Cross dehydrogenative coupling of sugar enol ethers with terminal alkenes in the synthesis of pseudo-disaccharides, chiral oxadecalins and a conjugated triene†
Abstract
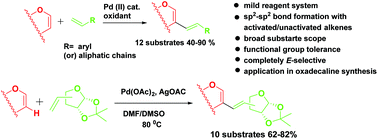
An efficient strategy for the synthesis of C-2 and C-3 branched sugar dienes via cross dehydrogenative coupling of sugar enol ethers with terminal alkenes was developed. Both pyran and furan based enol ethers coupled smoothly with electron rich as well as deficient alkene sources yielding sugar dienes with complete E-stereoselectivity. This coupling reaction was applied successfully for the synthesis of orthogonally protected pseudo-C-saccharides as an alternative to olefin metathesis. Substrate scope was further enhanced by reacting exoglycals with methyl acrylate to generate C-5 branched sugars with moderate diastereoselectivity. These diene subunits were reacted with maleic anhydride under [4 + 2] cycloaddition conditions to generate highly functionalised chiral oxadecalin cores. Finally, utilizing this C–C bond formation, a sugar based conjugated triene was synthesized in excellent yield and selectivity.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...