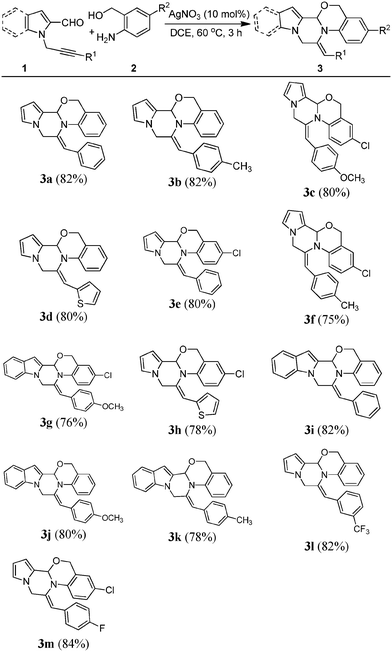
Silver-catalyzed synthesis of pyrrolopiperazine fused with oxazine/imidazole via a domino approach: evaluation of anti-cancer activity†
K.
Shiva Kumar
 *a,
N. Praveen
Kumar
a,
Bandari
Rajesham
a,
Gugulothu
Kishan
a,
Sravani
Akula
b and
Rama Krishna
Kancha
b
*a,
N. Praveen
Kumar
a,
Bandari
Rajesham
a,
Gugulothu
Kishan
a,
Sravani
Akula
b and
Rama Krishna
Kancha
b
aDepartment of Chemistry, Osmania University, Hyderabad-500007, India. E-mail: shivakumarkota@yahoo.co.in; Tel: +91 40 27682337
bMolecular Medicine and Therapeutics Laboratory, CPMB, Osmania University, Hyderabad-500007, India
First published on 29th November 2017
Abstract
Silver-catalyzed domino synthesis of pyrrolopiperazine fused with oxazine/imidazole by the reaction of pyrrole-derived δ-alkynyl aldehydes and nucleophilic amines was performed. This domino strategy involves the formation of two new C–N bonds and one C–O/C–N bond, resulting in the formation of two new interesting fused heterocycles. Some of the synthesized compounds exhibit promising growth inhibitory activity against leukemia and breast cancer cell lines.
Cascade reactions are high in demand in modern organic synthesis, because they can directly and efficiently construct complex molecules, without isolating any intermediates, from readily accessible starting materials and most importantly with excellent atom economy.1
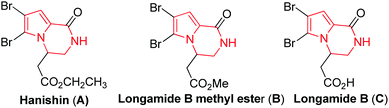
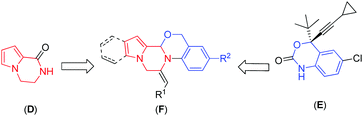
The pyrrolopiperazine framework has been found to be an integral part of several natural products, bioactive compounds and drugs.2 For example, hanishin3 (A) and longamide B methyl ester4 (B), two natural molecules isolated from marine sponges, exhibit cytotoxic activity against NSCLCN6 human nonsmall-cell lung carcinoma (IC50 9.7 μg mL−1) and P-388 lymphocytic leukemia cells (ED50 30 μg mL−1), respectively. Longamide B (C) exhibits activity against African trypanosomes (IC50 1.53 μg mL−1) (Fig. 1). The pyrrolopiperazine skeleton (D, Fig. 2) is also widely present in agelastatin alkaloids,5 which are known for their anticancer activity. Additionally, the synthetic derivatives thieno[3,2-e]pyrrolo[1,2-a]pyrazines6 and pyrido[2,3-e]pyrrolo[1,2-a]pyrazines7 have been found to be selective 5-HT3 receptor agonists and tricyclic 1,2,3,4-tetrahydropyrazino[1,2-a]indoles act as melatoninergic ligands (Ki = 7–11 nM).8 On the other hand, oxazine is considered to be of great biological and pharmacological interest,9e.g. Efavirenz (E, Fig. 2) is used for the treatment of AIDS,9e and A-62176 and A-74932 are phenoxazine derivatives and are potent inhibitors of mammalian DNA topoisomerase II in vivo that exhibit strong cytotoxic activity against tumor cell lines.10 Thus, a combination of the structural features of both pyrrolopiperazine and oxazine in a single molecular entity represented by F (Fig. 2) was expected to provide a new chemical space that might lead to the identification of novel molecules possessing pharmacological properties. Accordingly, we decided to synthesize pyrrolopiperazine-fused oxazine derivatives and evaluate their anticancer properties.
While as an individual class of heterocycles pyrrolopiperazine11 and benzoxazine12 are well known in the literature, their combined forms, i.e. pyrrolo[2′,1′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3,4]pyrazino[2,1-b][1,3]oxazine and imidazo[1,2-a]pyrrolo[2,1-c]pyrazine, are rather uncommon.
3,4]pyrazino[2,1-b][1,3]oxazine and imidazo[1,2-a]pyrrolo[2,1-c]pyrazine, are rather uncommon.
One of the major challenges and aims of the present study is therefore to develop a suitable methodology leading to the heterocyclic structure F (Fig. 2). In continuation of our interest in the synthesis of fused heterocyclic scaffolds,13 we envisaged that the one-pot synthesis of pyrrolopiperazine fused with oxazine/imidazole based on Fvia Ag-catalysed δ-alkynyl aldehydes and amines resulting in the formation of an imine, which has embedded nucleophiles (oxygen/nitrogen) through intermolecular condensation, would provide the oxazine/imidazole, which in the presence of an appropriate alkyne via 6-exo-dig cyclization would afford pyrrolopiperazine fused with oxazine/imidazole (Scheme 1). This domino approach would involve the regioselective formation of two new C–N bonds and one C–O/C–N bond, resulting in the formation of two heterocyclic rings. To the best of our knowledge, the use of this or a similar strategy for the construction of a core pyrrolopiperazine fused oxazine/imidazole ring system is unreported. Moreover, we also report the preliminary results of the evaluation of the pharmacological activities of the synthesized compounds.
Transition-metal-catalyzed domino reactions in the synthesis of structurally diverse polyheterocyclic scaffolds from δ-alkynyl aldehydes were used extensively by Larock,14 Yamamoto,15 Wu,16 and Verma.17 However, the use of δ-alkynyl aldehydes is less studied towards the synthesis of polyheterocyclic scaffolds via a domino approach. Nagarajan et al.11a reported the copper-catalysed tandem synthesis of fused benzimidazolopiperazine via tandem imidazole formation followed by 6-exo-dig cyclization. Abbiati and co-workers11b developed a microwave-mediated synthesis of pyrazino[1,2-a]indoles via the intramolecular cyclization of 2-carbonyl-1-alkynylindoles in the presence of ammonia. In 2009, the same group reported the microwave-promoted synthesis of pyrrolo[1,2-a]pyrazines and isoquinolines through the domino imination/annulation of alkynes bearing a proximate carbonyl group in the presence of ammonia.11c Recently, Balci et al.11d have demonstrated that the gold-catalyzed intramolecular cyclization of N-propargyl indole provides a pyrazino[1,2-a]indole. However, these methods suffer from a lack of regioselective cyclization leading to the formation of a mixture of isomers or require harsh reaction conditions and are not suitable for the preparation of our target compounds F (Fig. 2). We therefore decided to develop a highly regioselective, mild, practical and one-pot method for the synthesis of pyrrolopiperazine fused with oxazine/imidazole derivatives.
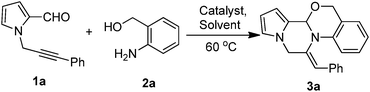
The metal-catalyzed domino reaction of 3a was then performed under a variety of conditions (Table 1) to establish the optimized reaction conditions. Initially, 1-(3-phenylprop-2-yn-1-yl)-1H-pyrrole-2-carbaldehyde (1a, 1.0 equiv.) was made to react with (2-aminophenyl)methanol (2a, 1.3 equiv.) in 1,2-dichloroethane (5 mL) in the presence of 10 mol% CuI at 60 °C for 3 h, and the product 3a was isolated in 35% yield (Table 1, entry 1). Inspired by these results, further optimization of the reaction conditions was carried out. Among various transitional metal catalysts such as Cu(OTf)3, AgOAc, Ag(OTf)3 and AgNO3 (Table 1, entries 2–5), AgNO3 was found to be the optimal catalyst for the reaction process. Solvents such as DMF, toluene, EtOAc, CH3CN and DCM were also evaluated (Table 1, entries 6–10), and an 82% yield of 3a was afforded when DCE was used (Table 1, entry 5). A decrease in AgNO3 loading decreased the product yield (Table 1, entry 11) and a higher catalyst loading did not increase the yield (Table 1, entry 12). A further increase in the reaction time and temperature did not improve the yield (Table 1, entry 13). Overall, it was observed that the conditions of entry 5 are the optimum reaction conditions for obtaining the desired product 3a in the best yield. Compound 3a was characterized by the appearance of (i) a singlet at δ 5.85 due to the proton attached to the tertiary carbon, (ii) diastereotopic doublets of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–CH2– at δ 5.17 and 5.07 and diastereotopic doublets of –O–CH2– at δ 4.77 and 4.60, (iii) a singlet at δ 6.19 due to the vinylic proton, (iv) 13C NMR signals at 51.4 and 66.7 ppm due to
N–CH2– at δ 5.17 and 5.07 and diastereotopic doublets of –O–CH2– at δ 4.77 and 4.60, (iii) a singlet at δ 6.19 due to the vinylic proton, (iv) 13C NMR signals at 51.4 and 66.7 ppm due to ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–CH2
N–CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) and –O–CH2–, respectively, and (v) a tertiary carbon signal appearing at 81.9 ppm.
and –O–CH2–, respectively, and (v) a tertiary carbon signal appearing at 81.9 ppm.
| Entry | Catalyst | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a All the reactions were carried out using compound 1a (1.0 equiv.), 2a (1.3 equiv.) and a catalyst in a solvent (5 mL) at 60 °C. b Isolated yield. c The reaction was carried out at 80 °C. | ||||
| 1 | CuI (10 mol%) | ClCH2CH2Cl | 3 | 35 |
| 2 | Cu(OTf)3 (10 mol%) | ClCH2CH2Cl | 3 | 50 |
| 3 | AgOAc (10 mol%) | ClCH2CH2Cl | 3 | 70 |
| 4 | Ag(OTf)3 (10 mol%) | ClCH2CH2Cl | 3 | 78 |
| 5 | AgNO3 (10 mol%) | ClCH2CH2Cl | 3 | 82 |
| 6 | AgNO3 (10 mol%) | DMF | 3 | 40 |
| 7 | AgNO3 (10 mol%) | Toluene | 3 | 50 |
| 8 | AgNO3 (10 mol%) | EtOAc | 3 | 65 |
| 9 | AgNO3 (10 mol%) | CH3CN | 3 | 78 |
| 10 | AgNO3 (10 mol%) | DCM | 3 | 75 |
| 11 | AgNO3 (5 mol%) | ClCH2CH2Cl | 3 | 70 |
| 12 | AgNO3 (15 mol%) | ClCH2CH2Cl | 3 | 80 |
| 13 | AgNO3 (10 mol%) | ClCH2CH2Cl | 4 | 78c |
With the optimized conditions in hand, we decided to explore the scope and generality of the present AgNO3-catalyzed domino reaction. Accordingly, a variety of δ-alkynyl aldehydes (1) were reacted with aminobenzyl alcohols. A variety of different aryl-substituted groups at the R1 position such as phenyl (3a, 3e and 3i), p-anisyl (3c, 3g and 3j), p-tolyl (3b, 3f and 3k), m-trifluoro (3l) and p-fluoro (3m) were successfully converted into desired products in moderate to good yields. Interestingly, the R1 replaced with thiophene (3d and 3h) substrates were smoothly converted into the corresponding pyrrolopiperazine fused with oxazines. The δ-alkynyl aldehydes used other than pyrrole include indole (1b) to generate the corresponding products (3g, 3i, 3j and 3k) in good yields. Furthermore, the scope of the reaction was demonstrated with (2-amino-5-chlorophenyl)methanol (2b) and the respective compounds were isolated (3c, 3e, 3f, 3g, 3h and 3m) (Table 2).
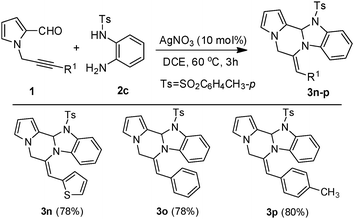
In order to explore more diversity and complexity, we treated δ-alkynyl aldehydes (1) with N-(2-aminophenyl)benzenesulfonamide (2c) to give pyrazino[2,1-b][1,3]imidazole fused with pyrrole (3n–p) (Scheme 2).
The structure and the olefin geometry of 3p were further conformed by the single-crystal X-ray data analysis (Fig. 3).18
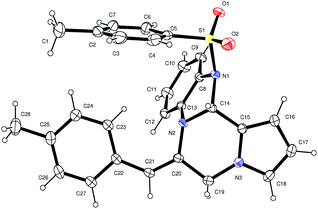 | ||
| Fig. 3 X-ray crystal structure of 3p (ORTEP diagram). Thermal ellipsoids are drawn at the 50% probability level. | ||
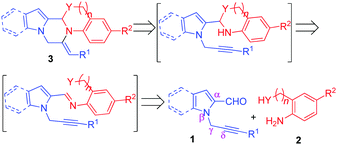
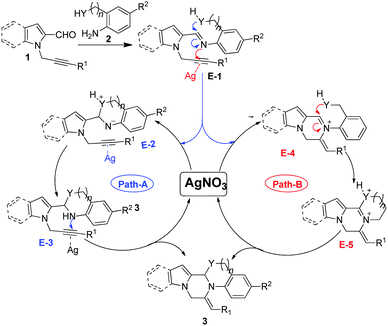
Based on the literature reports and our experimental observations, a plausible mechanism is proposed in Scheme 3.17 Initially, the reaction of δ-alkynyl aldehydes 1 with nucleophilic amine 2 produced condensation species E-1. After this, two possibilities exist for the formation of compound 3, either the C–O/C–N bond forms first and then the C–N bond (path-A) or vice versa (path-B). (i.e., either ring A forms first and then ring B or vice versa). Path-A involves (i) a nucleophile (Y) attack at the imine carbon to give E-2, (ii) a subsequent intramolecular proton transfer leading to E-3, and then (iii) the activation of the triple bond by AgNO3, which would undergo a second intramolecular nucleophilic attack of the nitrogen onto the triple bond to afford 3. Path-B involves (i) the activation of the triple bond by AgNO3 to give E-4, and (ii) subsequent nucleophile (Y) attack at the imine carbon to furnish E-5, which (iii) on subsequent deprotonation gives the desired product 3.
Most of the pyrrolopiperazine fused with oxazine/imidazole structures (3) synthesized were tested for their anti-proliferative properties in vitro using three different cancer cell lines including K562 (leukemia cells), BT-474 (human breast cancer cells) and MCF-7 (breast cancer cells). 4OH-Tamoxifene was used as a reference compound. Some of the compounds were examined at various concentrations using an Alamar Blue (AB) assay, and the IC50 values obtained for each compound are presented in Table 3. Some compounds exhibited inhibitory activity against leukemia cancer cell growth as reflected by their IC50 values, but the best results were obtained for compounds 3e, 3g, 3h and 3i (IC50 < 15 μM, Table 3). Compounds 3g (IC50 17.6 μM, Tables 3) and 3h (IC50 < 21 μM, Table 3) also exhibited inhibitory activity against the human breast cancer cells. Among all the compounds, 3i exhibited interesting activities against the leukemia cells and human breast cancer cells (IC50 < 4 μM, Table 3). It is interesting to note that compound 3i exhibited the maximum activity in p53-deficient cell lines K562 (IC50 = 3.0 μM) and BT-474 (IC50 = 4.0 μM), but not in p53-wildtype MCF7 cells (IC50 = 19.6 ± 1.5 μM) (Table 3). Similar observations were made with 3g and 3k where cytotoxic activity was specifically observed in p53-deficient cells, hence the present class of molecules exhibit both ‘cell-type-specific’ and ‘p53-status-dependent’ anti-cancer activity (Table 3). These findings suggest that pyrrolopiperazine fused with an oxazine/imidazole framework could be a new template for the design and development of molecules for the identification of novel anticancer agents.
| Compound | IC50 valuesa (μM) | ||
|---|---|---|---|
| K562 | BT-474 | MCF-7 | |
| a IC50 values are expressed as mean_SD. | |||
| 3b | 12.3 ± 3.2 | >50 | >50 |
| 3c | 19.3 ± 3.0 | >50 | >50 |
| 3e | 14.6 ± 2.5 | >50 | >50 |
| 3f | 20.6 ± 1.5 | 41.3 ± 0.5 | >50 |
| 3g | 9 ± 1.7 | 17.6 ± 0.5 | >50 |
| 3h | 14 ± 2 | 21.3 ± 0.5 | 29.6 ± 0.5 |
| 3i | 3 ± 0 | 4 ± 0 | 19.6 ± 1.5 |
| 3k | 22 ± 2.6 | 8.3 ± 1.5 | 41.3 ± 1.5 |
| 3n | 23 ± 2.6 | 27 ± 4.3 | >50 |
| 4OH-Tamoxifene | 6.0 ± 0 | 6.0 ± 0 | 8.0 ± 0 |
In conclusion, we have successfully developed a domino protocol for the synthesis of various pyrrolopiperazines fused with oxazine/imidazole via Ag-catalysed C–N bond formation followed by regioselective 6-exo-dig cyclization. The present methodology is a regioselective, mild, practical and one-pot method. Some of these compounds showed anti-proliferative properties when tested against leukemia and breast cancer cells.
Conflicts of interest
The authors confirm that this article content has no conflict of interest.Acknowledgements
KSK thanks the University Grants Commission (UGC), New Delhi, for the award of an Assistant Professorship under FRP and CSIR, India, for the financial support [02(0234)/15/EMR-II]. B. R. thanks CSIR, New Delhi, for a Senior Research Fellowship.Notes and references
-
(a) R. A. Sheldon, Pure Appl. Chem., 2000, 72, 1233–1246 CrossRef CAS
; (b) B. M. Trost, Science, 1991, 254, 1471–1477 CAS
; (c) B. M. Trost, Angew. Chem., Int. Ed., 1995, 34, 259–281 CrossRef CAS
.
-
(a)
C. Hansch, P. G. Sammes and J. B. Taylor, Comprehensive Medicinal Chemistry, Pergamon, Oxford, 1990, ch. 7, vol. 2 Search PubMed
; (b) D. A. Erlanson, R. S. Mc Dowell and T. O’Brien, J. Med. Chem., 2004, 4, 3463–3482 CrossRef PubMed
; (c) M. S. Butler, J. Nat. Prod., 2004, 67, 2141–2153 CrossRef CAS PubMed
.
- I. Mancini, G. Guella, P. Amade, C. Roussakis and F. Pietra, Tetrahedron Lett., 1997, 38, 6271–6274 CrossRef CAS
.
-
(a) N. S. Reddy and Y. Venkateswarlu, Biochem. Syst. Ecol., 2000, 28, 1035–1037 CrossRef CAS
; (b) A. Umeyama, S. Ito, E. Yuasa, S. Arihara and T. Yamada, J. Nat. Prod., 1998, 61, 1433–1434 CrossRef CAS PubMed
.
- S. Han, D. S. Siegel, K. C. Morrison, P. J. Hergenrother and M. Movassaghi, J. Org. Chem., 2013, 78, 11970–11984 CrossRef CAS PubMed
.
- S. Rault, J.-C. Lancelot, H. Prunier, M. Robba, P. Renard, P. Delagrange, B. Pfeiffer, D.-H. Caignard, B. Guardiola-Lemaitre and M. Hamon, J. Med. Chem., 1996, 39, 2068–2080 CrossRef CAS PubMed
.
- G. Campiani, E. Morelli, S. Gemma, V. Nacci, S. Butini, M. Hamon, E. Novellino, G. Greco, A. Cagnotto, M. Goegan, L. Cervo, F. D. Valle, C. Fracasso, S. Caccia and T. Mennini, J. Med. Chem., 1999, 42, 4362–4379 CrossRef CAS PubMed
.
- C. Markl, M. I. Attia, J. Julius, S. Sethi, P. A. Witt-Enderby and D. P. Zlotos, Bioorg. Med. Chem., 2009, 17, 4583–4594 CrossRef CAS PubMed
.
-
(a) K. F. Croom and K. L. Goa, Drugs, 2003, 63, 2769–2802 CrossRef CAS PubMed
; (b) M. Hajós, J. C. Fleishaker, J. K. Filipiak-Reisner, M. T. Brown and E. H. F. Wong, CNS Drug Rev., 2004, 10, 23–44 CrossRef
; (c) E. Testa, L. Fontanella, G. Cristiani and G. Gallo, J. Org. Chem., 1959, 24, 1928 CrossRef CAS
; (d) G. Raynaud, M. Sergant, C. Douzon and C. Fauran, U.S. Patent 3, 1974, 821, 215
; (e) S. M. Vrouenraets, W. F. Wit, J. van Tongeren and J. M. Lange, Expert Opin. Pharmacother., 2007, 8, 851–871 CrossRef CAS PubMed
; (f) S.-H. Zhao, J. Berger, R. D. Clark, S. G. Sethofer, N. E. Krauss, J. M. Brothers, R. S. Martin, D. L. Misner, D. Schwab and L. Alexandrova, Bioorg. Med. Chem. Lett., 2007, 17, 3504–3509 CrossRef CAS PubMed
.
-
(a) D. T. W. Chu, R. Hallas, J. J. Clement, L. Alder, E. Mc Donald and J. J. Plattner, Drugs Exp. Clin. Res., 1992, 18, 275–282 CAS
; (b) P. A. Permana, R. M. Snapka, L. L. Shen, D. T. Chu, J. J. Clement and J. J. Plattner, Biochemistry, 1994, 33, 11333–11339 CrossRef CAS PubMed
; (c) J. J. Clement, N. Burres, K. Jarvis, D. T. W. Chu, J. Swiniarski and J. Adler, Cancer Res., 1995, 55, 830–835 CAS
.
-
(a) S. Ramesh, S. K. Ghosh and R. Nagarajan, Org. Biomol. Chem., 2013, 11, 7712–7720 RSC
; (b) Y. Xiong and H. W. Moore, J. Org. Chem., 2005, 70, 4088–4095 CrossRef PubMed
; (c) M. Alfonsi, M. Dell’ Acqua, D. Facoetti, A. Arcadi, G. Abbiati and E. Rossi, Eur. J. Org. Chem., 2009, 2852–2862 CrossRef CAS
; (d) S. Basceken, S. Kaya and M. Balci, J. Org. Chem., 2015, 80, 12552–12561 CrossRef CAS PubMed
.
-
(a) Y. Tian, X. Wang, Q. Xiao, C. Sun and D. Yin, J. Org. Chem., 2014, 79, 9678–9685 CrossRef CAS PubMed
; (b) B. Liu, M. Yin, H. Gao, W. Wu and H. Jiang, J. Org. Chem., 2013, 78, 3009–3020 CrossRef CAS PubMed
; (c) A. Sharifi, M. S. Abaee, Z. Mokhtare, M. Mirzaei, S. K. Singh, A. K. Bajpai and R. Saini, Tetrahedron Lett., 2013, 54, 7132–7135 CrossRef
; (d) K. Brahma, B. Das and C. Chowdhury, Tetrahedron, 2014, 70, 5863–5871 CrossRef CAS
.
-
(a) K. S. Kumar, S. R. Meesa, B. Rajesham, B. Bhasker, M. A. Ashfaq, A. A. Khan, S. S. Rao and M. Pal, RSC Adv., 2016, 6, 48324–48328 RSC
; (b) K. S. Kumar, B. Bhaskar, M. S. Ramulu, N. P. Kumar, M. A. Ashfaq and M. Pal, Org. Biomol. Chem., 2017, 15, 82–87 RSC
; (c) K. S. Kumar, M. S. Ramulu, B. Rajesham, N. P. Kumar, V. Voora and R. K. Kancha, Org. Biomol. Chem., 2017, 15, 4468–4476 RSC
.
-
(a) S. Roy, S. Roy, B. Neuenswander, D. Hill and R. C. Larock, J. Comb. Chem., 2009, 11, 1061–1065 CrossRef CAS PubMed
; (b) N. A. Markina, R. Mancuso, B. Neuenswander, G. H. Lushington and R. C. Larock, ACS Comb. Sci., 2011, 13, 265–271 CrossRef CAS PubMed
; (c) K. R. Roesch and R. C. Larock, J. Org. Chem., 2002, 67, 86–94 CrossRef CAS PubMed
; (d) Q. Huang, J. A. Hunter and R. C. Larock, J. Org. Chem., 2002, 67, 3437–3444 CrossRef CAS PubMed
; (e) G. Dai and R. C. Larock, J. Org. Chem., 2002, 67, 7042–7047 CrossRef CAS PubMed
; (f) G. Dai and R. C. Larock, J. Org. Chem., 2003, 68, 920–928 CrossRef CAS PubMed
; (g) T. Yao and R. C. Larock, J. Org. Chem., 2003, 68, 5936–5942 CrossRef CAS PubMed
; (h) Q. Huang and R. C. Larock, J. Org. Chem., 2003, 68, 980–988 CrossRef CAS PubMed
; (i) H. Zhang and R. C. Larock, J. Org. Chem., 2003, 68, 5132–5138 CrossRef CAS PubMed
; (j) G. Dai and R. C. Larock, Org. Lett., 2002, 4, 193–196 CrossRef CAS PubMed
.
-
(a) N. T. Patil and Y. Yamamoto, Chem. Rev., 2008, 108, 3395–3442 CrossRef CAS PubMed
; (b) N. Asao, S. S. Yudha, T. Nogami and Y. Yamamoto, Angew. Chem., Int. Ed., 2005, 44, 5526–5528 CrossRef CAS PubMed
; (c) K. Sato, N. Asao and Y. Yamamoto, J. Org. Chem., 2005, 70, 8977–8981 CrossRef CAS PubMed
; (d) N. Asao, K. Sato and Y. Yamamoto, J. Org. Chem., 2005, 70, 3682–3685 CrossRef CAS PubMed
; (e) N. Asao, H. Aikawa and Y. Yamamoto, J. Am. Chem. Soc., 2004, 126, 7458–7459 CrossRef CAS PubMed
; (f) N. Asao, C. S. Chan, K. Takahashi and Y. Yamamoto, Tetrahedron, 2005, 61, 11322–11326 CrossRef CAS
; (g) M. Ohtaka, H. Nakamura and Y. Yamamoto, Tetrahedron Lett., 2004, 45, 7339–7344 CrossRef CAS
.
-
(a) Q. Ding and J. Wu, Org. Lett., 2007, 9, 4959–4962 CrossRef CAS PubMed
; (b) K. Gao and J. Wu, J. Org. Chem., 2007, 72, 8611–8613 CrossRef CAS PubMed
; (c) W. Sun, Q. Ding, X. Sun, R. Fan and J. Wu, J. Comb. Chem., 2007, 9, 690–694 CrossRef CAS PubMed
; (d) Z. Chen, X. Yang and J. Wu, Chem. Commun., 2009, 3469–3471 RSC
; (e) X. Yu and J. Wu, J. Comb. Chem., 2010, 12, 238–244 CrossRef CAS PubMed
; (f) Q. Ding, B. Wang and J. Wu, Tetrahedron, 2007, 63, 12166–12171 CrossRef CAS
.
-
(a) A. K. Verma, D. Choudhary, R. K. Saunthwal, V. Rustagi, M. Patel and R. K. Tiwari, J. Org. Chem., 2013, 78, 6657–6669 CrossRef CAS PubMed
; (b) P. K. Mishra and A. K. Verma, Green Chem., 2016, 18, 6367–6372 RSC
; (c) V. Rustagi, T. Aggarwal and A. K. Verma, Green Chem., 2011, 13, 1640–1643 RSC
; (d) R. R. Jha, R. K. Saunthwal and A. K. Verma, Org. Biomol. Chem., 2014, 12, 552–556 RSC
.
- Crystal data of (3p): CCDC 1555220, single crystals suitable for X-ray diffraction of 3p DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (1
EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Molecular formula = C28H25N3O2S, formula weight = 467.57, crystal system = triclinic, space group = P
1). Molecular formula = C28H25N3O2S, formula weight = 467.57, crystal system = triclinic, space group = P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 9.0323(6) Å, b = 10.9047(7) Å, c = 12.9519(9) Å, V = 1185.55(14) Å3, T = 296(2) K, Z = 2, Dc = 1.310 Mg m−3, 18
, a = 9.0323(6) Å, b = 10.9047(7) Å, c = 12.9519(9) Å, V = 1185.55(14) Å3, T = 296(2) K, Z = 2, Dc = 1.310 Mg m−3, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 387 reflections collected, 3324 [R(int) = 0.1105] independent reflections, and goodness of fit = 1.01.
387 reflections collected, 3324 [R(int) = 0.1105] independent reflections, and goodness of fit = 1.01.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectral data for all new compounds, and copies of the spectra. CCDC 1555220. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7nj03608f |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 |