig. 3I and J) with two kinds of overlap arrangement, which led to the formation of molecular columns along the b-axis (Fig. 3K). The neighbouring molecules in the column also displayed intermolecular π–π stacking (3.396 Å and 3.285 Å) (Fig. 3L). The different molecular overlap in DCA-R resulted in a slip angle (59.7°) along the long molecular axis (Fig. 3L), while a very small slip was observed along the short axis (Fig. 3M). Multiple intermolecular C–H⋯O interactions (2.461 Å, 2.505 Å and 2.520 Å) have also been found throughout the crystal of DCA-R (Fig. S4B, ESI†), which hold the columns together in a 3D structure. Compared to the packing of DCA-Y, the degree of molecular overlap in DCA-R is relatively larger. In addition, DCA-R exhibited stronger π–π stacking due to the short distance between neighbouring molecules, which indicated larger electronic excited-state delocalization of DCA-R and resulted in its red-shifted emission.
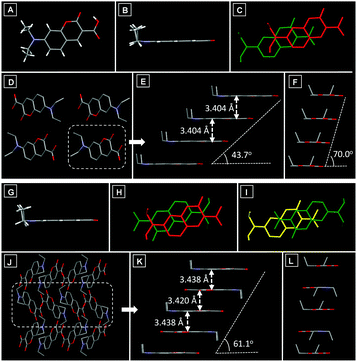
X-ray diffraction analysis revealed that molecules in DCCA-G exhibited a coplanar conformation (Fig. 4A and B). In the DCCA-G, molecules were in a head-to-head parallel packing mode (Fig. 4C), which resulted in polymer columns along the a-axis direction (Fig. 4D). The distance between adjacent molecules in the column was 3.404 Å (Fig. 4E). Meanwhile, the small molecular overlap gave a slip angle of 43.7° along the long molecular axis (Fig. 4E), and the slip angle was 70.0° along the short molecular axis (Fig. 4F). Multiple intermolecular C–H⋯O interactions (2.659 Å, 2.675 Å and 2.680 Å) have also been found throughout the crystal of DCCA-G (Fig. S5A, ESI†), which helped the columns to form a 3D structure.
The conformation of DCCA-R was also coplanar (Fig. 4G). Nevertheless, the packing mode in the crystal was very different from that of DCCA-G. When viewed along the a-axis, the molecules in DCCA-R packed into a 1D chain along the c-axis direction (Fig. 4J), which adopted a head-to-tail parallel packing pattern (Fig. 4H and I) with two types of overlap arrangement. The distances between adjacent molecules were 3.438 Å and 3.420 Å (Fig. 4K). The higher degree of molecular overlap led to a larger slip angle (61.1°) along the long molecular axis (Fig. 4K), while there was a very small slip along the short molecular axis (Fig. 4L). Different from DCCA-G, multiple C–H⋯O interactions (2.460 Å, 2.515 Å and 2.630 Å) existed throughout the crystal (Fig. S5B, ESI†), which held the chains in a 3D structure and helped to rigidify the molecular conformation. Upon carefully checking the crystal packing it was revealed that the distance of π–π stacking interactions in DCCA-G and DCCA-R was similar, but the molecular overlap degree in DCCA-R was higher than that in DCCA-G. This feature led to the relatively larger electronic delocalization in DCCA-R and thus it achieved a bathochromic-shift emission.
Tunable solid-state emission under external stimuli
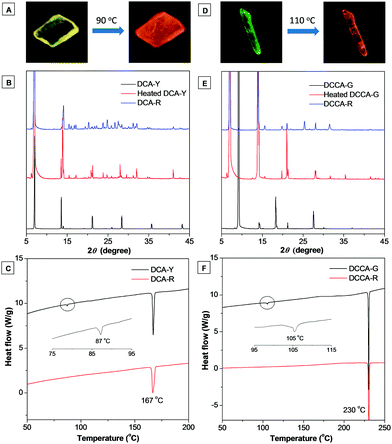
Both DCA and DCCA presented intriguing crystal-to-crystal transformation under thermal stimuli. As shown in Fig. 5A, DCA-Y displayed an attractive yellow emission color (λem = 564 nm). However, upon heating at 90 °C for ∼2 minutes, the yellow emissive crystal quickly changed into a reddish emissive crystal. Meanwhile, the emission peak of the heated crystal (λem = 600 nm) overlapped fairly well with the emission profile of DCA-R (Fig. S6B, ESI†). A similar phenomenon was achieved for DCCA-G. After heating at 110 °C for ∼2 minutes, the original green emissive crystal turned into a red emissive one (λem = 584 nm), and the emission spectrum of the heated sample was almost the same as that of DCCA-R (Fig. 5D and Fig. S6D, ESI†). It's noteworthy that the emission color of both heated crystals could not change back at the ambient temperature, which fetched them a potential application in the fabrication of thermographic recording materials. As shown in Fig. 5B and E, the XRD analysis indicated that thermally treated DCA-Y and DCCA-G possessed good crystallinity and the profiles were similar to those of DCA-R and DCCA-R, respectively. The above results confirmed the existence of a crystal-to-crystal transformation.15Such crystal-to-crystal conversion was also investigated using differential scanning calorimetry (DSC). As seen in Fig. 5C, two types of crystal (DCA-Y and DCA-R) gave consistent melting points at 167 °C. During the process of heating, DCA-Y displayed a distinct exothermic peak at approximately 87 °C, whereas no peak was detected for DCA-R. Combining the emission change, this exothermic peak could be attributed to the thermal phase transition from DCA-Y to the thermodynamically stable state of DCA-R. The same melting point at 230 °C was observed for crystals of DCCA-G and DCCA-R (Fig. 5F). However, DCCA-G exhibited an exothermic peak at about 105 °C but not DCCA-R, suggesting that DCCA-G experienced a phase transition to the relatively stable state of DCCA-R.
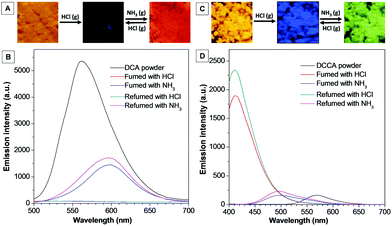
The solid-state emission of DCA and DCCA could also be tuned by fuming with HCl and NH3 gas. As shown in Fig. 6A and B, the powder of DCA emitted an intense orange emission at 560 nm. However, upon exposing to HCl gas for ∼2 minutes, the fluorescence was completely quenched. After being fumed with NH3 gas for a few minutes, the sample gave a red emission color and the emission peak appeared at 600 nm, which red-shifted by about 50 nm compared with the initial sample of DCA. This “off–on” emission switch could be repeated by fuming HCl–NH3 gas. In sharp contrast, a different phenomenon was found for DCCA under the same conditions (Fig. 6C and D). When DCCA powder was fumed with HCl gas, the emission color was converted from orange to blue, and the emission peak shifted from 570 nm to 420 nm. Subsequently, treating the sample with NH3 gas brought about a green emission peaked at 500 nm. The emission color between blue and green could be repeated by using HCl–NH3 gas. The above results confirmed that DCA and DCCA can be employed as a fluorescent sensor for HCl and NH3 gas with high sensitivity.
In order to understand the mechanism of the above fluorescence response, 1H NMR analysis of DCA and DCCA at different states was performed. As shown in Fig. S7 (ESI†), DCA possessed a typical aldehyde proton signal at 10 ppm. After the treatment with HCl gas, however, the aldehyde proton disappeared and the signal of aromatic proton shifted significantly. This suggested that both amino and aldehyde groups of DCA could undergo a protonation process in an acidic environment. The protonated structure destroyed the ICT process and led to the quenching of fluorescence. The aldehyde proton signal was not recovered after the treatment with NH3 gas, which could be ascribed to the reaction between NH3 and the aldehyde group under acidic conditions to give the imine species. The resulting product exhibited stronger ICT character and gave a red-shifted emission compared to its initial state. For DCCA, fuming with HCl gas shifted all proton signals down field except for those of the methyl group, suggesting that the protonation of the amino group occurred (Fig. S8, ESI†). As a result, the ICT process was decreased and remarkable blue-shifted emission was observed. After being treated with NH3 gas, the carboxylic acid group might be ionized due to the neutralization which reduced the ICT effect. Consequently, the green emission was observed.
The above results confirmed that the solid-state emission of DCA and DCCA could be efficiently tuned by various external stimuli including heating and fuming with acidic or basic gas. In particular for DCCA, a considerably wide range of emission was achieved (from 420 nm to 584 nm), which is extremely rare in previous reports about tunable organic solid-state emitters.
Self-assemblies and optical waveguides
Based on crystal analysis, the π–π stacking combined with multiple non-covalent interactions made the two fluorophores capable of self-assembling into ordered structures. Thus, the two fluorophores were employed to fabricate organic micromaterials by using the classic reprecipitation method.16 By rapidly injecting a specific amount of DCA or DCCA in THF solution into an ultrasonic aqueous solution and aging the mixture for several hours, floc aggregates were generated. As shown in Fig. 7, fluorescence microscopy and scanning electron microscopy (SEM) images indicated that ordered 1D microrods and microfibers were assembled for DCA and DCCA, respectively, while the length of these micromaterials was up to 100 μm. Different from the blue light of the two fluorophores in diluted THF solution, yellow (DCA, λem = 555 nm) and orange (DCCA, λem = 570 nm) emission colors were achieved for their micromaterials (Fig. S9, ESI†).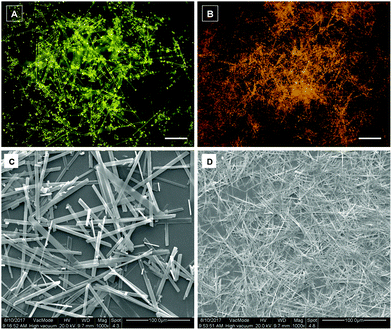 | ||
| Fig. 7 Fluorescence microscopy and SEM images of DCA (A and C) and DCCA (B and D) micromaterials. Scale bar in A and B: 20 μm. | ||
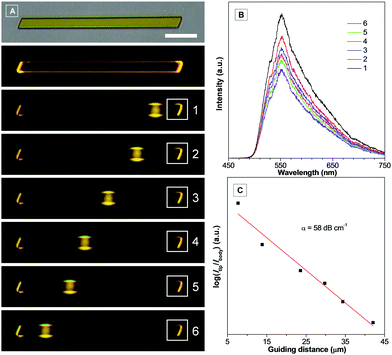
According to the fluorescence microscopy images, bright emission spots at both ends of the micromaterials, while a relatively weak emission from the body, implied that the micromaterials might exhibit optical waveguide behaviour.17 In order to prove such properties, a distance-dependent fluorescence image of a single microrod (DCA) was taken using a near-field scanning optical microscope. As shown in Fig. 8A, the chosen microrod was excited using a focused laser at six different positions along its length. Obviously, a yellow emission was observed at the end of the rod except for the excited sites. This phenomenon indicated that the microrod propagated the light to the end of the rod, indicative of the optical waveguide behaviour. The emission spectra were collected in the fixed emitting ends with varied excitation positions (labelled with 1–6) (Fig. 8B). The results showed that the emission intensity was decreased with increasing distance between the excited site and the emitted end, which originated from the optical loss during the propagation process. The emission intensities (550 nm) at the fixed end (Iend) and the excited site (Ibody) were recorded, and the optical loss coefficient (a) was calculated by using the equation log(Iend/Ibody) = −αx, where x is the distance between the excited site and emitting end (Fig. 8C). The α value of DCA was determined to be 58 dB cm−1, which was quite low compared with previous reports.6c,15b,17a,18 The well-ordered arrangement of microrods as well as their smooth surface should be responsible for such excellent optical waveguide behaviour.
Conclusions
In summary, multiple solid-state fluorescence properties were explored in two fluorophores that were derived from aminocoumarin (DCA and DCCA). Both fluorophores exhibited typical solvent and concentration-dependent emission effects. Importantly, they displayed a remarkable polymorphism phenomenon with tunable solid-state emission, which was attributed to their diverse molecular packing mode. In addition, crystal-to-crystal transformations were achieved for both fluorophores by thermal treatment. Meanwhile, the emission of the solid powder could be tuned efficiently by HCl and NH3 gas. Moreover, both DCA and DCCA assembled into ordered 1D micromaterials, and DCA gave excellent optical waveguide properties. These unique features endowed both fluorophores with broad potential applications in fields of optical recording, pH sensing and photoelectric devices. The strategy of exploring new properties in known molecular systems also provides an ideal way to construct multifunctional organic solid fluorophores.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (21672135, 21402115 and 51403122), the Fundamental Research Funds for the Central Universities (GK201703024 and GK201702002) and the Natural Science Foundation of Shaanxi Province (2016JQ2020).Notes and references
- (a) Z. Y. Yang, Z. Mao, Z. L. Xie, Y. Zhang, S. W. Liu, J. Zhao, J. R. Xu, Z. G. Chi and M. P. Aldredb, Chem. Soc. Rev., 2017, 46, 915–1016 RSC; (b) M. C. Gather, A. Köhnen and K. Meerholz, Adv. Mater., 2011, 23, 233–248 CrossRef CAS PubMed; (c) M. R. Zhu and C. L. Yang, Chem. Soc. Rev., 2013, 42, 4963–4976 RSC; (d) M. Stępień, E. Gońka, M. Żyła and N. Sprutta, Chem. Rev., 2017, 117, 3479–3716 CrossRef PubMed.
- (a) A. J. C. Kuehne and M. C. Gather, Chem. Rev., 2016, 116, 12823–12864 CrossRef CAS PubMed; (b) I. D. W. Samuel and G. A. Turnbull, Chem. Rev., 2007, 107, 1272–1295 CrossRef CAS PubMed.
- (a) B. Cho, S. Song, Y. Ji, T. W. Kim and T. Lee, Adv. Funct. Mater., 2011, 21, 2806–2829 CrossRef CAS; (b) H. L. Dong, H. F. Zhu, Q. Meng, X. Gong and W. P. Hu, Chem. Soc. Rev., 2012, 41, 1754–1808 RSC.
- (a) L. Basabe-Desmonts, D. N. Reinhoudt and M. Crego-Calama, Chem. Soc. Rev., 2007, 36, 993–1017 RSC; (b) V. S. Padalkar and S. Seki, Chem. Soc. Rev., 2016, 45, 169–202 RSC; (c) C. Ge, Y. Liu, X. Ye, X. Zheng, Q. Han, J. Liu and X. Tao, Mater. Chem. Front., 2017, 1, 530–537 RSC.
- (a) S. J. Yoon, J. W. Chung, J. Gierschner, K. S. Kim, M. G. Choi, D. H. Kim and S. Y. Park, J. Am. Chem. Soc., 2010, 132, 1367–13683 Search PubMed; (b) Y. N. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332–4353 RSC; (c) H. Sasabe and J. Kido, Chem. Mater., 2011, 23, 621–630 CrossRef CAS; (d) D. Yan and D. Evans, Mater. Horiz., 2014, 1, 46–57 RSC; (e) Z. J. Zhao, B. R. He and B. Z. Tang, Chem. Sci., 2015, 6, 5347–5365 RSC.
- (a) Y. Sagara, S. Yamane, M. Mitani, C. Weder and T. Kato, Adv. Mater., 2016, 28, 1073–1095 CrossRef CAS PubMed; (b) T. Seki, T. Ozaki, T. Okura, K. Asakura, A. Sakon, H. Uekusa and H. Ito, Chem. Sci., 2015, 6, 2187–2195 RSC; (c) P. Z. Chen, H. Zhang, L. Y. Niu, Y. Zhang, Y. Z. Chen, H. B. Fu and Q. Z. Yang, Adv. Funct. Mater., 2017, 27, 1700332 CrossRef; (d) K. Wang, H. Zhang, S. Chen, G. Yang, J. Zhang, W. Tian, Z. Su and Y. Wang, Adv. Mater., 2014, 26, 6168–6173 CrossRef CAS PubMed; (e) P. Galer, R. C. Korošec, M. Vidmar and B. Šket, J. Am. Chem. Soc., 2014, 136, 7383–7394 CrossRef CAS PubMed; (f) C. Wang and Z. Li, Mater. Chem. Front., 2017, 1, 2174–2194 RSC; (g) H. Naito, Y. Morisaki and Y. Chujo, Angew. Chem., Int. Ed., 2015, 54, 5084–5087 CrossRef CAS PubMed; (h) W. A. Morris, T. Butler, M. Kolpaczynskaa and C. L. Fraser, Mater. Chem. Front., 2017, 1, 158–166 RSC.
- (a) G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS PubMed; (b) R. W. Sinkeldam, N. J. Greco and Y. Tor, Chem. Rev., 2010, 110, 2579–2619 CrossRef CAS PubMed.
- (a) J. D. Luo, Z. L. Xie, J. W. Y. Lam, L. Cheng, H. Y. Chen, C. F. Qiu, H. S. Kwok, X. W. Zhan, Y. Q. Liu, Y. Q. Liu, D. B. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC; (b) J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed; (c) D. Ding, K. Li, B. Liu and B. Z. Tang, Acc. Chem. Res., 2013, 46, 2441–2453 CrossRef CAS PubMed.
- (a) F. G. Medina, J. G. Marrero, M. Macías-Alonso, M. C. González, I. Córdova-Guerrero, A. G. T. Garcíaa and S. Osegueda-Roblesa, Nat. Prod. Rep., 2015, 32, 1472–1507 RSC; (b) J. Liu, Y. Q. Sun, Y. Y. Huo, H. X. Zhang, L. F. Wang, P. Zhang, D. Song, Y. W. Shi and W. Guo, J. Am. Chem. Soc., 2014, 136, 574–577 CrossRef CAS PubMed; (c) Z. S. Wang, Y. Cui, K. Hara, Y. Dan-oh, C. Kasada and A. Shinpo, Adv. Mater., 2007, 19, 1138–1141 CrossRef CAS; (d) M. E. Riveiro, N. De Kimpe, A. Moglioni, R. Vazquez, F. Monczor, C. Shayo and C. Davio, Curr. Med. Chem., 2010, 17, 1325–1338 CrossRef CAS PubMed; (e) M. Tasior, D. Kim, S. Singha, M. Krzeszewski, K. H. Ahn and D. T. Gryko, J. Mater. Chem. C, 2015, 3, 1421–1446 RSC.
- (a) C. T. Chen, C. L. Chiang, Y. C. Lin, L. H. Chan, C. Huang, Z. W. Tsai and C. T. Chen, Org. Lett., 2003, 5, 1261–1264 CrossRef CAS PubMed; (b) M. R. Shreykar and N. Sekar, Dyes Pigm., 2017, 142, 121–125 CrossRef CAS; (c) U. Venkataramudu, M. Annadhasan, H. Maddali and R. Chandrasekar, J. Mater. Chem. C, 2017, 5, 7262–7269 RSC; (d) F. Bu, R. Duan, Y. Xie, Y. Yi, Q. Peng, R. Hu, A. Qin, Z. Zhao and B. Z. Tang, Angew. Chem., Int. Ed., 2015, 54, 14492–14497 CrossRef CAS PubMed; (e) H. Yan, X. Meng, B. Li, S. Ge and Y. Lu, Dyes Pigm., 2017, 146, 479–490 CrossRef CAS; (f) S. Kumar, P. Singh, R. Srivastava, R. R. Koner, A. Pramanik, J. Mathew, S. Sinha, M. Rawat, R. S. Anande and S. Ghosh, J. Mater. Chem. C, 2014, 2, 6637–6647 RSC; (g) H. Moon, Q. P. Xuan, D. Kim, Y. Kim, J. W. Park, C. H. Lee, H. J. Kim, A. Kawamata, S. Y. Park and K. H. Ahn, Cryst. Growth Des., 2014, 14, 6613–6619 CrossRef CAS; (h) B. Ventura, Y. M. Poronik, I. Deperasińska and D. T. Gryko, Chem. – Eur. J., 2016, 22, 15380–15388 CrossRef CAS PubMed.
- (a) M. Cigan, J. Donovalova, V. Szocs, J. Gasper, K. Jakusova and A. Gaplovsky, J. Phys. Chem. A, 2013, 117, 4870–4883 CrossRef CAS PubMed; (b) X. C. Li, L. Yang, X. W. Zhang, L. F. Li, Y. Zhou and Y. A. Son, Z. Kristallogr. – New Cryst. Struct., 2014, 229, 241–242 CAS; (c) R. Arcos-Ramos, M. Maldonado-Domínguez, J. Ordóñze-Hernández, M. Romero-Ávila, N. Farfán and M. D. P. Carreón-Castro, J. Mol. Struct., 2017, 1130, 914–921 CrossRef CAS; (d) H. Zhang, T. Z. Yu, Y. L. Zhao, D. W. Fan, L. L. Chen, Y. Q. Qiu, L. Qian, K. Zhang and C. H. Yang, Spectrochim. Acta, Part A, 2008, 69, 1136–1139 CrossRef PubMed; (e) X. G. Liu, J. M. Cole, P. C. Y. Chow, L. Zhang, Y. Z. Tan and T. Zhao, J. Phys. Chem. C, 2014, 118, 13042–13051 CrossRef CAS.
- (a) B. Raju and T. S. Varadarajan, J. Phys. Chem., 1994, 98, 8903–8905 CrossRef; (b) A. Chatterjee and D. Seth, Photochem. Photobiol., 2013, 89, 280–293 CrossRef CAS PubMed; (c) K. S. Hettie and T. E. Glass, Chem. – Eur. J., 2014, 20, 17488–17499 CrossRef CAS PubMed; (d) A. Chatterjee, B. Maity and D. Seth, Phys. Chem. Chem. Phys., 2013, 15, 1894–1906 RSC.
- H. Yu, W. Ren, H. Lu, Y. Liang and Q. Wang, Chem. Commun., 2016, 52, 7387–7389 RSC.
- (a) H. Pan, P. Liu, Y. Li, Y. Wu, B. S. Ong, S. Zhu and G. Xu, Adv. Mater., 2007, 19, 3240–3243 CrossRef CAS; (b) C. Näther and I. Jeβ, Angew. Chem., Int. Ed., 2006, 45, 6381–6383 CrossRef PubMed; (c) R. H. Li, S. Z. Xiao, Y. Li, Q. F. Lin, R. H. Zhang, J. Zhao, C. Y. Yang, K. Zou, D. S. Li and T. Yi, Chem. Sci., 2014, 5, 3922–3928 RSC; (d) G. Q. Zhang, J. W. Lu, M. Sabat and C. L. Fraser, J. Am. Chem. Soc., 2010, 132, 2160–2162 CrossRef CAS PubMed; (e) J. N. Zhang, H. Kang, N. Li, S. M. Zhou, H. M. Sun, S. W. Yin, N. Zhao and B. Z. Tang, Chem. Sci., 2017, 8, 577–582 RSC.
- (a) T. H. Kim, Y. W. Shin, J. H. Jung, J. S. Kim and J. Kim, Angew. Chem., Int. Ed., 2008, 47, 685–688 CrossRef CAS PubMed; (b) X. Gu, J. Yao, G. Zhang, Y. Yan, C. Zhang, Q. Peng, Q. Liao, Y. Wu, Z. Xu, Y. Zhao, H. Fu and D. Zhang, Adv. Funct. Mater., 2012, 22, 4862–4872 CrossRef CAS.
- Y. Zhao, X. Mu, C. Bao, Y. Fan, J. Zhang and Y. Wang, Langmuir, 2009, 25, 3264–3270 CrossRef CAS PubMed.
- (a) Y. S. Zhao, J. J. Xu, A. D. Peng, H. B. Fu, Y. Ma, L. Jiang and J. N. Yao, Angew. Chem., Int. Ed., 2008, 47, 7301–7305 CrossRef CAS PubMed; (b) N. Zhao, M. Li, Y. l. Yan, J. W. Y. Lam, Y. L. Zhang, Y. S. Zhao, K. S. Wong and B. Z. Tang, J. Mater. Chem. C, 2013, 1, 4640–4646 RSC.
- (a) C. Shi, Z. Guo, Y. Yan, S. Zhu, Y. Xie, Y. S. Zhao, W. Zhu and H. Tian, ACS Appl. Mater. Interfaces, 2013, 5, 192–198 CrossRef CAS PubMed; (b) S. Chen, N. Chen, Y. L. Yan, T. Liu, Y. Yu, Y. Li, H. Liu, Y. S. Zhao and Y. Li, Chem. Commun., 2012, 48, 9011–9013 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization, optical spectra, and crystal data. CCDC 1557654. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7qm00617a |
| This journal is © the Partner Organisations 2018 |