Styrylisoxazole-based fluorescent probes for the detection of hydrogen sulfide†
Received
1st September 2017
, Accepted 6th November 2017
First published on 7th November 2017
Abstract
Styrylisoxazoles bearing a nitro group linked to bulky aromatic rings have been synthesized and examined for their absorption and emission studies in organic solvents and water. The molecules showed emission in the visible region with significant solvatochromic emission shifts influenced by the extended conjugation of aromatic rings and intramolecular charge transfer. These absorption and emission changes were used for the efficient and sensitive detection of trace concentrations of hydrogen sulfide (H2S) through the reduction of the nitro group to the amine group in the presence of aqueous sodium sulfide. The experimental results indicated that the probes exhibit an excellent emission response with large shifts in the emission and sensitivity with a micromolar detection limit.
Introduction
π-Conjugated small organic molecules bearing donor and acceptor substituents have been extensively investigated due to their unique photoresponsive ability and consequent promise in the fields of materials science and life sciences.1–3 In particular, systems bearing a diarylethene scaffold have attracted attention as promising candidates because of their synthetic tunability, absorption and emission in solution and in the solid state, tunable redox properties, and photochemical interconversion, which have enabled their use for optoelectronic and biological applications.4–8 Their properties can be modulated further by incorporating heteroaromatic rings.9–12 Many biologically active substrates have a heterocyclic system as one of the components that play a key role in several biological processes. Isoxazole is a five-membered heterocyclic unit having oxygen and nitrogen atoms in the 1,2-positions, respectively. Molecules based on this heterocyclic ring are used as building blocks for the synthesis of many natural products13–16 and were found to have multiple biological functions, such as to induce genes that support neuroendocrine and β-cell phenotypes or to act as cyclooxygenase inhibitors.17,18 The nitro group attached to the heteroaromatic core on the vinyl chain can act as a good electron sink and has been proven to be an effective inducer of apoptosis.19 The nitroaromatics create a localization of the negative region employed to improve the binding affinity of any ligand to its putative target.20 Furthermore, isoxazole derivatives coupled with calixarenes and pyrroles were utilized as chemosensors for the efficient detection of metal ion analytes,21 anion sensors22 or ‘turn-off’ sensors in micellar media.23 Considering the unique electronic and biological properties of isoxazoles, in this work, we synthesized styrylisoxazoles incorporating a nitro group [Fig. 1]. We envisaged two uses of the incorporation of the nitro group. First, it acts as a strong electron withdrawing group helping in emission in the lower energy regions; second is its ability to detect and react with hydrogen sulfide (H2S). Hydrogen sulfide (H2S) is a cytotoxic gasotransmitter besides CO and NO produced mainly by two enzymes: cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE) using L-cysteine (L-Cys) as the substrate.24 The accumulation of H2S in trace quantities in biological media was associated with some physiological and pathological processes such as vasodilatation, apoptosis, and various diseases. For the detection of H2S, several techniques were employed that include colorimetric and fluorimetric assays,25,26 redox or nucleophilic reactions,27 and electrochemical assays.28,29 However, considering their greater selectivity and environment sensitivity, absorption or fluorescence-based techniques became prevalent.30–32 Fluorescence-based techniques take advantage of distinct changes in the absorption or emission process through specific structural changes.33 Various strategies include H2S-mediated reduction, nucleophilic addition, copper-mediated precipitations, and thiolysis among other methods.34–40 However, most of these fluorescent probes are limited by emission in the narrower energy domains in the UV-visible region and longer response times. Despite the availability of various fluorescent probes, a design strategy with tunable emission maxima provides opportunities to develop different industrially or biologically relevant analyte sensing probes.41 Herein, we report a set of isoxazole-based fluorescent sensors (Fig. 1–4) containing a NO2 group for the quantitative detection of H2S that shows solvent sensitivity as well as enabling the reduction of the nitro group. Even though the presence of the nitro group yields lower fluorescence due to strong non-radiative transitions, the mild conditions required for the reduction allow the design of such fluorophores for H2S detection. Furthermore, the strong electron withdrawing nature of the nitro group induces strong solvatochromism with emission in the lower energy regions. Taking advantage of this, we tested these fluorophores for H2S detection. The synthesized fluorophores exhibited immediate selectivity to H2S with a short response time correlated with the changes in the emission. The results are described in the following sections.
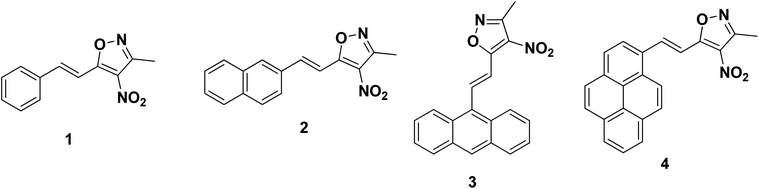 |
| | Fig. 1 Structures of the molecules investigated. | |
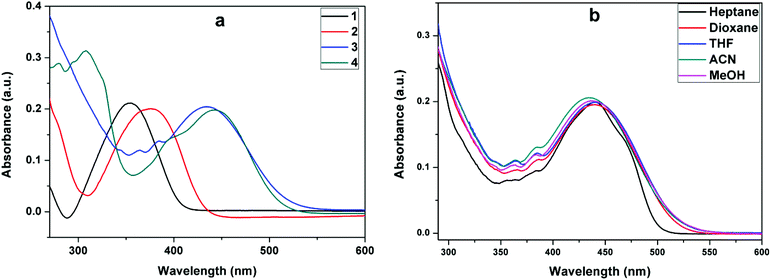 |
| | Fig. 2 Absorption of (a) 1–4 in acetonitrile and (b) (3) in solvents of different polarities. | |
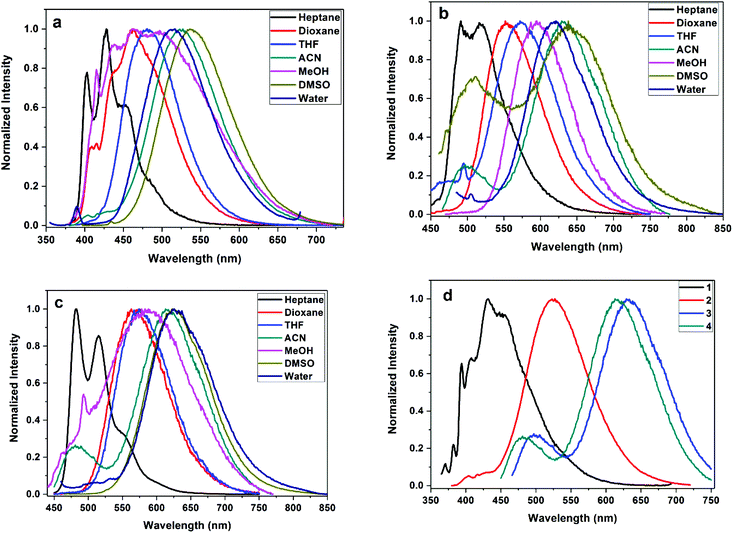 |
| | Fig. 3 Normalized emission of styrylisoxazole derivatives 2 (λex 350 nm), 3 (λex 440 nm) and 4 (λex 440 nm) in different solvents (a–c) and (d) normalized emission of styrenes (1–4) in acetonitrile. | |
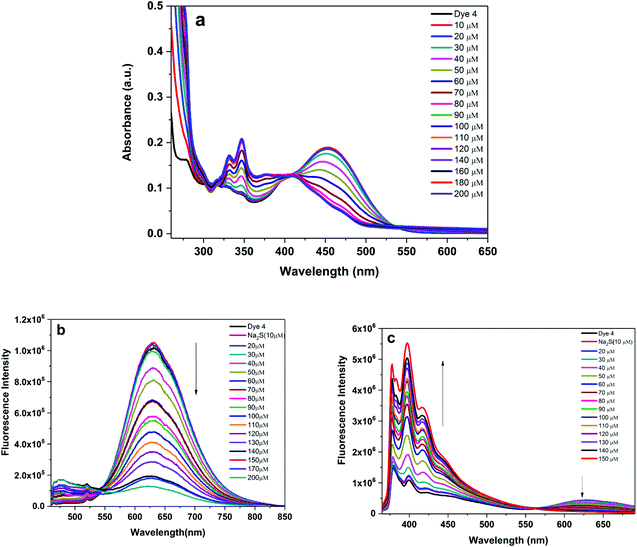 |
| | Fig. 4 UV-vis absorption (a) and emission response (b): λex 450 nm and (c): λex 350 nm of probe 4 (10 μM) with increasing concentration (0 to 200 μM) of Na2S in a DMSO + H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) binary mixture. 1) binary mixture. | |
Experimental section
The reagents and chemicals utilized in this study were obtained from SD Fine, Sigma-Aldrich or Alfa Aesar. All the solvents employed for the synthesis and optical spectroscopic studies were dried thoroughly using reported procedures. 1H NMR and 13C NMR spectra were recorded on a Bruker (500 MHz) spectrophotometer using tetramethylsilane (TMS, δ = 0 ppm) as an internal standard with chloroform-D (CDCl3) as the solvent. Mass-spectral data were obtained using an ESI-QToF (Waters-synapt G2S) high-resolution mass spectrometer. UV-vis absorption spectra were recorded using an Analytik Jena Specord 210 plus, and fluorescence emission studies were performed using a Horiba-Jobin Yvon Fluolorog-3 spectrofluorometer with a slit-width of 1 nm or 2 nm.
Synthesis of the compounds (1–4)
In a typical synthetic strategy, the corresponding aldehydes [benzaldehyde, naphthaldehyde, 9-anthraldehyde and pyrene-1-aldehyde (R-CHO)] were condensed with 3,5-dimethyl-4-nitroisoxazole (6) yielding the corresponding styryl derivatives (1–4) (Scheme 1). The details of the synthesis of the starting materials (pyrene aldehyde, 3,5-dimethylisoxazole and 3,5-dimethyl-4-nitroisoxazole) are given in the ESI†.
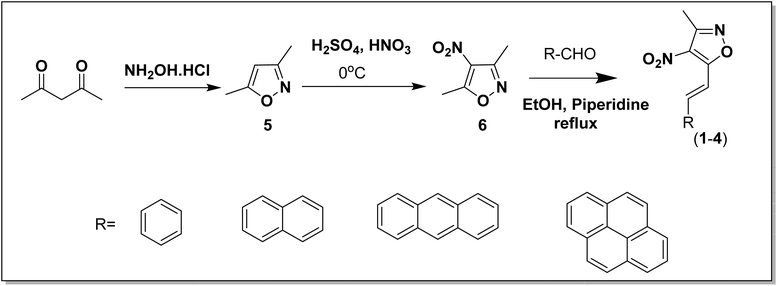 |
| | Scheme 1 Synthetic strategy for the preparation of the styrylisoxazole derivatives (1–4). | |
Synthesis of 3-methyl-4-nitrostyrylisoxazoles (1–4).
To a solution of 3,5-dimethyl-4-nitroisoxazole (1.4 g, 10 mmol) in EtOH (10 mL) were added the desired aldehyde (1.0 equiv.) and piperidine (98.0 μl, 0.01 equiv.) successively at ambient temperature. The reaction mixture was heated at 65 °C for 1 h overnight. After cooling the reaction mixture to room temperature, the precipitate was collected by filtration and washed several times with Et2O to give 3-methyl-4-nitro-5-alkenyl-isoxazoles (1–4) as colored solids.
(E)-3-Methyl-4-nitro-5-styrylisoxazole (1).
1H NMR (500 MHz, CDCl3-d) δ 7.81 (s, 1H), 7.72 (s, 1H), 7.69 (m, 3H), 7.49 (m, 3H), 2.91 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 167.18, 156.14, 143.10, 134.45, 131.12, 129.18, 128.42, 110.98, 95.69, 11.84. HRMS (ESI-MS) m/z: calcd for [M + H]+, 230.0691; found, 231.0881.
(E)-3-Methyl-5-(2-(naphthalen-2-yl)vinyl)-4-nitroisoxazole (2).
1H NMR (500 MHz, CDCl3) δ 8.04 (s, 1H), 7.95 (m, 1H), 7.90 (d, J = 8.5 Hz, 2H), 7.87 (d, J = 7.5 Hz, 1H), 7.83–7.75 (m, 2H), 7.57–7.52 (m, 2H), 2.61 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 167.20, 156.10, 143.09, 134.63, 133.35, 132.02, 130.68, 129.04, 128.75, 127.87, 127.78, 126.97, 123.33, 111.09, 11.76. HRMS (ESI-MS) m/z: calcd for [M + H]+, 280.0848; found, 281.1012.
(E)-5-(2-(Anthracen-9-yl)vinyl)-3-methyl-4-nitroisoxazole (3).
1H NMR (500 MHz, CDCl3) δ 8.78 (d, J = 16.5 Hz, 1H), 8.51 (s, 1H), 8.31 (d, J = 7.7 Hz, 2H), 8.05 (d, J = 7.5 Hz, 2H), 7.69 (d, J = 16.5 Hz, 1H), 7.60–7.50 (m, 4H), 2.67 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 166.63, 156.28, 140.18, 131.34, 129.70, 129.45, 129.11, 128.88, 126.98, 125.56, 125.33, 124.81, 119.58, 11.85. HRMS (ESI-MS) m/z: calcd for [M]+, 330.1004; found, 330.1080.
(E)-3-Methyl-4-nitro-5-(2-(pyren-1-yl)vinyl)isoxazole (4).
1H NMR (500 MHz, CDCl3) δ 8.91 (d, J = 15.3 Hz, 1H), 8.50 (d, J = 9.0 Hz, 1H), 8.45 (d, J = 8.0 Hz, 1H), 8.26–8.19 (m, 4H), 8.15 (d, J = 9.0, 1H), 8.09–8.04 (m, 2H), 7.91 (d, J = 16.2 Hz, 1H), 2.64 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 167.30, 156.24, 139.27, 133.44, 131.33, 130.66, 130.31, 129.20, 127.94, 127.39, 126.48, 126.46, 126.23, 125.32, 125.01, 124.57, 124.09, 122.01, 112.23, 11.92. HRMS (ESI-MS) m/z: calcd for [M]+, 354.1004; found, 354.1067.
Results and discussion
Absorption and emission of (1–4)
Fig. 1 gives the structural details of the synthesized molecules. The phenyl group in (1), naphthalene in (2), anthracene in (3) and pyrene in (4) were connected to the isoxazole ring through a π-spacer (double bond). The nitro group present on the isoxazole unit acts as a strong electron acceptor. Through this arrangement, we expect to see the effect of extended conjugation on the absorption and emission properties of the styrenes. Table 1 gives the absorption data of styrenes (1–4) in organic solvents. In general, depending on the nature of the substituents, the absorption maxima were observed in the wavelength range of 345–446 nm [Fig. 2a]. Styrene (1) containing a simple phenyl ring absorbs at ∼355 nm in acetonitrile. The substitution of the naphthyl ring results in a +21 nm shift in absorption. Furthermore, an increase in conjugation through the introduction of other fused ring systems such as anthracene and pyrene results in a +88 nm increase in the absorption maxima. Despite the presence of an additional aromatic ring, the absorption of (4) shows only a slight effect on the absorption (λa) maxima in comparison with (3). With the change in polarity, moderate absorption changes (+4–10 nm) were noted for the substituted styrenes [Fig. 2b and Fig. S1†].
Table 1 Absorption and emission data of the synthesized molecules (1–4)
| |
Solvent |
λ
a (nm) |
λ
f (nm) |
Stokes shift (cm−1) |
|
Solvent |
λ
a (nm) |
λ
f (nm) |
Stokes shift (cm−1) |
| (1) |
Heptane |
345 |
415 |
5354 |
(2) |
Heptane |
365 |
398, 427, 460 |
4618 |
| Dioxane |
356 |
436 |
5154 |
Dioxane |
371 |
461 |
5262 |
| THF |
356 |
436 |
5154 |
THF |
370 |
479 |
6150 |
| CH3CN |
355 |
461 |
6477 |
CH3CN |
376 |
524 |
7511 |
| MeOH |
352 |
462 |
6764 |
MeOH |
369 |
465, 496 |
6938 |
| DMSO |
349 |
475 |
7600 |
DMSO |
385 |
536 |
7319 |
| Water |
363 |
464 |
5996 |
Water |
347 |
513 |
9325 |
| |
| (3) |
Heptane |
441 |
490, 518 |
5464 |
(4) |
Heptane |
440 |
480, 513 |
3234 |
| Dioxane |
441 |
552 |
4559 |
Dioxane |
447 |
564 |
4640 |
| THF |
441 |
572 |
5193 |
THF |
446 |
574 |
4999 |
| CH3CN |
434 |
631 |
7193 |
CH3CN |
443 |
612 |
6233 |
| MeOH |
438 |
595 |
6024 |
MeOH |
442 |
593 |
5761 |
| DMSO |
436 |
509, 637 |
7237 |
DMSO |
453 |
623 |
6023 |
| Water |
453 |
618 |
5893 |
Water |
442 |
626 |
6649 |
Fluorescence properties in solution
Styrene (1) bearing a phenyl ring shows emission (λf) at ∼461 nm in acetonitrile. Styrene (2) containing a naphthalene moiety emits at ∼524 nm, styrene (3) bearing an anthracene system emits at ∼631 nm and pyrenyl styrene (4) emits at 612 nm [Table 1]. The bathochromic shift in emission reflects the extended delocalization due to the increased aromaticity. Solvent polarity has moderate to strong influence on the emission behavior of these styrenes. In non-polar heptane, the structured emission is noted for (3) and the increase in solvent polarity results in bathochromic emission shifts (λf). Styrene (1) shows a bathochromic shift of +46 nm upon changing the solvent polarity from heptane to acetonitrile. Styrene (2) shows structured emission at 399, 427 and 460 nm in heptane but shows a significant emission (+64 nm) shift in acetonitrile [Fig. 3a]. Similarly, anthryl substituted (3) and pyrenyl substituted (4) show about +113 and +99 nm shifts in the emission with an increase in solvent polarity [Fig. 3b and c], respectively. The order is phenyl < naphthyl < pyrenyl < anthryl [Fig. 3d].
This strong solvent polarity dependent emission indicates the presence of an excited state with a strong intramolecular charge transfer (ICT) character and a large dipole moment relative to the ground state. In particular, the presence of the nitro group also has a strong influence on the intramolecular charge transfer.42,43 These compounds show weak and blue-shifted emission in methanol compared to that in acetonitrile. This emission behavior is influenced by the involvement of solute (fluorophore)–solvent (methanol) dipole–dipole interactions and intermolecular hydrogen bonding interactions.
Detection of H2S
H2S, a third member of the gasotransmitter family along with NO and CO, plays an important role in some biological and pharmacological processes.44,45 For this reason, it is important to detect the presence of H2S, and we have utilized the spectroscopic properties of the synthesized styrylisoxazoles (1–4). The absorption and emission spectra of styrylisoxazoles were recorded in a DMSO–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture. Pyrenyl isoxazole (4) absorbs at ∼450 nm and emits at ∼631 nm in a DMSO–H2O binary mixture. Upon titrating with aqueous sodium sulfide [typically the emission spectra are recorded after about 3 minutes post addition of Na2S], the absorbance initially decreases (up to 50 μM) in intensity and later shows a hypsochromic shift from ∼60 μM. Beyond this concentration, the primary absorption band at ∼450 nm merely appears as a shoulder. The absorption at ∼350 nm shows a progressive increase with a well-defined isosbestic point at ∼407 nm [Fig. 4a]. This shift in absorption may indicate the formation of a new species, and the blue-shifted absorption is attributed to a loss in delocalization. This is a consequence of the shifts in the change of the structure from the initial electron withdrawing nitro (NO2) group which is reduced to the electron donating amino group (NH2). The changes in the emission spectra of (4) in the absence or presence of Na2S (0–170 μM) are displayed in Fig. 4b. Under 450 nm excitation, compound (4) shows emission maxima at 631 nm in the absence of Na2S. However, the addition of Na2S results in a significant loss of emission intensity with the eventual loss of emission. Noticeably, the emission in the blue region of the spectrum (at or near 400–450 nm) seems to be increasing. To get a more accurate picture of this emission behavior, we excited compound (4) at 350 nm which is a dominant absorption band post addition of Na2S. The emission shows two peaks: a long charge transfer band at ∼630 nm and a shorter wavelength structured emission at 380, 400 and 420 nm. These shorter wavelength emission peaks show an enhancement in intensity upon the addition of sodium sulfide, and a concomitant decrease in emission intensity at 630 nm was noted. Similar observations were reported for anthryl (3) and naphthyl (2) substituted isoxazoles. In the case of (3), the emission at ∼630 nm decreases in intensity, and new emissive peaks appear in shorter wavelength regions at ∼500 nm with a sudden surge in emission intensity at 80 μM [Fig. 5a]. The excitation at 350 nm shows emission peaks primarily due to the anthracene [Fig. S3†]. The naphthyl derivative (2) also shows a drop in the emission intensity of the charge transfer band (542 nm) with a slight shift of the wavelength to the blue region [Fig. 5b] at higher concentrations. The increase in the emission intensity at higher energy is conspicuous for (3) or (4) compared to that for (2). The changes in the UV-spectral profile upon the reduction of the nitro group to the amino group are shown in the ESI [Fig. S2†]. H2S acts as a weak acid and may contribute to the observed emission changes. Our experiments showed that the emission is unaffected with pH variation [Fig S8†]. To check the sensitivity for other analytes such as Na2SO3, cysteine, homocysteine and glutathione, the isoxazole derivative (4) was titrated with different analyte concentrations. The results are shown in the ESI.† Similar to the earlier emission results, the emission band at ∼630 nm is quenched with the analytes used [Fig S9a†]. A comparative bar chart [Fig S9b†] for a given concentration of the analyte reveals greater sensitivity for H2S. While this result is encouraging, the nature of product formation is uncertain. For instance, cysteine or homocysteine was earlier detected by a Michael addition, a cyclization or a sequential substitution mechanism.46–48 The results obtained from the mass spectral analysis gave a molecular weight much higher than the sole amino product. The product formed cannot be conclusively confirmed based on the mass spectral data alone and requires further experimentation.
1) mixture. Pyrenyl isoxazole (4) absorbs at ∼450 nm and emits at ∼631 nm in a DMSO–H2O binary mixture. Upon titrating with aqueous sodium sulfide [typically the emission spectra are recorded after about 3 minutes post addition of Na2S], the absorbance initially decreases (up to 50 μM) in intensity and later shows a hypsochromic shift from ∼60 μM. Beyond this concentration, the primary absorption band at ∼450 nm merely appears as a shoulder. The absorption at ∼350 nm shows a progressive increase with a well-defined isosbestic point at ∼407 nm [Fig. 4a]. This shift in absorption may indicate the formation of a new species, and the blue-shifted absorption is attributed to a loss in delocalization. This is a consequence of the shifts in the change of the structure from the initial electron withdrawing nitro (NO2) group which is reduced to the electron donating amino group (NH2). The changes in the emission spectra of (4) in the absence or presence of Na2S (0–170 μM) are displayed in Fig. 4b. Under 450 nm excitation, compound (4) shows emission maxima at 631 nm in the absence of Na2S. However, the addition of Na2S results in a significant loss of emission intensity with the eventual loss of emission. Noticeably, the emission in the blue region of the spectrum (at or near 400–450 nm) seems to be increasing. To get a more accurate picture of this emission behavior, we excited compound (4) at 350 nm which is a dominant absorption band post addition of Na2S. The emission shows two peaks: a long charge transfer band at ∼630 nm and a shorter wavelength structured emission at 380, 400 and 420 nm. These shorter wavelength emission peaks show an enhancement in intensity upon the addition of sodium sulfide, and a concomitant decrease in emission intensity at 630 nm was noted. Similar observations were reported for anthryl (3) and naphthyl (2) substituted isoxazoles. In the case of (3), the emission at ∼630 nm decreases in intensity, and new emissive peaks appear in shorter wavelength regions at ∼500 nm with a sudden surge in emission intensity at 80 μM [Fig. 5a]. The excitation at 350 nm shows emission peaks primarily due to the anthracene [Fig. S3†]. The naphthyl derivative (2) also shows a drop in the emission intensity of the charge transfer band (542 nm) with a slight shift of the wavelength to the blue region [Fig. 5b] at higher concentrations. The increase in the emission intensity at higher energy is conspicuous for (3) or (4) compared to that for (2). The changes in the UV-spectral profile upon the reduction of the nitro group to the amino group are shown in the ESI [Fig. S2†]. H2S acts as a weak acid and may contribute to the observed emission changes. Our experiments showed that the emission is unaffected with pH variation [Fig S8†]. To check the sensitivity for other analytes such as Na2SO3, cysteine, homocysteine and glutathione, the isoxazole derivative (4) was titrated with different analyte concentrations. The results are shown in the ESI.† Similar to the earlier emission results, the emission band at ∼630 nm is quenched with the analytes used [Fig S9a†]. A comparative bar chart [Fig S9b†] for a given concentration of the analyte reveals greater sensitivity for H2S. While this result is encouraging, the nature of product formation is uncertain. For instance, cysteine or homocysteine was earlier detected by a Michael addition, a cyclization or a sequential substitution mechanism.46–48 The results obtained from the mass spectral analysis gave a molecular weight much higher than the sole amino product. The product formed cannot be conclusively confirmed based on the mass spectral data alone and requires further experimentation.
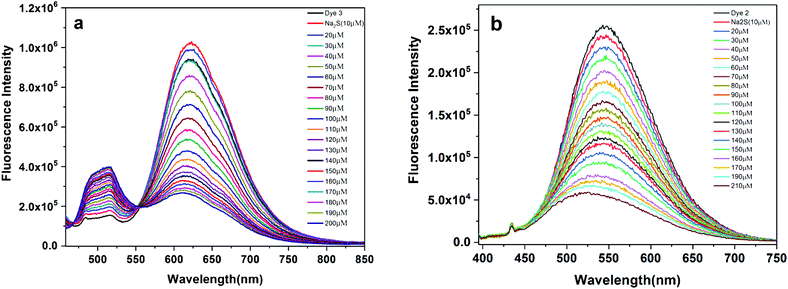 |
| | Fig. 5 Fluorescence response of probe (a) 3 [λex 440 nm] and (b) 2 [λex 380 nm] with increasing concentration (0 to 220 μM) of Na2S in a DMSO + H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) binary mixture. 1) binary mixture. | |
Sensing mechanism
The spectral changes involving the emission response are attributed to the loss of charge transfer due to the conversion of the electron withdrawing nitro group to the electron donating amine group [Fig. 6]. After the reaction with H2S, the donor–acceptor electron system was inhibited by the donor–donor electron system. This expectedly disturbs the charge transfer yielding the loss in the emission intensity and the emission spectral shifts.49 This reduction of the nitro group to the amino group is facilitated by the generation of the sulfide anion.50,51 To prove the product formation, mass spectral analysis was performed and the HRMS analysis predicts the formation of the amino product (Fig. 7 and Fig S4–S6†). The product cannot be isolated, and NMR characterization could not be performed as the sample concentrations are too low. For qualitative visualization, the obtained products are subjected to the ninhydrin test and the products show a strong color [Fig. S7†]. These results pave the way for identifying quicker means of H2S detection with relevance to industrial and biological applications.
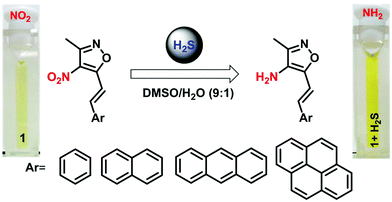 |
| | Fig. 6 Schematic representation of the reduction of the nitro group for (1–4) to an amino group by H2S in DMSO/H2O mixtures. | |
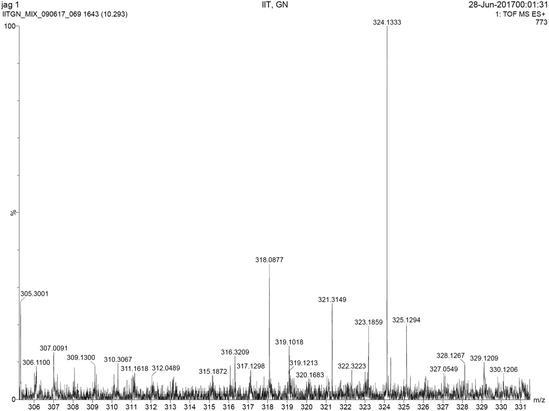 |
| | Fig. 7 HRMS spectra of the amine compound obtained after the reduction of 4. The mass spectral data of other compounds are given in the ESI.† | |
Conclusions
In summary, we have demonstrated the detection of micromolar (60 μM) quantities of hydrogen sulfide through a rapid fluorescence response through the reduction of the nitro group to the amine functional group. The molecular systems bearing fused aromatic ring systems and the nitro group are sensitive to solvent polarity variations resulting in significant solvatochromic shifts and emission shifts attributed to intramolecular charge transfer. This unique charge transfer emission is quenched by the addition of H2S resulting in a fast and significant emission response. The formation of the amine product is confirmed by mass spectral analysis.
Conflicts of interest
No conflict of interest to declare.
Acknowledgements
The authors acknowledge a financial grant from BRNS (Board of Research in Nuclear Sciences, India; 37(2)/14/05/2016) and IIT Gandhinagar for overall support. We thank the reviewers for useful suggestions.
References
- C. Jia, A. Migliore, N. Xin, S. Huang, J. Wang, Q. Yang, S. Wang, H. Chen, D. Wang, B. Feng, Z. Liu, G. Zhang, D.-H. Qu, H. Tian, M. A. Ratner, H. Q. Xu, A. Nitzan and X. Guo, Covalently Bonded Single-Molecule Junctions with Stable and Reversible Photoswitched Conductivity, Science, 2016, 352, 1443–1445 CrossRef CAS PubMed.
- Y. Li, T. Liu, H. Liu, M.-Z. Tian and Y. Li, Self-Assembly of Intramolecular Charge-Transfer Compounds into Functional Molecular Systems, Acc. Chem. Res., 2014, 47, 1186–1198 CrossRef CAS PubMed.
- D. Bléger and S. Hecht, Visible-Light-Activated Molecular Switches, Angew. Chem., Int. Ed., 2015, 54, 11338–11349 CrossRef PubMed.
- U. Al-Atar, R. Fernandes, B. Johnsen, D. Baillie and N. R. Branda, A Photocontrolled Molecular Switch Regulates Paralysis in a Living Organism, J. Am. Chem. Soc., 2009, 131, 15966–15967 CrossRef CAS PubMed.
- N. Xie and Y. Chen, Construction and Photoswitching Properties of Fluorescent Diarylethenes, J. Mater. Chem., 2007, 17, 861–865 RSC.
- M. Bossi, V. Belov, S. Polyakova and S. W. Hell, Reversible Red Fluorescent Molecular Switches, Angew. Chem., Int. Ed., 2006, 45, 7462–7465 CrossRef CAS PubMed.
- M. Berberich and F. Würthner, Tuning the Redox Properties of Photochromic Diarylethenes by Introducing Electron-Withdrawing Substituents, Asian J. Org. Chem., 2013, 2, 250–256 CrossRef CAS.
- M. Zubair Khalid Baig, D. Majhi, R. N. P. Tulichala, M. Sarkar and M. Chakravarty, Easy Access to New Anthracenyl π-Conjugates: Generation of Distinct AIE-Active Materials, J. Mater. Chem. C, 2017, 5, 2380–2387 RSC.
- S. Achelle, C. Baudequin and N. Plé, Luminescent Materials Incorporating Pyrazine or Quinoxaline Moieties, Dyes Pigm., 2013, 98, 575–600 CrossRef CAS.
- S. K. Pathak, R. K. Gupta, S. Nath, D. S. S. Rao, S. K. Prasad and A. S. Achalkumar, Columnar Self-Assembly of Star-Shaped Luminescent Oxadiazole and Thiadiazole Derivatives, J. Mater. Chem. C, 2015, 3, 2940–2952 RSC.
- T. Ichikawa, M. Morimoto, H. Sotome, S. Ito, H. Miyasaka and M. Irie, Photochromism of Diarylethene Derivatives Having Benzophosphole and Benzothiophene Groups, Dyes Pigm., 2016, 126, 186–193 CrossRef CAS.
- D. Šarlah, A. Juranovič, B. Kožar, L. Rejc, A. Golobič and A. Petrič, Synthesis of Naphthalene-Based Push-Pull Molecules with a Heteroaromatic Electron Acceptor, Molecules, 2016, 21, 267 CrossRef PubMed.
- H. Rivera, S. Dhar, J. J. La Clair, S.-C. Tsai and M. D. Burkart, An Unusual Intramolecular Trans-Amidation, Tetrahedron, 2016, 72, 3605–3608 CrossRef CAS PubMed.
- S. Xue, J. Liu and C. Wang, Dbu-Mediated [3 + 2] Cycloaddition Reactions of Donor–Acceptor Cyclopropanes with Nitromethane: Efficient Strategy for the Construction of Isoxazole Skeletons, Eur. J. Org. Chem., 2016, 2450–2456 CrossRef CAS.
- S. Batra, S. K. Rastogi, B. Kundu, A. Patra and A. P. Bhaduri, A Novel Isoxazole-Based Scaffold for Combinatorial Chemistry, Tetrahedron Lett., 2000, 41, 5971–5974 CrossRef CAS.
- S. Nagaraju, N. Satyanarayana, B. Paplal, A. K. Vasu, S. Kanvah, B. Sridhar, P. Sripadi and D. Kashinath, One-Pot Synthesis of Functionalized Isoxazole–Thiolane Hybrids Via Knoevenagel Condensation and Domino Sulfa-1, 6-Michael/Intramolecular Vinylogous Henry Reactions, RSC Adv., 2015, 5, 94474–94478 RSC.
- M. G. Perrone, P. Vitale, A. Panella, S. Ferorelli, M. Contino, A. Lavecchia and A. Scilimati, Isoxazole-Based-Scaffold Inhibitors Targeting Cyclooxygenases (Coxs), ChemMedChem, 2016, 11, 1172–1187 CrossRef PubMed.
- M. A. Kalwat, Z. Huang, C. Wichaidit, K. McGlynn, S. Earnest, C. Savoia, E. M. Dioum, J. W. Schneider, M. R. Hutchison and M. H. Cobb, Isoxazole Alters Metabolites and Gene Expression, Decreasing Proliferation and Promoting a Neuroendocrine Phenotype in B-Cells, ACS Chem. Biol., 2016, 11, 1128–1136 CrossRef CAS PubMed.
- D. Simoni, R. Rondanin, R. Baruchello, M. Rizzi, G. Grisolia, M. Eleopra, S. Grimaudo, A. D. Cristina, M. R. Pipitone, M. R. Bongiorno, M. Aricò, F. P. Invidiata and M. Tolomeo, Novel Terphenyls and 3,5-Diaryl Isoxazole Derivatives Endowed with Growth Supporting and Antiapoptotic Properties, J. Med. Chem., 2008, 51, 4796–4803 CrossRef CAS PubMed.
-
K. J. Coe, Metabolism and Cytotoxicity of the Nitroaromatic Drug Flutamide and Its Cyano Analog in Hepatocyte Cell Lines, ProQuest, 2008 Search PubMed.
- N. J. Maher, H. Diao, J. O'Sullivan, E. Fadda, F. Heaney and J. McGinley, Lower Rim Isoxazole-Calix [4] Arene Derivatives as Fluorescence Sensors for Copper(II) Ions, Tetrahedron, 2015, 71, 9223–9233 CrossRef CAS.
- Z. Yang, K. Zhang, F. Gong, S. Li, J. Chen, J. S. Ma, L. N. Sobenina, A. I. Mikhaleva, G. Yang and B. A. Trofimov, A New Fluorescent Chemosensor for Fluoride Anion Based on a Pyrrole–Isoxazole Derivative, Beilstein J. Org. Chem., 2011, 7, 46–52 CrossRef CAS PubMed.
- M. Gómez, E. G. Perez, V. Arancibia, C. Iribarren, C. Bravo-Díaz, O. García-Beltrán and M. E. Aliaga, New Fluorescent Turn-Off Probes for Highly Sensitive and Selective Detection of SO2 Derivatives in a Micellar Media, Sens. Actuators, B, 2017, 238, 578–587 CrossRef.
- A. Xiao, H. Wang, X. Lu, J. Zhu, D. Huang, T. Xu, J. Guo, C. Liu and J. Li, H2S, a Novel Gasotransmitter, Involves in Gastric Accommodation, Sci. Rep., 2015, 5, 16086 CrossRef CAS PubMed.
- F. Yu, P. Li, P. Song, B. Wang, J. Zhao and K. Han, An Ict-Based Strategy to a Colorimetric and Ratiometric Fluorescence Probe for Hydrogen Sulfide in Living Cells, Chem. Commun., 2012, 48, 2852–2854 RSC.
- Q. Fu, G. Chen, Y. Liu, Z. Cao, X. Zhao, G. Li, F. Yu, L. Chen, H. Wang and J. You, In Situ Quantification and Evaluation of Clo−/H2S Homeostasis in Inflammatory Gastric Tissue by Applying a Rationally Designed Dual-Response Fluorescence Probe Featuring a Novel H+-Activated Mechanism, Analyst, 2017, 142, 1619–1627 RSC.
- C. Zhang, R. Wang, L. Cheng, B. Li, Z. Xi and L. Yi, A Redox-Nucleophilic Dual-Reactable Probe for Highly Selective and Sensitive Detection of H2S: Synthesis, Spectra and Bioimaging, Sci. Rep., 2016, 6, 30148 CrossRef CAS PubMed.
- X. Chen, Y. Zhou, X. Peng and J. Yoon, Fluorescent and Colorimetric Probes for Detection of Thiols, Chem. Soc. Rev., 2010, 39, 2120–2135 RSC.
- H. Liu, L. Weng and C. Yang, A Review on Nanomaterial-Based Electrochemical Sensors for H2o2, H2s and No inside Cells or Released by Cells, Microchim. Acta, 2017, 1–17 Search PubMed.
- H. Peng, Y. Cheng, C. Dai, A. L. King, B. L. Predmore, D. J. Lefer and B. Wang, A Fluorescent Probe for Fast and Quantitative Detection of Hydrogen Sulfide in Blood, Angew. Chem., Int. Ed., 2011, 50, 9672–9675 CrossRef CAS PubMed.
- V. S. Lin and C. J. Chang, Fluorescent Probes for Sensing and Imaging Biological Hydrogen Sulfide, Curr. Opin. Chem. Biol., 2012, 16, 595–601 CrossRef CAS PubMed.
- R. Wang, F. Yu, L. Chen, H. Chen, L. Wang and W. Zhang, A Highly Selective Turn-on near-Infrared Fluorescent Probe for Hydrogen Sulfide Detection and Imaging in Living Cells, Chem. Commun., 2012, 48, 11757–11759 RSC.
- F. Yu, X. Han and L. Chen, Fluorescent Probes for Hydrogen Sulfide Detection and Bioimaging, Chem. Commun., 2014, 50, 12234–12249 RSC.
- D.-P. Li, J.-F. Zhang, J. Cui, X.-F. Ma, J.-T. Liu, J.-Y. Miao and B.-X. Zhao, A Ratiometric Fluorescent Probe for Fast Detection of Hydrogen Sulfide and Recognition of Biological Thiols, Sens. Actuators, B, 2016, 234, 231–238 CrossRef CAS.
- S. Ding, W. Feng and G. Feng, Rapid and Highly Selective Detection of H2S by Nitrobenzofurazan (NBD) Ether-Based Fluorescent Probes with an Aldehyde Group, Sens. Actuators, B, 2017, 238, 619–625 CrossRef CAS.
- J. Gao, Q. Li, C. Wang and H. Tan, Copper(II)-Mediated Fluorescence of Lanthanide Coordination Polymers Doped with Carbon Dots for Ratiometric Detection of Hydrogen Sulfide, Sens. Actuators, B, 2017, 253, 27–33 CrossRef CAS.
- H. Wang, D. Yang, R. Tan, Z. J. Zhou, R. Xu, J. F. Zhang and Y. Zhou, A Cyanine-Based Colorimetric and Fluorescence Probe for Detection of Hydrogen Sulfide in Vivo, Sens. Actuators, B, 2017, 247, 883–888 CrossRef CAS.
- C. Yin, F. Huo, M. Xu, C. L. Barnes and T. E. Glass, A NIR, Special Recognition on Hs-/Cn- Colorimetric and Fluorescent Imaging Material for Endogenous H2S Based on Nucleophilic Addition, Sens. Actuators, B, 2017, 252, 592–599 CrossRef CAS.
- Y. Huang, F. Yu, J. Wang and L. Chen, Near-Infrared Fluorescence Probe for in Situ Detection of Superoxide Anion and Hydrogen Polysulfides in Mitochondrial Oxidative Stress, Anal. Chem., 2016, 88, 4122–4129 CrossRef CAS PubMed.
- M. Gao, F. Yu, H. Chen and L. Chen, Near-Infrared Fluorescent Probe for Imaging Mitochondrial Hydrogen Polysulfides in Living Cells and in Vivo, Anal. Chem., 2015, 87, 3631–3638 CrossRef CAS PubMed.
- H. Chen, X. He, M. Su, W. Zhai, H. Zhang and C. Li, A General Strategy toward Highly Fluorogenic Bioprobes Emitting across the Visible Spectrum, J. Am. Chem. Soc., 2017, 139, 10157–10163 CrossRef CAS PubMed.
- Y. Sonoda, M. Goto, S. Tsuzuki and N. Tamaoki, Fluorescence Spectroscopic Properties and Crystal Structure of a Series of Donor-Acceptor Diphenylpolyenes, J. Phys. Chem. A, 2006, 110, 13379–13387 CrossRef CAS PubMed.
- H. Agnihotri, M. Paramasivam, V. Palakollu and S. Kanvah, Photoisomerization of Trans Ortho-, Meta-, Para-Nitro Diarylbutadienes: A Case of Regioselectivity, Photochem. Photobiol., 2015, 91, 1324–1331 CrossRef CAS PubMed.
- L. Li and P. K. Moore, Putative Biological Roles of Hydrogen Sulfide in Health and Disease: A Breath of Not So Fresh Air?, Trends Pharm. Sci., 2008, 29, 84–90 CrossRef CAS PubMed.
- G. Yang, L. Wu, B. Jiang, W. Yang, J. Qi, K. Cao, Q. Meng, A. K. Mustafa, W. Mu, S. Zhang, S. H. Snyder and R. Wang, H2s as a Physiologic Vasorelaxant: Hypertension in Mice with Deletion of Cystathionine Γ-Lyase, Science, 2008, 322, 587 CrossRef CAS PubMed.
- Q. Wu, Y. Wu, C. Yu, Z. Wang, E. Hao and L. Jiao, A Highly Selective Visible Light Excitable Boron Dipyrromethene Probe for Cysteine over Homocysteine and Glutathione Based on a Michael Addition Reaction, Sens. Actuators, B, 2017, 253, 1079–1086 CrossRef CAS.
- O. Rusin, N. N. S. Luce, R. A. Agbaria, J. O. Escobedo, S. Jiang, I. M. Warner, F. B. Dawan, K. Lian and R. M. Strongin, Visual Detection of Cysteine and Homocysteine, J. Am. Chem. Soc., 2004, 126, 438 CrossRef CAS PubMed.
- L. Ma, J. Qian, H. Tian, M. Lan and W. Zhang, A Colorimetric and Fluorescent Dual Probe for Specific Detection of Cysteine Based on Intramolecular Nucleophilic Aromatic Substitution, Analyst, 2012, 137, 5046–5050 RSC.
- M.-Y. Wu, K. Li, J.-T. Hou, Z. Huang and X.-Q. Yu, A Selective Colorimetric and Ratiometric Fluorescent Probe for Hydrogen Sulfide, Org. Biomol. Chem., 2012, 10, 8342–8347 CAS.
- N. C. Pradhan and M. M. Sharma, Reactions of Nitrochlorobenzenes with Sodium Sulfide: Change in Selectivity with Phase-Transfer Catalysts, Ind. Eng. Chem. Res., 1992, 31, 1606–1609 CrossRef CAS.
- L. A. Montoya and M. D. Pluth, Selective Turn-on Fluorescent Probes for Imaging Hydrogen Sulfide in Living Cells, Chem. Commun., 2012, 48, 4767–4769 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectral characterization data, and supporting absorption and emission figures. See DOI: 10.1039/c7pp00331e |
|
| This journal is © The Royal Society of Chemistry and Owner Societies 2018 |
 *
*


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) binary mixture.
1) binary mixture.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture. Pyrenyl isoxazole (4) absorbs at ∼450 nm and emits at ∼631 nm in a DMSO–H2O binary mixture. Upon titrating with aqueous sodium sulfide [typically the emission spectra are recorded after about 3 minutes post addition of Na2S], the absorbance initially decreases (up to 50 μM) in intensity and later shows a hypsochromic shift from ∼60 μM. Beyond this concentration, the primary absorption band at ∼450 nm merely appears as a shoulder. The absorption at ∼350 nm shows a progressive increase with a well-defined isosbestic point at ∼407 nm [Fig. 4a]. This shift in absorption may indicate the formation of a new species, and the blue-shifted absorption is attributed to a loss in delocalization. This is a consequence of the shifts in the change of the structure from the initial electron withdrawing nitro (NO2) group which is reduced to the electron donating amino group (NH2). The changes in the emission spectra of (4) in the absence or presence of Na2S (0–170 μM) are displayed in Fig. 4b. Under 450 nm excitation, compound (4) shows emission maxima at 631 nm in the absence of Na2S. However, the addition of Na2S results in a significant loss of emission intensity with the eventual loss of emission. Noticeably, the emission in the blue region of the spectrum (at or near 400–450 nm) seems to be increasing. To get a more accurate picture of this emission behavior, we excited compound (4) at 350 nm which is a dominant absorption band post addition of Na2S. The emission shows two peaks: a long charge transfer band at ∼630 nm and a shorter wavelength structured emission at 380, 400 and 420 nm. These shorter wavelength emission peaks show an enhancement in intensity upon the addition of sodium sulfide, and a concomitant decrease in emission intensity at 630 nm was noted. Similar observations were reported for anthryl (3) and naphthyl (2) substituted isoxazoles. In the case of (3), the emission at ∼630 nm decreases in intensity, and new emissive peaks appear in shorter wavelength regions at ∼500 nm with a sudden surge in emission intensity at 80 μM [Fig. 5a]. The excitation at 350 nm shows emission peaks primarily due to the anthracene [Fig. S3†]. The naphthyl derivative (2) also shows a drop in the emission intensity of the charge transfer band (542 nm) with a slight shift of the wavelength to the blue region [Fig. 5b] at higher concentrations. The increase in the emission intensity at higher energy is conspicuous for (3) or (4) compared to that for (2). The changes in the UV-spectral profile upon the reduction of the nitro group to the amino group are shown in the ESI [Fig. S2†]. H2S acts as a weak acid and may contribute to the observed emission changes. Our experiments showed that the emission is unaffected with pH variation [Fig S8†]. To check the sensitivity for other analytes such as Na2SO3, cysteine, homocysteine and glutathione, the isoxazole derivative (4) was titrated with different analyte concentrations. The results are shown in the ESI.† Similar to the earlier emission results, the emission band at ∼630 nm is quenched with the analytes used [Fig S9a†]. A comparative bar chart [Fig S9b†] for a given concentration of the analyte reveals greater sensitivity for H2S. While this result is encouraging, the nature of product formation is uncertain. For instance, cysteine or homocysteine was earlier detected by a Michael addition, a cyclization or a sequential substitution mechanism.46–48 The results obtained from the mass spectral analysis gave a molecular weight much higher than the sole amino product. The product formed cannot be conclusively confirmed based on the mass spectral data alone and requires further experimentation.
1) mixture. Pyrenyl isoxazole (4) absorbs at ∼450 nm and emits at ∼631 nm in a DMSO–H2O binary mixture. Upon titrating with aqueous sodium sulfide [typically the emission spectra are recorded after about 3 minutes post addition of Na2S], the absorbance initially decreases (up to 50 μM) in intensity and later shows a hypsochromic shift from ∼60 μM. Beyond this concentration, the primary absorption band at ∼450 nm merely appears as a shoulder. The absorption at ∼350 nm shows a progressive increase with a well-defined isosbestic point at ∼407 nm [Fig. 4a]. This shift in absorption may indicate the formation of a new species, and the blue-shifted absorption is attributed to a loss in delocalization. This is a consequence of the shifts in the change of the structure from the initial electron withdrawing nitro (NO2) group which is reduced to the electron donating amino group (NH2). The changes in the emission spectra of (4) in the absence or presence of Na2S (0–170 μM) are displayed in Fig. 4b. Under 450 nm excitation, compound (4) shows emission maxima at 631 nm in the absence of Na2S. However, the addition of Na2S results in a significant loss of emission intensity with the eventual loss of emission. Noticeably, the emission in the blue region of the spectrum (at or near 400–450 nm) seems to be increasing. To get a more accurate picture of this emission behavior, we excited compound (4) at 350 nm which is a dominant absorption band post addition of Na2S. The emission shows two peaks: a long charge transfer band at ∼630 nm and a shorter wavelength structured emission at 380, 400 and 420 nm. These shorter wavelength emission peaks show an enhancement in intensity upon the addition of sodium sulfide, and a concomitant decrease in emission intensity at 630 nm was noted. Similar observations were reported for anthryl (3) and naphthyl (2) substituted isoxazoles. In the case of (3), the emission at ∼630 nm decreases in intensity, and new emissive peaks appear in shorter wavelength regions at ∼500 nm with a sudden surge in emission intensity at 80 μM [Fig. 5a]. The excitation at 350 nm shows emission peaks primarily due to the anthracene [Fig. S3†]. The naphthyl derivative (2) also shows a drop in the emission intensity of the charge transfer band (542 nm) with a slight shift of the wavelength to the blue region [Fig. 5b] at higher concentrations. The increase in the emission intensity at higher energy is conspicuous for (3) or (4) compared to that for (2). The changes in the UV-spectral profile upon the reduction of the nitro group to the amino group are shown in the ESI [Fig. S2†]. H2S acts as a weak acid and may contribute to the observed emission changes. Our experiments showed that the emission is unaffected with pH variation [Fig S8†]. To check the sensitivity for other analytes such as Na2SO3, cysteine, homocysteine and glutathione, the isoxazole derivative (4) was titrated with different analyte concentrations. The results are shown in the ESI.† Similar to the earlier emission results, the emission band at ∼630 nm is quenched with the analytes used [Fig S9a†]. A comparative bar chart [Fig S9b†] for a given concentration of the analyte reveals greater sensitivity for H2S. While this result is encouraging, the nature of product formation is uncertain. For instance, cysteine or homocysteine was earlier detected by a Michael addition, a cyclization or a sequential substitution mechanism.46–48 The results obtained from the mass spectral analysis gave a molecular weight much higher than the sole amino product. The product formed cannot be conclusively confirmed based on the mass spectral data alone and requires further experimentation.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) binary mixture.
1) binary mixture.




