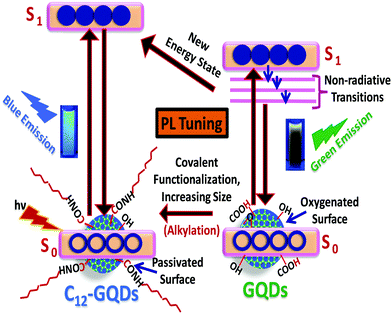
Tuning the wettability and photoluminescence of graphene quantum dots via covalent modification†
Manash Jyoti
Deka
,
Ananya
Dutta
and
Devasish
Chowdhury
 *
*
Material Nanochemistry Laboratory, Physical Sciences Division, Institute of Advanced Study in Science and Technology, Paschim Boragaon, Garchuk, Guwahati-781035, India. E-mail: devasish@iasst.gov.in
First published on 13th November 2017
Abstract
Tuning of the photoluminescence of graphene quantum dots (GQDs) through modulation of the bandgap and chemical doping is important for the GQDs use in optoelectronic devices. Herein, tuning of dual properties, i.e., wettability (hydrophilic to hydrophobic) and photoluminescence, of graphene quantum dots is demonstrated. We synthesized graphene quantum dots (GQDs) and alkylated graphene quantum dots (C12-GQDs) by simple chemical exfoliation (top-down technique) of graphite nanopowder. The synthesized GQDs are highly hydrophilic due to the presence of oxygenated functional moieties. We successfully functionalized the synthesized GQDs covalently with dodecyl amine (DDA) and simultaneously reduced them with glycine to render hydrophobicity on the GQD surface. Herein, glycine plays a dual role as a chemical functionalizer and reducing agent. All the characterizations of GQDs and C12-GQDs were carried out by UV-visible absorption spectroscopy, scanning electron microscopy, Fourier transform infrared spectroscopy, dynamic light scattering, Raman spectroscopy, and contact angle analysis. Interestingly, after covalent modification, we also observed the tuning of photoluminescence (from green to blue) of GQDs. Thus, we can easily tune the dual properties, i.e., wettability and photoluminescence, of GQDs by simple covalent modification with long-chain alkyl (–C12H27) groups.
1 Introduction
Graphene quantum dots (GQDs) are the newest member of the carbon nanohybrid family with a great combination of many attractive and extraordinary properties. They are a kind of quantum-sized fragments of graphene sheets having 0D, atomically thin, hexagonal, and planar shapes.1–3 Depending upon the various synthetic strategies used for their preparation, the size of graphene quantum dots may vary in the range from a few nanometer to ∼100 nm. Some of the interesting properties of GQDs are the surface/edge effect,4 quantum confinement effect,5 tunable photoluminescence, well aqueous dispersion, chemical inertness, low toxicity,6,7etc. Due to these properties, graphene quantum dots have attracted significant attention from the scientific community ever since their serendipitous discovery. A wide range of applications of GQDs have been explored in different fields, and with the transition of time, the area of applications has become more diverse. Some of the most promising applications of graphene quantum dots are in the fields of bioimaging,8 sensors,9 fuel cells,10 drug delivery,11 photo-catalysis,12 photovoltaics,13,14 solar cell,15 energy storage device16etc. Different synthetic routes have been proposed for the synthesis of GQDs. These synthetic routes are either top-down or bottom-up techniques. For example, hydrothermal cutting,17 solvothermal cutting, nanolithography, ultrasonic shearing,18 and chemical exfoliation of graphite are most familiar top-down strategies. The stepwise organic synthesis, cage opening of fullerene,19 carbonization of citric acid20 and unsubstituted hexa-peri-hexabenzocoronene,21 microwave irradiation, etc. have led to the formation of GQDs by a bottom-up approach. However, GQDs synthesized by all these techniques (top-down and bottom-up) are mostly hydrophilic in nature due to the presence of oxygenated functionalities on their surface. However, to date, only a few routes for the synthesis of hydrophobic graphene quantum dots have been reported. Our group has already demonstrated the synthesis of hydrophobic GQDs using the CVD technique to sense aromatic amino acids.22 Na-Jung Kuo and his coworkers have recently prepared N-doped hydrophilic and hydrophobic graphene quantum dots using a chemical exfoliation method.23 Recently, the development of a newer strategy for the synthesis of hydrophobic graphene quantum dots has become a challenge since these systems can be effectively used as efficient drug delivery systems for the transport of hydrophobic drugs.The photoluminescence of GQDs can be tuned by doping, creating defects, and tuning of its size, shape, attached chemical functionalities, etc.24 These factors also affect the PL of GQD excitation-dependent/independent behavior, but the exact origin of this is still unclear. Tunable photoluminescence is necessary to use GQDs in optoelectronic devices. Jin et al. have reported that charge transfer (CT) mechanism of functional groups is also responsible for tuning of the PL of graphene quantum dots.25 Again, there is a recent report by Wang et al. stating that functional groups play a dual role in the overall emission of GQDs.26 For N-doped GQDs, C–N configuration also plays an important role in the luminescence properties of GQDs prepared by the hydrothermal process, as demonstrated by Permatasari and his co-workers.27 Hwang and his group prepared hexafluorohydroxypropanyl benzene-functionalized GQDs via the diazonium coupling reaction and demonstrated these GQDs as a new PL sensory probe.28
In this study, we have synthesized GQDs by chemical exfoliation (top-down technique) of graphite nanopowder already employed for the synthesis of graphene oxide.29,30 The presence of oxygenated functional moieties makes the GQDs highly hydrophilic. We then successfully functionalized these GQDs covalently with dodecyl amine (DDA) and reduced them with glycine to introduce hydrophobicity on the GQDs surface. Herein, glycine plays a dual role as a chemical functionalizer and reducing agent. Interestingly, after covalent modification, it is observed that wettability (hydrophilic to hydrophobic), as well as photoluminescence (green to blue) of GQDs, can be tuned. Thus, we can tune the dual properties, such as wettability and photoluminescence, of GQDs by simple covalent modification with long-chain alkyl (C12H27) groups.
2 Experimental
2.1 Materials
All the chemicals and solvents were used as received without further purification. Graphite nanopowder and dodecyl amine (C12H27N) were purchased from SRL, India. Sulphuric acid (H2SO4), nitric acid (HNO3), glycine (NH2–CH2–COOH), n-hexane (C6H14), toluene (C6H5–CH3), and sodium hydroxide (NaOH) were purchased from Merck, India.2.2 Preparation of hydrophilic (GQDs) and hydrophobic graphene quantum dots (C12-GQDs)
The hydrophilic graphene quantum dots were prepared by a simple chemical oxidation and exfoliation technique. In this method, 50 ml dispersion of graphite nanoplatelets was prepared by dispersing 0.01 g of commercially available graphene nanoplatelets in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acid mixture of concentrated H2SO4 (98 wt%) and HNO3 (70 wt%). The mixture was then sonicated in a water bath for 24 hours. The sonicated dispersion was then diluted by adding 100 ml of deionized water. The diluted dispersion was then centrifuged at 2500 rpm for 45 minutes at room temperature to separate the lighter exfoliated sheets. The supernatant solution was then separated out from the heavier particles, and it showed green fluorescence.28 This dispersion contained smaller particles of exfoliated graphene oxide, and it was hydrophilic in nature.
1 acid mixture of concentrated H2SO4 (98 wt%) and HNO3 (70 wt%). The mixture was then sonicated in a water bath for 24 hours. The sonicated dispersion was then diluted by adding 100 ml of deionized water. The diluted dispersion was then centrifuged at 2500 rpm for 45 minutes at room temperature to separate the lighter exfoliated sheets. The supernatant solution was then separated out from the heavier particles, and it showed green fluorescence.28 This dispersion contained smaller particles of exfoliated graphene oxide, and it was hydrophilic in nature.
The as-prepared GO was used as a starting material for the preparation of hydrophobic GQDs. Briefly, 0.002 g of GO in 40 ml of organic solvents (e.g., hexane and toluene) was subjected to ultrasonication for half an hour. Another dispersion of 0.001 g of dodecyl amine (DDA) was prepared in 25 ml of n-hexane solution.31,32 Then, this dispersion of DDA was mixed with the ultrasonicated GO dispersion, and 0.0003 g of glycine was simultaneously added. Herein, glycine acts as a chemical functionalizer and reducing agent of GQDs. The mixture was then stirred mechanically for 45 minutes. Then, it was refluxed at 120 °C for 10 hours. The solution was then centrifuged at 7000 rpm for 7 minutes at room temperature. The supernatant dispersion was obtained for further use and characterization. This dispersion contains alkyl (–C12H27)-functionalized graphene quantum dots, which are hydrophobic in nature and show blue fluorescence under a UV lamp.
2.3 Characterization
The characterization of the synthesized GQDs and C12-GQDs was carried out by UV–visible absorption spectroscopy (Shimadzu, UV-2600), Fourier transform infrared spectroscopy (Nicolet 6700), scanning electron microscopy (Carl Zeiss Sigma-VP), and transmission electron microscopy (JEM 2100). Particle size and zeta potential measurement were performed using dynamic light scattering (Malvern Zetasizer Nano series, Nano ZS90). Fluorescence spectra were obtained using the Jasco spectrofluorometer FP-8300. The Optics Technology UV lamp (365 nm) was used to check the fluorescence intensity of the prepared GQDs. Powder XRD spectra of the GQDs were obtained using a Bruker D8 Advance diffractometer. The static contact angles of h-GQDs were measured using a contact angle analyzer of model DSA30, KRUSS.3 Results and discussion
In this study, an attempt was made to synthesize hydrophobic graphene quantum dots using a simple chemical exfoliation and functionalization strategy. The synthesized graphene oxide is further functionalized with long-chain alkyl groups (with DDA) using a simple chemical reaction. We used a trace amount of glycine that acts as a reducing agent as well as a chemical functionalizer to GO as reported earlier.33 The synthesized alkylated GOs are further sonicated and centrifuged to synthesize C12-GQDs in different organic solvents, e.g., hexane and toluene, and are labeled as C12-GQDsHexane and C12-GQDsToluene, respectively. The synthesis of graphene oxide from commercially available graphite nanopowder and then functionalization with alkyl groups to synthesize hydrophobic graphene quantum (C12-GQDs) dots are demonstrated in Scheme 1.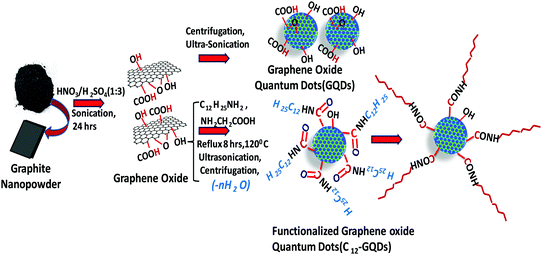 | ||
| Scheme 1 Schematic of the synthesis of hydrophilic (GQDs) and hydrophobic graphene quantum dots (C12-GQDs) from commercially available graphite nanopowder. | ||
UV-visible spectroscopy was performed for GQDs, C12-GQDsHexane, and C12-GQDsToluene, and the spectra are shown in Fig. 1A. From the figure, it is clear that for the GQDs, the main characteristic peak is at 221 nm (π–π* transition of the aromatic C–C bond), and one shoulder peak at 305 nm responsible for the n–π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O originates due to oxidation.34 However, after covalent modification with DDA due to alkylation, the 305 nm peak is shifted to 284 nm, and it becomes more prominent. This peak is due to the n–π* transition of the C–N bond, which is formed after functionalization. Interestingly, the π–π* transition peak at 221 nm drastically drops and shifts to 226 nm for C12-GQDsHexane and C12-GQDsToluene because after covalent functionalization, the long-chain bulky alkyl group (–C12 H27) is attached on the surface of GQDs and hence interferes and disrupts the conjugation of GQDs.
O originates due to oxidation.34 However, after covalent modification with DDA due to alkylation, the 305 nm peak is shifted to 284 nm, and it becomes more prominent. This peak is due to the n–π* transition of the C–N bond, which is formed after functionalization. Interestingly, the π–π* transition peak at 221 nm drastically drops and shifts to 226 nm for C12-GQDsHexane and C12-GQDsToluene because after covalent functionalization, the long-chain bulky alkyl group (–C12 H27) is attached on the surface of GQDs and hence interferes and disrupts the conjugation of GQDs.
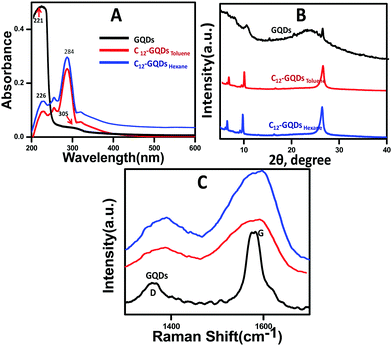 | ||
| Fig. 1 (A) UV-vis Spectra, (B) powder XRD patterns, and (C) Raman spectra of GQDs, C12-GQDsHexane, and C12-GQDsToluene. | ||
Powder XRD analysis of GQDs, C12-GQDsHexane, and C12-GQDsToluene is shown in Fig. 1B. The GQDs show a weak diffraction peak at 2θ = 11.2° (d-spacing approximately 0.79 nm). The larger interlayer spacing for GQDs as compared to that of graphene (0.34 nm) is due to the presence of oxygenated moieties in the basal plane, as depicted in the figure. A new diffraction peak at 2θ = 5.5° (interlayer spacing (d) of approximately 1.6 nm) indicates the attachment of dodecyl (C12H27) groups to the GQD basal plane.35 In the case of GQDs, the 2θ in between 25° and 30° indicates the structure of a graphitic crystal as we have synthesized GQDs from commercially available graphite nanopowder. The peaks may be broad or small depending on the amount of oxygen-containing moieties or other defects on the GQD surface.36
Raman spectroscopy analysis of GQDs, C12-GQDsHexane, and C12-GQDsToluene was carried out, and the results are depicted in Fig. 1C. As shown in Fig. 1C, we studied the D and G band of GQDs and alkylated GQDs. A sharp existing band of GQDs near 1580 cm−l (G-band) is for the E2g vibrational mode of sp2-bonded carbon of the graphitic structure. There was also a weak band near 1360 cm−1 (D-band) due to the presence of oxygenated functionalities in the carbon network (sp3 hybridized) that added disorder on the GQD surface.37 The ID/IG ratio (amount of disorder) is calculated for GQDs, C12-GQDsHexane, and C12-GQDsToluene and tabulated in Table 1. It is evident from Table 1 that after covalent functionalization of GQDs, ID/IG ratio increases from 0.52 to 0.82 and 0.86 (C12-GQDsHexane, and C12-GQDsToluene), and D and G bands are shifted to a longer wavenumber; this is attributed to the attachment of the long-chain alkyl group to the basal plane of GQDs that makes the system more disordered (localized sp3 defects). Raman spectra show good agreement with the XRD patterns, which provide indirect evidence of functionalization of GQDs.
| Sample name | D-band (cm−1) | G-band (cm−1) | I D/IG |
|---|---|---|---|
| GQDs | 1360 | 1582 | 0.52 |
| C12-GQDsHexane | 1382 | 1585 | 0.82 |
| C12-GQDsToluene | 1379 | 1590 | 0.86 |
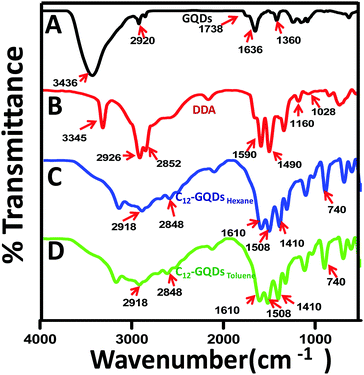
FTIR spectrum for GQDs is depicted in Fig. 2A. For GQDs, the prominent peaks are due to the presence of oxygenated functionalities after oxidation of the graphite nanopowder. The main peaks appeared at 3436 cm−1, 1738 cm−1, 1636 cm−1, and 1360 cm−1. The assignment of the peaks has been carried out as reported earlier as the peak near 3436 cm−1 (–OH stretching vibrations), peak at 1738 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations), peak near 1636 cm−1 (C
O stretching vibrations), peak near 1636 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations) from unoxidized sp2 domains of C–C bonds, and peak at 1360 cm−1 (C–O stretching vibrations). DDA had different characteristic peaks in the FTIR spectrum, as shown in Fig. 2B.38 The peaks at 2926 cm−1 and 2852 cm−1 are mainly responsible for –CH2 stretching vibration. The asymmetric and symmetric stretching vibration of NH2 is at 3345 cm−1. The peak in the range from 1590 and 3345 cm−1 to 1490 cm−1 may be due to the bending mode of vibration of NH2. The C–N stretching vibration peak is from around 1160 cm−1 to 1028 cm−1. After covalent modification of GQDs with DDA in hexane and toluene (C12-GQDsHexane and C12-GQDsToluene), some peaks disappeared, and some new peaks became prominent; however, interestingly, in both solvents, the same FTIR characteristic peaks were observed. The OH vibration peaks of GQDs (at 3436 cm−1) are not seen in C12-GQDs; this indicates that the –OH functionality of GQDs is gradually removed after reduction and functionalization. The band of GQD at 1738 cm−1 vanishes, and a new band appears at 1610 cm−1, which supports the asymmetric stretching vibration mode of carboxylic acid salt (COO–). In addition, a few new bands due to –CH2 groups are observed at 2918 cm−1, 2848 cm−1, and 740 cm−1. Another prominent peak appears at 1410 cm−1 due to C–N stretching vibration of amide in the C12-GQDs.39
C stretching vibrations) from unoxidized sp2 domains of C–C bonds, and peak at 1360 cm−1 (C–O stretching vibrations). DDA had different characteristic peaks in the FTIR spectrum, as shown in Fig. 2B.38 The peaks at 2926 cm−1 and 2852 cm−1 are mainly responsible for –CH2 stretching vibration. The asymmetric and symmetric stretching vibration of NH2 is at 3345 cm−1. The peak in the range from 1590 and 3345 cm−1 to 1490 cm−1 may be due to the bending mode of vibration of NH2. The C–N stretching vibration peak is from around 1160 cm−1 to 1028 cm−1. After covalent modification of GQDs with DDA in hexane and toluene (C12-GQDsHexane and C12-GQDsToluene), some peaks disappeared, and some new peaks became prominent; however, interestingly, in both solvents, the same FTIR characteristic peaks were observed. The OH vibration peaks of GQDs (at 3436 cm−1) are not seen in C12-GQDs; this indicates that the –OH functionality of GQDs is gradually removed after reduction and functionalization. The band of GQD at 1738 cm−1 vanishes, and a new band appears at 1610 cm−1, which supports the asymmetric stretching vibration mode of carboxylic acid salt (COO–). In addition, a few new bands due to –CH2 groups are observed at 2918 cm−1, 2848 cm−1, and 740 cm−1. Another prominent peak appears at 1410 cm−1 due to C–N stretching vibration of amide in the C12-GQDs.39
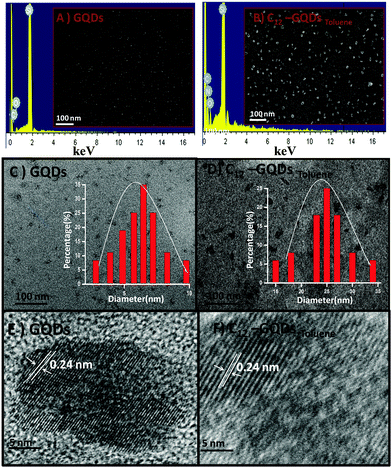
Surface morphology of chemically prepared GQDs was investigated using SEM microscopy images. Representative SEM images and EDX spectra of GQDs and C12-GQDsToluene are depicted in Fig. 3A and B. From SEM images, it is clear that GQDs form a homogeneous dispersion, and their average size is below 10 nm. However, for functionalized GQDs (C12-GQDsToluene), the average size becomes larger; this is due to attachment of an alkyl chain (–C12H27) on the GQD surface. The successful functionalization of GQDs is also confirmed by energy-dispersive X-ray spectroscopy (EDX) (Fig. 3A and B). From EDX, it is confirmed that modification with DDA and glycine introduces N into the GQD surface, as well as the amount of oxygenated groups is also reduced. The spectrum (Fig. 3B) clearly shows the presence of the elements C, O, and N, and the corresponding weight percentages of these elements in GQDs and C12-GQDsToluene are shown in Table 2. Transmission electron microscopic (TEM) studies were also carried out on GQDs and C12-GQDsToluene. The TEM images obtained also supported the SEM image, and clearly showed that the size was below 10 nm (Fig. 3C). In addition, after functionalization, the size of GQDs increased (Fig. 3D). From the size distribution curve, the average sizes of GQDs and C12-GQDsToluene are found to be 6.5 nm and 24.5 nm (Fig. 3C and D), respectively. We also investigated the average sizes of GQDs, and C12-GQDsToluene using the DLS technique, as demonstrated in Fig. S1A and B (ESI†). The DLS size also supports the successful functionalization. The average size of GQDs is less than 10 nm, but for C12-GQDsToluene, the average size becomes greater. It should be noted that the SEM, DLS, and TEM results are in agreement with each other.
| Sample name | C (W%) | O (W%) | N (W%) | Si (W%) from substrate |
|---|---|---|---|---|
| GQDs | 6.98 | 4.10 | — | 88.92 |
| C12-GQDsToluene | 19.92 | 3.96 | 2.27 | 73.85 |
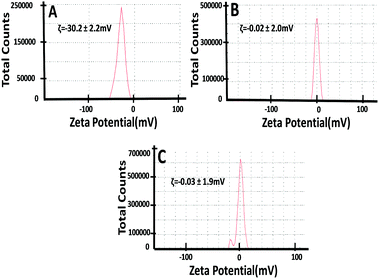
The surface charges of GQDs and functionalized GQDs (C12-GQDsHexane and C12-GQDsToluene) are measured using the DLS technique and depicted in Fig. 4. The zeta potential value of GQDs dispersion was highly negative (ζ = −30.2 ± 2.2 mV) due to the presence of ample surface oxygen functionalities such as hydroxyls, epoxides, and carbonyls (Fig. 4A). However, after functionalization (Fig. 4B and C), the surface charge becomes less negative (for C12-GQDsHexaneζ = −0.02 ± 2.0 mV and C12-GQDsTolueneζ = −0.03 ± 1.9 mV); this indicates reduction of oxygenated functional groups and alkylation of GQDs. This evidence supports the successful functionalization of GQDs.
The wettability of GQDs, C12-GQDsHexane, and C12-GQDsToluene was measured by calculating the contact angles at the water–air interface, as shown in Fig. 5. For this purpose, the bare glass substrates are coated with our synthesized GQDs and C12-GQDs. The contact angle for GQDs is 34° (Fig. 5B), which implies that the high hydrophilicity is due to the presence of highly hydrophilic oxygenated functional groups. On the other hand, the contact angle of alkyl functionalized GQDs (C12-GQDsHexane) is found to be 97° (Fig. 5C), and that of C12-GQDsToluene is 98° (Fig. 5D), which are notably larger than that of GQDs as well as a bare glass substrate. Improvement in contact angles suggests that functionalization and reduction of graphene quantum dots with the dodecyl (C12H27) group is successful, and this functionality is responsible for hydrophobicity in the GQDs. In this study, we introduced long-chain alkyl groups through covalent modification and simultaneously reduced the oxygenated GQDs using glycine. Herein, glycine acts as a reducing agent and a chemical functionalizer. As the long-chain alkyl groups are hydrophobic, after covalent modification, GQD surface is passivated and introduces hydrophobicity in GQDs. This is the reason that we are able to obtain contact angles greater than 90° (hydrophobic). However, we have not achieved superhydrophobic GQDs as some unreacted oxygenated groups may remain on the GQD surface after covalent modification. However, via only simple thermal reduction, it is not easy to obtain hydrophobic GQDs. Contact angles may increase as compared to those of oxygenated GQDs, but they are not sufficient to obtain hydrophobic GQDs.
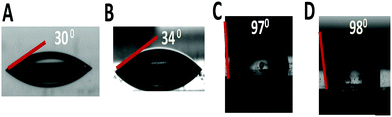 | ||
| Fig. 5 Water static contact angle measurement on (A) bare glass, (B) GQD-coated, (C) C12-GQDsHexane, and (D) C12-GQDsToluene-coated glass substrates. | ||
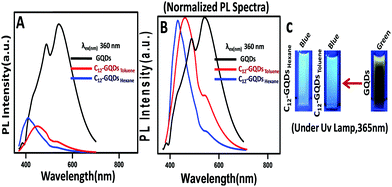
The PL property of GQDs, C12-GQDsHexane, and C12-GQDsToluene was studied, and the results are shown in Fig. 6. The emission spectra were obtained at different excitation wavelengths ranging from 330 nm to 440 nm. The GQDs interestingly show an excitation-independent PL property, as shown in some of the earlier reports (Fig. 6A).40 For GQDs, the maximum emission is found at 550 nm at an excitation wavelength of 360 nm. On the other hand, C12-GQDs (Fig. 6B and C) show an excitation-dependent PL behavior. The excitation wavelength-dependent or independent behavior of PL mainly originates from surface defects, disruption of symmetry, quantum size effects, and particle size distribution leads to different emissive states, zigzag sites, solvent relaxation, etc. However, the exact mechanism is not fully explored.41,42 The quantum yield (φ) of GQDs, C12-GQDsHexane, and C12-GQDsToluene is also calculated and found to be 11.76%, 16.17%, and 18.10%, respectively. The procedure used to determine the quantum yield of GQDs, C12-GQDsHexane, and C12-GQDsToluene is given in the ESI† (Fig. S2–S4).
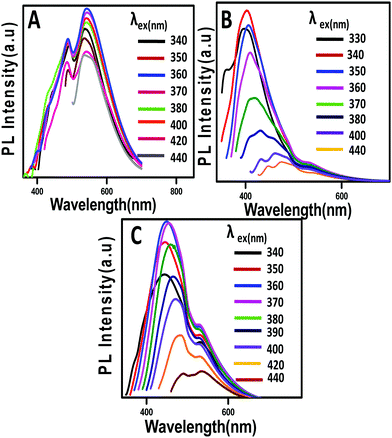 | ||
| Fig. 6 Stacked PL emission spectra of (A) GQDs, (B) C12-GQDsHexane, and (C) C12-GQDsToluene excited at different wavelengths. | ||
The stacked PL emission spectra of C12-GQDsHexane and C12-GQDsToluene show an initial increase in emission intensity and shifting to the larger wavelength in the excitation wavelength range from 330 nm to 440 nm. It was observed that emission spectra attained a maximum value (λexmax) and a gradual decrease in emission intensity showing an excitation-dependent PL behavior. The maximum emission values for C12-GQDsHexane and C12-GQDsToluene are observed at the excitation wavelengths of 340 nm and 360 nm, respectively. The GQDs emission maximum is observed at 550 nm, but interestingly, for C12-GQDsHexane and C12-GQDsToluene, emission is found at 415 nm and 445 nm, respectively. This change in the emission spectrum upon functionalization is also reflected in the color of the GQDs, C12-GQDsHexane, and C12-GQDsToluene under a UV lamp. The GQDs show green fluorescence under UV light. However, after functionalization with alkyl groups, C12-GQDsHexane, and C12-GQDsToluene show bluish fluorescence under UV light. The images of GQDs, C12-GQDsHexane, and the C12-GQDsToluene solution are depicted in Fig. 7C.
The PL properties of the synthesized GQDs, C12-GQDsHexane, and C12-GQDsToluene are also investigated at different pH values (2, 5, 7, 10, and 12) at a common excitation wavelength of 360 nm, as shown in Fig. 8. GQDs show pH-dependent PL behavior, and the shifting of PL spectra while changing the pH of the GQDs is shown. At a pH of 10 and 12, maximum red shifting of emission spectra is observed. The reason may be deprotonation of carboxylic acid groups (COO−) of GQDs as well as the presence of electronic charge on the carboxylic acid groups, as reported by Jin et al.25 On the other hand, at a pH of 2, the carboxylic acid group in the GQDs is very hard to protonate. Therefore, the shifting of the PL spectra of GQDs at a pH of 2 is minimum as compared to that at the pH values of 10 and 12. However, interestingly, the alkyl-functionalized GQDs (C12-GQDsHexane and C12-GQDsToluene) show a pH-independent PL property, as shown in Fig. 8B and C. In this case, COOH groups are functionalized with CONHC12H25 (GQD surfaces are passivated by long-chain alkyl groups). Therefore, there are fewer chances of deprotonation or protonation changing the pH value of C12-GQDs.
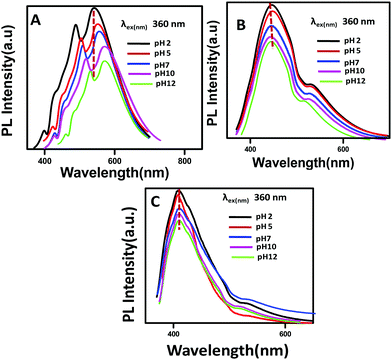 | ||
| Fig. 8 Stacked PL emission spectra of (A) GQDs, (B) C12-GQDsHexane, and (C) C12-GQDsToluene at pH 2, 5, 7, 10, and 12 (at an excitation wavelength of 360 nm). | ||
4 Mechanism
We have tried to explain the observed PL property of GQDs and functionalized GQDs (C12-GQDs), and the proposed PL mechanism is depicted in Scheme 2. The various mechanisms have been put forward by different research groups to understand the GQDs PL behavior and its origin. The different shapes, sizes, zig zag edges, surface functionalities, and photo-induced electron transfer mechanism of GQDs are mainly responsible for its bright photoluminescence.43 However, the real fact remains that the origin of GQDs PL is not yet fully known and understood.In this study, we synthesized graphene quantum dots from graphite nanopowder by a simple chemical exfoliation and oxidation strategy, and the prepared GQDs show green fluorescence under exposure to a UV lamp (365 nm). There are several reports in which the probable cause of the green fluorescence of GQDs has been discussed. The oxygenated functional groups on the GQD surface act as the non-radiative photoluminescence centers, and it causes green fluorescence.
Santiago and his co-workers show that delocalized extrinsic states originating on the basal plane of GQDs due to oxidation are also responsible for green photoluminescence.44 GQDs upon functionalization with DDA and reduction with glycine result in bluish fluorescence under exposure to a UV lamp. The change in fluorescence and shifting of the bandgap to a higher energy region upon alkylation of GQDs with DDA may be due to passivation of the GQD surface with a long-chain alkyl group, and glycine removes the remaining oxygenated functional groups. Again, GQD modification with DDA and glycine introduces N into the GQD surface. N-Doping has a direct impact on the band gap of GQDs, resulting in PL tuning. The tuning of fluorescence may be due to charge transfer of N-doped functional groups of GQDs, as reported by Jin et al.25 Again, tuning of emission of N-doped GQDs is reported by Ain et al. They showed that for N-doped GQD, there were more than one recombination pathways for the charge carriers, and this might have an effect on the PL.45
5 Conclusions
In summary, we demonstrated the synthesis of hydrophobic graphene quantum dots (C12-GQDs) using a simple chemical exfoliation process. At first, we have synthesized hydrophilic GQDs and then functionalized them covalently with a dodecyl amine (DDA) and simultaneously reduced them with glycine to synthesize the hydrophobic GQDs. Our functionalization method is simple, cost-effective, and easy. It is observed that the prepared C12-GQDs when coated on the glass substrate have the contact angles of 97° (C12-GQDsHexane) and 98° (C12-GQDsToluene). Hence, the wettability can be changed from GQDs (hydrophilic) to C12-GQDs (hydrophobic). Moreover, the photoluminescence property of GQDs (green fluorescence) changes after covalent modification (blue fluorescence). Thus, we have successfully modified the dual properties of GQDs (wettability and photoluminescence) by a simple chemical functionalization process. Thus, this material can be used in optoelectronics and as a hydrophobic fluorescence coating material.Conflicts of interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the study.Acknowledgements
The authors thank SERB, New Delhi, Grant No. SB/S1/PC-69/2012 and BRNS, Mumbai, Grant No. 34/14/20/2014-BRNS. MJD wants to thank SERB for the fellowship.References
- Z. Zhang, J. Zhang, N. Chen and L. Qu, Energy Environ. Sci., 2012, 5, 8869 CAS.
- J. Shen, Y. Zhu, C. Chen, X. Yang and C. Li, Chem. Commun., 2011, 47, 2580 RSC.
- Y. Li, Y. Zhao, H. Cheng, Y. Hu, G. Shi, L. Dai and L. Qu, J. Am. Chem. Soc., 2012, 134, 15 CrossRef CAS PubMed.
- S. Zhu, Y. Song, J. Wang, H. Wan, Y. Zhang, Y. Ning and B. Yang, Nano Today, 2017, 13, 10 CrossRef CAS.
- Z. Huang, J. Qu, X. Peng, W. Liu, K. Zhang, X. Wei and J. Zhong, Phys. Status Solidi RRL, 2014, 8, 436 CrossRef CAS.
- Y. Chong, Y. Ma, H. Shen, X. Tu, X. Zhou, J. Xu, J. Dai, S. Fan and Z. Zhang, Biomaterials, 2014, 35, 5041 CrossRef CAS PubMed.
- Z. Fei, Z. Li, L. Wang, C. Hu, Z. Zhang, C. Li and L. Qu, Chem. Commun., 2015, 51, 13201 RSC.
- M. K. Kumawat, M. Thakur, R. B. Gurung and R. Srivastava, ACS Sustainable Chem. Eng., 2017, 5, 1382 CrossRef CAS.
- F. Shi, Y. Zhang, W. Na, X. Zhang, Y. Li and X. Su, J. Mater. Chem. B, 2016, 4, 3278 RSC.
- A. T. Çolak, T. Eren, M. L. Yola, E. Beşli, O. Şahin and N. Atar, J. Electrochem. Soc., 2016, 163, 1237 CrossRef.
- D. Iannazzo, A. Pistone, M. Salamò, S. Galvagno, R. Romeo, S. V. Giofré, C. Branca, G. Visalli and A. D. Pietro, Int. J. Pharm., 2017, 518, 185 CrossRef CAS PubMed.
- Q. Lu, Y. Zhang and S. Liu, J. Mater. Chem. A, 2015, 3, 8552 CAS.
- V. Gupta, N. Chaudhary, R. Srivastava, G. D. Sharma, R. Bhardwaj and S. Chand, J. Am. Chem. Soc., 2011, 133, 9960 CrossRef CAS PubMed.
- Y. Li, Y. Hu, Y. Zhao, G. Shi, L. Deng, Y. Hou and L. Qu, Adv. Mater., 2011, 23, 776 CrossRef CAS PubMed.
- Y. Qin, Y. Cheng, L. Jiang, X. Jin, M. Li, X. Luo, G. Liao, T. Wei and Q. Li, ACS Sustainable Chem. Eng., 2015, 3, 637 CrossRef CAS.
- C. Hu, L. Song, Z. Zhang, N. Chen, Z. Feng and L. Qu, Energy Environ. Sci., 2015, 8, 31 CAS.
- D. Pan, J. Zhang, Z. Li and M. Wu, Adv. Mater., 2010, 22, 734 CrossRef CAS PubMed.
- L. Lu, Y. Zhu, C. Shi and Y. T. Pei, Carbon, 2016, 109, 373 CrossRef CAS.
- C. K. Chua, Z. Sofer, P. Simek, O. Jankovsky, K. Klímova, S. Bakardjieva, S. H. Kuckova and M. Pumera, ACS Nano, 2015, 9, 2548 CrossRef CAS PubMed.
- M. Zhou, Z. Zhou, A. Gong, Y. Zhang and Q. Li, Talanta, 2015, 143, 107 CrossRef CAS PubMed.
- R. Liu, D. Wu, X. Feng and K. Mullen, J. Am. Chem. Soc., 2011, 133, 15221 CrossRef CAS PubMed.
- M. J. Deka and D. Chowdhury, ChemistrySelect, 2017, 2, 1999 CrossRef CAS.
- N.-J. Kuo, Y.-S. Chen, C.-W. Wu, C.-Y. Huang, Y.-H. Chan, I-W.P. Chen and P. Chen, Sci. Rep., 2016, 6, 1 CrossRef PubMed.
- M. A. Sk, A. Ananthanarayanan, L. Huang, K. H. Lim and P. Chen, J. Mater. Chem. C, 2014, 2, 6954 RSC.
- S. H. Jin, D. H. Kim, G. H. Jun, S. H. Hong and S. Jeon, ACS Nano, 2013, 7, 1239 CrossRef CAS PubMed.
- S. Wang, I. S. Cole, D. Zhao and Q. Li, Nanoscale, 2016, 8, 7449 RSC.
- F. A. Permatasari, A. H. Aimon, F. Iskandar, T. Ogi and K. Okuyama, Sci. Rep., 2016, 6, 21042 CrossRef CAS PubMed.
- E. Hwang, H. M. Hwang, Y. Shin, Y. Yoon, H. Lee, J. Yang, S. Bak and H. Lee, Sci. Rep., 2016, 6, 1 CrossRef PubMed.
- M. J. Deka and D. Chowdhury, J. Phys. Chem. C, 2016, 120, 4121 CAS.
- M. J. Deka, U. Baruah and D. Chowdhury, Mater. Chem. Phys., 2015, 163, 236 CrossRef CAS.
- H. P. Mungse, N. Kumar and O. P. Khatri, RSC Adv., 2015, 5, 25565 RSC.
- J. Jang, V. H. Pham, B. Rajagopalan, S. H. Hur and J. S. Chung, Nanoscale Res. Lett., 2014, 9, 265 CrossRef PubMed.
- S. Bose, T. Kuila, A. K. Mishra, N. H. Kim and J. H. Lee, J. Mater. Chem., 2012, 22, 9696 RSC.
- U. Baruah and D. Chowdhury, Nanotechnology, 2016, 27, 145501 CrossRef PubMed.
- C. Wang, Z. Liu, S. Wang and Y. Zhang, J. Appl. Polym. Sci., 2016, 42907, 1 Search PubMed.
- B. Zhang, C. Xiao, Y. Xiang, B. Dong, S. Ding and Y. Tang, ChemElectroChem, 2016, 3, 864 CrossRef CAS.
- R. Yan, H. Wu, Q. Zheng, J. Wang, J. Huang, K. Ding, Q. Guo and J. Wang, RSC Adv., 2014, 4, 23097 RSC.
- H. Yuehua, S. Wei, J. Hao, J. D. Miller and F. A. Keqing, Int. J. Miner. Process., 2005, 76, 163 CrossRef.
- S.-P. Zhang and H.-O. Song, New J. Chem., 2012, 36, 1733 RSC.
- S. Chen, X. Hai, C. Xia, X.-W. Chen and J.-H. Wang, Chem. – Eur. J., 2013, 19, 15918 CrossRef CAS PubMed.
- J. Wang, S. Cao, Y. Ding, F. Ma, W. Lu and M. Sun, Sci. Rep., 2016, 6, 24850 CrossRef CAS PubMed.
- L. Wang, S.-J. Zhu, H.-Y. Wang, S.-N. Qu, Y.-L. Zhang, J.-H. Zhang, Q.-D. Chen, H.-L. Xu, W. Han, B. Yang and H.-B. Sun, ACS Nano, 2014, 8, 2541 CrossRef CAS PubMed.
- Z. Wang, H. Zeng and L. Sun, J. Mater. Chem. C, 2015, 3, 1157 RSC.
- S. R. M. Santiago, T. N. Lin, C. T. Yuan, J. L. Shen, H. Y. Huang and C. A. J. Lin, Phys. Chem. Chem. Phys., 2016, 18, 22599 RSC.
- Noor-Ul-Ain, M. O. Eriksson, S. Schmidt, M. Asghar, P.-C. Lin, P. O. Holtz, M. Syväjärvi and G. Reza Yazdi, Nanomaterials, 2016, 6, 198 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7nj03280c |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 |