Styrene and substituted styrene grafted functional polyolefins via nitroxide mediated polymerization†
Abstract
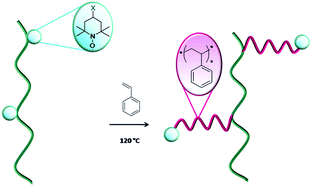
The grafting of (functionalized) polystyrene from bulk or surface-functionalized polyolefins via nitroxide mediated polymerization is described. High density polyethylene and a poly(ethylene-co-α-olefin) copolymer (EOC) modified with different functional 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO) derivatives were used as macroinitiators for the “grafting from” polymerization of various styrene-based monomers to yield polyolefin-g-polystyrene graft copolymers. The successful grafting of styrene and styrene derivatives was demonstrated by complementary analyses such as infrared (ATR-FTIR) and NMR spectroscopy, size exclusion chromatography, thermogravimetric analysis, and differential scanning calorimetry. Typically, IR spectroscopy of the grafted copolymers showed the bands attributable to the aromatic moieties and the obtained thermograms evidenced a lower degradation temperature for the grafted copolymers compared to that of polyolefin starting materials. In addition, solid state 19F-NMR was chosen to confirm the growth of polystyrene (PS) chains when EOC functionalized with a fluoroalkyl TEMPO was used for NMP. The extent of grafting of PS chains onto the polyolefin backbone was found to depend on the nature of the macroinitiator, especially on the structure and cleavage temperature of alkoxyamine derivatives created by TEMPO functionalization, on its content and on the adopted experimental conditions.


 Please wait while we load your content...
Please wait while we load your content...