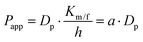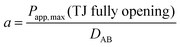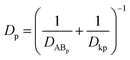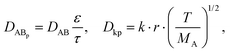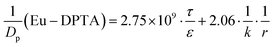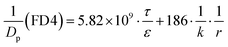Zinc ions regulate opening of tight junction favouring efflux of macromolecules via the GSK3β/snail-mediated pathway
Ruyue
Xiao
a,
Lan
Yuan
a,
Weijiang
He
 b and
Xiaoda
Yang
b and
Xiaoda
Yang
 *a
*a
aState Key laboratories of Natural and Mimetic Drugs and Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University Health Science Center, Beijing 100191, China. E-mail: xyang@bjmu.edu.cn; Fax: +86-10-62015584; Tel: +86-10-82805611
bState Key Laboratory of Coordination Chemistry, Coordination Chemistry Institute, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, P. R. China
First published on 13th December 2017
Abstract
Zinc is an essential trace element presenting in particularly high concentration in the brain. In some regions, e.g. lateral amygdala, subiculum and hippocampus, rapidly-exchangeable zinc may transiently reach even up to 600 μM. To explore the possible roles of high-concentration Zn2+ in regulating the blood–brain barrier (BBB), we investigated the effects of Zn2+ on the functions and structures of the tight junction (TJ) with an in vitro model of a Madin-Darby canine kidney (MDCK) cell monolayer. The experimental results indicated that high concentrations (>200 μM) of Zn2+ can affect the TJ integrity in a polarized manner. Basolateral addition of Zn2+ led to reversible TJ opening with pore paths of r ∼ 2 nm or more depending on Zn2+ concentration. The efflux/influx ratios of different sized probes were found to be ∼4.6 for FD4 (MW 4000) and ∼1.8 for Eu–DTPA (MW 560), suggesting that the Zn2+-induced paracelluar channels favour efflux especially for macromolecules. Further mechanistic studies revealed that the elevated intracellular Zn2+ taken from the basolateral side can increase phosphorylation of glycogen synthase kinase (GSK) 3β, primarily due to the inhibition of calcineurin (CaN), thus resulting in the elevation of the snail transcriptional repressors. Subsequently, Zn2+ can cause the down-regulation of claudin-1, breakage of occludin and ZO-1 rings, and collapse of basolateral F-actin structures. These overall factors result in the formation of a trumpet-like paracellular channel, which allows asymmetric solute permeation. The ERK1/2 and JNK1/2 pathways may also be involved in the Zn2+-induced TJ opening process, while the activation of matrix metalloproteinase was not observed. Our results may suggest a potential role of zinc in regulation of BBB permeability associated with brain clearance of metabolites through the glymphatic system.
Significance to metallomicsZinc is a well-known essential element with some as-yet-unclear biological functions. The present work investigated the biological significance of high concentrations of Zn2+ for the tight junction (TJ), which is a critical structural element of the blood–brain barrier. Our results showed that high concentrations (>200 μM) of zinc caused reversible TJ opening in a polarized manner through the GSK3β/snail-mediated pathway. Zn2+-Induced asymmetric paracellular pore paths favour the efflux of molecules, especially macromolecules. The present work may indicate a new role of Zn2+ in regulating BBB permeability and potential role of Zn2+ in brain clearance of metabolites through the glymphatic system. |
Introduction
As an essential micro-nutrient, zinc (Zn) is very important in a variety of biological processes due to its indispensable catalytic, structural and regulatory functions. The roles and the underlying mechanisms of Zn in biological systems have fascinated researchers for decades.1 With significant abundance in the brain, zinc has the second highest concentration after iron among all transition metals. However, the exact concentration of free Zn2+ in the brain is controversial.2–9 While some researchers reported relatively low concentrations of Zn (nanomolar level),2–4 others showed evidence of higher concentrations of Zn (more than 100 μM).5–9 Some cytoarchitectonic regions of the brain, for example the neuropil of the lateral amygdala, subiculum, and the mossy fiber neuropil of the hippocampus, are speculated to contain 200–600 μM of mobile Zn.2,5–8 During neuronal activity, Zn2+ ions are released into the synaptic cleft, resulting in transient local Zn2+ concentrations in the range of 100–300 μM.9 In certain pathological conditions, such as brain ischemia and reperfusion injury, a significant and sustained increase of extracellular Zn2+ concentration has been observed.2,10 Overall, the biological significance of the high concentration of Zn2+ in the brain has so far remained mysterious;11 in particular, it is of interest to establish how temporarily high levels of Zn2+ would affect the structure and functions of the blood–brain barrier (BBB).It is well described that the BBB controls the flux and transport of liquids, ions and larger solutes into and out of the central nervous system (CNS) and protects the CNS from toxins and pathogens.12 The tight junction (TJ) between endothelial cells of brain capillaries is one of the most important structural elements of the BBB.13 Structurally, the TJ is an intricate complex consisting of transmembrane proteins and cytoplasmic accessory proteins primarily including claudins, occludin, zonula occludens (ZO) proteins and junctional adhesion molecules (JAMs).14 These TJ proteins are linked to an actin-based cytoskeleton, allowing the formation of TJ seals.15 Alterations in TJ structures and functions are associated with many diseases16,17 and metal toxicities.18–21 Previous studies indicated that low concentrations of Zn2+ strengthened the TJ,22–26 for example, 100 μM of Zn2+ enhanced the TJ functions that operate through the PI3K/AKT/mTOR signalling pathway in caco-2 cells.24 Nevertheless, considering the apico-basal polarization of epithelial cells and potential site-dependent action of Zn2+,27–30 it is appropriate to investigate the actions of Zn2+ in a bilateral manner, particularly at higher concentrations (100–300 μM).
In the present work, we investigated the effects of Zn2+ on the TJ. The experimental results showed that Zn2+ can cause alteration of TJ structures and functions in a polarized manner, and >200 μM concentrations of Zn2+ could increase the paracellular permeation favouring efflux of macromolecules via the GSK3β/snail-mediated pathway. Our results may suggest a potential role of zinc in regulation of BBB permeability associated with brain clearance of metabolites through the glymphatic system.
Materials and methods
Materials
Madin-Darby canine kidney (MDCK) cells were obtained from the Cell Culture Center of Peking Union Medical College, high glucose Dulbecco's modified Eagle's medium (DMEM) was from HyClone, penicillin–streptomycin was from Gibco (USA), fetal bovine serum (FBS) was from PAN (Germany), and Transwells (12 wells, pore diameter of 3 μm, polycarbonate) were from Corning Costar (USA). The MTS tetrazolium compound was from Promega (USA). All the other reagents were of analytical grade and came from commercial sources.Eu–DTPA was prepared according to the previous method.31 Briefly, 1 mL 10 mM EuCl3 solution was added to 1 mL 10 mM DTPA solution in Hank's balanced salt solution (HBSS; pH 7.0) under vigorous stirring. The mixture was centrifuged at 4000 rpm for 10 min, then the supernatant (Eu–DTPA) was collected.
Non-fluorescent DMEM (NF-DMEM) was prepared by excluding some amino acids (tryptophan, tyrosine, and phenylalanine) and vitamins (folic acid, pyridoxine hydrochloride, and riboflavin) from media preparation according to our previous method.32
MDCK cell culture and monolayer preparation
MDCK cells were cultured in high glucose DMEM supplied with 10% FBS, 1% nonessential amino acids, and penicillin–streptomycin (100 μg mL−1) at 37 °C in a 5% CO2 atmosphere. Cultured MDCK cells were used at passage 15–50 in all experiments. MDCK cell monolayers were prepared by plating the cells onto Transwell filters (aperture, 3 μm; diameter, 12 mm) at a density of 2.5 × 104 cells per well and allowing growth and differentiation for 2–3 d. The transepithelial electrical resistance (TEER) was measured with an EVOM Voltohmmeter (World Precision Instruments, USA). The cell monolayers with TEER of >200 Ω cm2 were used for later studies.Cytotoxicity assay
Cell viability was measured by MTS assay according to the manufacturer's instructions. Briefly, MDCK cells were seeded in 96-well plates at a density of 5 × 103 cells per well and left to grow for 24 h. The cells were then incubated with various concentrations of ZnSO4 (0–800 μM) diluted in DMEM with 5% FBS for 12 h. Then the cells were incubated with MTS solution for another 2 h at 37 °C. Finally, the absorbance at 490 nm was measured on a Thermo Multiskan Ascent plate reader (Thermo, USA). Cell viability was calculated as Atest/Acontrol × 100%. The 50% inhibition concentration (IC50) was calculated by plotting the cell viability versus Zn2+ concentrations and fitting the data to the Hill model using the MicroCal™ Origin program (Lab Corporation, USA).Measurement of apparent permeability coefficient (Papp) of paracellular permeation probes
MDCK cell monolayers were prepared as above. After rinsing twice with NF-DMEM, the apical (donor) chambers were supplied with 0.5 mL NF-DMEM containing 10 μM Eu–DTPA and 0.5 mM fluorescein isothiocyanate-dextran 4 kDa (FD4) while the basolateral (receiver) chambers were supplied with 1.5 mL NF-DMEM. For the bilateral flux tests, the basolateral chambers were supplied with 2 mL NF-DMEM to mimic the dynamic flux process in the brain.33,34 Samples were collected in the opposite receiver chambers after 1 h incubation. Then the Papp values of Eu–DTPA and FD4 were calculated using the following formula:| Papp = (ΔQ/Δt)/(AC0) |
The concentration of FD4 was determined by fluorescence spectrometric measurement on a Flexstation 3 microplate reader (Molecular Devices, USA) at λEx/Em = 490/525 nm. The concentration of Eu–DTPA in the samples above was determined by a time-resolved fluorescence assay. Briefly, samples were mixed with equal volumes of 2 × β-NTA fluorescence enhancement solution (30 μM β-NTA, 10 mM TOPO, 0.2% Triton X-100, and 0.1 M potassium hydrogen phthalate buffer, pH 3.0) and left at 37 °C for 1 h. Then the fluorescence intensity was measured on a Flexstation 3 microplate reader at λEx/Em = 340/616 nm with a measurement window from 600 to 1000 ms.
Calculation of TJ pore size and the retention capacity upon incubation with ZnSO4
MDCK cell monolayers were treated with various concentrations of ZnSO4 (added on the apical side or basolateral side or both sides) for 12 h. After rinsing with NF-DMEM, fluorescent probes (10 μM Eu–DTPA and 0.5 mM FD4) were added in the donor chamber and the Papp values were determined as described above.The calculation of TJ pore size and the retention capacity was performed according to the previous method.32 Briefly, the diffusion coefficient of a solute (Dp) was obtained by the following equation:
Given that the diffusion of the probes in tight junction channels is similar to that in porous medium and there is no specific interaction among the solutes, solvents and tight junction channels, the diffusion of the probes can be described by the amended Knudsen equation:
in which
where ε and τ represent the porosity and tortuosity of the porous medium, respectively; r is the radius of the pores; MA is the molecular mass of the solute. The ratio ε/τ is an overall reflection of the retention capacity of the porous medium for the solutes. Considering the size exclusion effect, the relationship between the ε/τ values of Eu–DTPA and FD4 is described as:
| (ε/τ)Eu–DTPA = (ε/τ)·10tr(Eu–DTPA)/tr(FD4) |
By assigning the size (r ∼ 4 Å) of primary TJ pores35 as the average size of sealed TJ pores, the calibration constant k was calculated to be 8.47 × 10−4. Then, the change of pore size (r) and retention capacity (ε/τ) could be calculated using the above equations.
Cellular zinc uptake
The cellular uptake of Zn2+ was measured using a Zn2+ fluorescent sensor (NBD-TPEA).36 Briefly, MDCK cell monolayers on Transwell filters were incubated with 250 μM ZnSO4 (in the apical chamber or basolateral chamber or both) in DMEM containing 5% FBS for 12 h. Then the cell monolayers were rinsed with Ca2+/Mg2+-free PBS buffer (0.0067 M) and incubated with 25 μM NBD-TPEA for 30 min at room temperature. After rinsing, the cells were observed under a confocal microscope (Leica TCS SP8 STED 3X; Leica, Wetzlar, Germany).Matrix metalloproteinase (MMP) activity assay
The MMP activity of MDCK cells upon Zn2+ treatment was measured with an Amplite™ Universal Fluorimetric MMP Activity Assay Kit (AAT Bioquest, USA) according to the manufacturer's instructions. Briefly, MDCK cells were seeded in 96-well plates at a density of 5 × 103 cells per well and incubated for 24 h. Then the cells were incubated with 0, 100, 250, 350 μM ZnSO4 in 50 μL of DMEM with 5% FBS for 12 h. Then 50 μL of MMP Green substrate solution was added. The fluorescence signal was read on a microplate reader (Thermo, USA) at λEx/Em = 490/525 nm. Cells pre-treated with 1 mM 4-aminophenylmercuric acetate (APMA) for 2 h were used as positive control.Calcineurin (CaN) activity assay
MDCK cells were seeded in 6-well plates at a density of 2 × 105 cells per well and incubated for 24 h. Then the cells were incubated with 0, 100, 250, 350 μM ZnSO4 in 2.5 mL of DMEM with 5% FBS for 12 h. After incubation with ZnSO4, the cells were lysed by lysis buffer (50 mM Tris, pH 7.5, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.2% NP-40) containing protease inhibitor cocktails and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm at 4 °C for 20 min. Then the intracellular CaN activity was measured using an assay kit (Nanjingjiancheng, China) following the manufacturer's instructions.
000 rpm at 4 °C for 20 min. Then the intracellular CaN activity was measured using an assay kit (Nanjingjiancheng, China) following the manufacturer's instructions.
Western blotting
Cells were lysed by RIPA buffer containing protease and phosphatase inhibitor cocktails and then centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm at 4 °C for 20 min. The supernatant protein concentration was determined using a BCA protein assay reagent kit (Beyotime Biotechnology, China). Samples containing 30 μg total protein were applied to an SDS-polyacrylamide gel electrophoresis instrument and the bands were transferred to polyvinylidene difluoride membranes (Millipore, USA). The membranes were blocked for 1 h with 5% skim milk, and then incubated overnight at 4 °C with the corresponding primary antibodies, i.e. antibodies against claudin-1 (Bioworld), occludin and ZO-1 (Invitrogen), p-Akt, p-ERK1/2, p-JNK1/2, and snail (Cell Signaling Technology), p-GSK3β (Abcam), and GAPDH (BioEasy). After washing with TBS containing 0.05% Tween-20, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (BioEasy, China) at room temperature for 1 h. Finally the protein bands were developed and analysed on a MiniChemi Imaging and Analysis System (Sage Creation Science Co., Ltd, China) using an ECL western blot substrate kit (Pierce, USA) according to the manufacturer's instructions.
000 rpm at 4 °C for 20 min. The supernatant protein concentration was determined using a BCA protein assay reagent kit (Beyotime Biotechnology, China). Samples containing 30 μg total protein were applied to an SDS-polyacrylamide gel electrophoresis instrument and the bands were transferred to polyvinylidene difluoride membranes (Millipore, USA). The membranes were blocked for 1 h with 5% skim milk, and then incubated overnight at 4 °C with the corresponding primary antibodies, i.e. antibodies against claudin-1 (Bioworld), occludin and ZO-1 (Invitrogen), p-Akt, p-ERK1/2, p-JNK1/2, and snail (Cell Signaling Technology), p-GSK3β (Abcam), and GAPDH (BioEasy). After washing with TBS containing 0.05% Tween-20, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (BioEasy, China) at room temperature for 1 h. Finally the protein bands were developed and analysed on a MiniChemi Imaging and Analysis System (Sage Creation Science Co., Ltd, China) using an ECL western blot substrate kit (Pierce, USA) according to the manufacturer's instructions.
RT-PCR assay for claudin-1 expression
The total RNA of the harvested MDCK cells was extracted with TransZol Up (Transgen Biotech, China) according to the manufacturer's instructions. The purity and concentration were determined by measuring the absorbance (A) at 260 nm and 280 nm (A260/A280). Then 2 μg of total RNA was reverse transcribed into the first-strand cDNA using a RevertAid First Strand cDNA Synthesis Kit (Thermo, USA) and quantitative PCR reactions were carried out using 2 × SYBR Green qPCR Mix (Aidlab, China) according to the manufacturer's protocols. Genes of interest were normalized to GAPDH expression.The following primer combinations were used: for claudin-1, F (ATGGAAGACGATGAGGTGC), R (GCAACTAAAACAGCCAGACC); for GAPDH, F (GTAGTGAAGCAGGCATCGGA), R (GTCGAAGGTGGAAGAGTGG).
Immuno-fluorescence imaging
MDCK cell monolayers were prepared on 6-well plates containing coverslips for 2–3 days. After incubation with different concentrations of ZnSO4 for 12 h, the coverslips were washed with cold PBS, fixed in 4% paraformaldehyde for 15 min at 4 °C and permeabilized with 0.1% Triton X-100 in PBS for 4 min. After blocking with PBS containing 1% bovine serum albumen (BSA) for 1 hour at room temperature, the cells were then incubated with the primary antibody in PBS containing 1% BSA solution overnight at 4 °C. After washing with PBS three times, AlexaFluor 488-conjugated goat anti-rabbit secondary antibodies in PBS were applied for 1 h at room temperature in the dark. Finally the cells were stained with DAPI (5 μg mL−1 in PBS) for 10 min at room temperature, washed with PBS and mounted with a drop of glycerol and placed on coverslips. The slides were observed on a confocal microscope (Leica TCS SP8 STED 3X; Leica, Wetzlar, Germany).Transmission electron microscopy (TEM)
MDCK cell monolayers on Transwell filters were incubated with 250 μM ZnSO4 in the basolateral chamber for 12 h. Then the cell monolayers were fixed in glutaraldehyde and treated with 1% osmium tetroxide. Samples were dehydrated with graded ethanol and propylene oxide, and embedded in pure epoxy resin. Ultrathin sections were stained with uranyl acetate, and observed in a JEM 1400 transmission electron microscope (JEOL, Japan).Statistical analysis
All the experiments were repeated at least three times. Results are presented as means ± SE. The differences between the groups were compared using one-way analysis of variance (ANOVA). A P-value less than 0.05 was considered as statistically significant.Results
The effect of ZnSO4 on cell viability and monolayer permeability
Zn2+ below 200 μM did not affect cell viability; however, at higher concentrations, treatment of MDCK cells with ZnSO4 for 12 h caused a dose-dependent decrease of cell viability (Fig. 1A). The IC50 value was calculated to be 347 ± 6 μM.The alteration of MDCK cell monolayer integrity was monitored by TEER measurement upon treatment with 100, 250 and 350 μM of Zn2+, corresponding to the residual cell viability of 100%, >80% and ∼50%, respectively. The results (Fig. 1B) showed that Zn2+ could cause paracellular leakage in both concentration and action-site-dependent manners: (i) Zn2+ at the apical chamber enhanced the TJ seal in the tested range of concentrations; (ii) Zn2+ at the basolateral chamber caused a significant decline of TEER at concentrations above 200 μM and (iii) compared with Zn2+ at both apical and basolateral chambers, Zn2+ at only the basolateral chamber had a similar effect on the TJ.
It was observed that 250 μM of Zn2+ on the basolateral side could cause a ∼50% decrease of the TEER value. However, when Zn2+ was removed from the media, TEER recovered completely in 24 h. The full recovery of the TJ seal was also verified by the permeability of the Eu–DTPA probe (Fig. 1C). The Papp of Eu–DTPA increased by three-fold upon incubation with 250 μM Zn2+ at the basolateral chamber, but returned to the control level after removal of Zn2+.
Alterations of TJ pore size and retention capacity
The changes of TJ pore size and retention capacity upon Zn2+ treatment were investigated using Eu–DTPA and FD4 as double probes as described.32 Zn2+ (Table 1) at the apical side did not affect TJ pore size but increased the retention capacity, which is consistent with the enhancement of TEER (Fig. 1B). The presence of 250 μM Zn2+ at the basolateral side increased the pore size to r ∼ 2 nm, which would allow the leakage of most biological macromolecules. Higher Zn2+ concentrations induced larger pores. Markedly, Zn2+ present at the basolateral side caused a significant decreasing of the retention capacity (ε/τ) of paracellular pathways, indicating that the TJ was open.| Zn2+ location | Apical side | Basolateral side | Both sides | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [Zn2+] (μM) | 0 | 100 | 250 | 350 | 100 | 250 | 350 | 100 | 250 | 350 |
| r (nm) (SD) | 0.4 (0.1) | 0.2 (0.1) | 0.7 (0.2) | 0.4 (0.1) | 0.9 (0.1) | 1.8 (0.2) | 6.1 (0.3) | 0.6 (0.1) | 2.6 (0.4) | 52.4 (3.0) |
| τ/ε (SD) | 0.03 (0.01) | 0.04 (0.01) | 0.24 (0.05) | 0.12 (0.03) | 0.10 (0.01) | <0.01 | <0.01 | 0.14 (0.01) | <0.01 | <0.01 |
Effects of Zn2+ on TJ architecture of MDCK cell monolayer
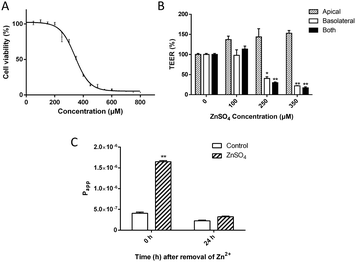
The change of spatial distribution of major TJ proteins, e.g. claudin-1, occludin, ZO-1 and F-actin,37–40 revealed the alteration of the TJ architecture. As shown in Fig. 2A, treatment with 250 μM Zn2+ caused TJ structural changes including: (i) significant decrease of the claudin-1 level; (ii) breakage and condensation of occludin and ZO-1 ring structures; (iii) re-arrangement and scattering of F-actin structure at the basolateral side. TEM observation (Fig. 2B) indicated the formation of a trumpet-like paracellular pathway with the bell mouth facing the basolateral side.Bilateral flux of Eu–DTPA and FD4 probes upon Zn2+-induced TJ opening
The asymmetric paracellular pathway structure upon Zn2+ treatment indicates potential discrimination between transmembrane efflux (basolateral-to-apical) and influx (apical-to-basolateral). Thus, the changes of bilateral flux of two different sized probes upon opening of the TJ by treatment with 250 μM Zn2+ on the basolateral side for 12 h were investigated under dynamic conditions mimicking those in the brain, as previously described.33,34 The results (Fig. 2C) revealed that both influx and efflux of the Eu–DTPA and FD4 probes increased markedly. For the small probe (Eu–DTPA, MW 560), the ratio of efflux/influx changed from 1.0 ± 0.5 (TJ closed) to 1.8 ± 0.3 (TJ); however, for the large probe (FD4, MW 4000), the ratio of efflux/influx changed from 1.9 ± 1.1 (TJ closed) to 4.6 ± 0.6 (TJ). These results clearly indicate that the Zn2+-induced TJ pore path favoured efflux, especially for macromolecules.The levels of claudin-1, occludin and ZO-1 expression in MDCK cells upon treatment with Zn2+
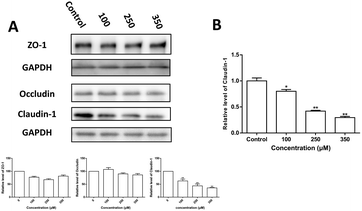
To further clarify the TJ protein alteration induced by Zn2+, the levels of claudin-1, occludin and ZO-1 expression were examined by western blot analysis and the results are shown in Fig. 3. It is observed that: (i) Zn2+ caused a dose-dependent decrease in both protein and mRNA levels of claudin-1; (ii) neither occludin nor ZO-1 protein levels were affected significantly by Zn2+ treatment.Cellular uptake of zinc
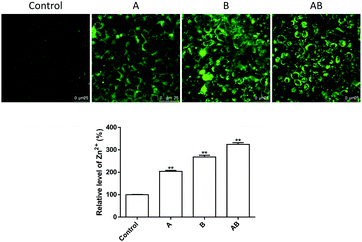
As shown in Fig. 4, the cellular Zn2+ level increased significantly upon incubation with 250 μM of ZnSO4. Addition of Zn2+ to the apical chamber (A), basolateral chamber (B) and both chambers (AB) caused elevation of intracellular Zn2+ levels to 204.2 ± 4.2%, 268.3 ± 7.4%, and 324.3 ± 7.5% respectively.The mechanism underlying Zn2+-induced TJ opening
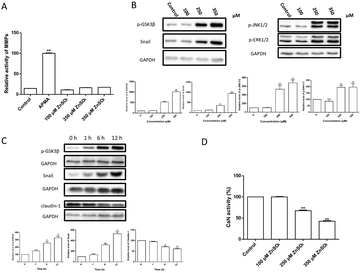
MMPs are normally responsible for the tissue remodeling and degradation of the extracellular matrix. Activation of MMPs has been shown to enhance BBB permeability.41,42 However, the present results (Fig. 5A) showed that the treatment of Zn2+ did not affect the MMP activity in MDCK cell monolayers.Zn2+ and its transporters are known to be involved in regulating cell adhesion in the epithelial–mesenchymal transition (EMT).43–45 Amongst these, snail is a transcriptional repressor that plays an important role in the EMT46 and modulation of tight junction proteins.47,48 Thus, the changes of snail and related signal transduction were observed. The results (Fig. 5B–D) revealed that: (i) Zn2+ at 250 μM or higher concentrations significantly increased the levels of snail, p-GSK3β, p-JNK1/2 and p-ERK1/2 in a dose-dependent way; (ii) the levels of snail, p-GSK3β and claudin-1 were significantly altered at ∼6 h after Zn2+ treatment; (iii) both the increase of snail and p-GSK3β and decrease of claudin-1 were well correlated; (iv) Zn2+ above 250 μM significantly inhibited the CaN activity.
Discussion
High concentration of Zn2+ causes reversible TJ opening favouring efflux of macromolecules by inducing a trumpet-like paracellular channel
The effect of Zn2+ on MDCK cell viability was tested over the entire potential physiological concentration range (0–600 μM) in neural systems. As Zn2+ caused a decline of cell viability in the range of 250–450 μM (Fig. 1A), the effects of Zn2+ on the TJ function of MDCK monolayers were observed at Zn2+ concentrations of 100, 250 and 350 μM, corresponding to 100%, >80%, and ∼50% of cell viability, respectively. The results (Fig. 5C) and preliminary tests suggested that the transcriptional effect of Zn2+ (not Zn2+ signalling) reached its maximal extent after 12 h. Hence, the effects of Zn2+ were most clearly observed at 12 h.The MDCK cells in the monolayer were apparently more resistant to Zn2+ toxicity because Zn2+ (0–350 μM) on the apical side actually improved the TJ seal, as indicated by the increase of TEER (Fig. 1B) and paracellular path retention capacity (τ/ε) (Table 1). Very interestingly, Zn2+ on the basolateral side caused a concentration-dependent reversible opening of the TJ (Fig. 1B and C). It is noteworthy that the TJ pore size (r ∼ 2 nm or more) induced by Zn2+ may allow the leakage of various biological macromolecules.
The changes of TJ architecture (Fig. 2A and B) and the major building proteins (Fig. 3) revealed that the Zn2+ treatment caused different effects on the two ends of the polarized MDCK cell monolayer. On the apical end, the TJ seal was broken, possibly due to loss of claudins. On the basolateral end, the collapse of F-actin structure probably indicated large expansion of the paracellular space. Also, the TEM observation (Fig. 2B) revealed the formation of a trumpet-like paracellular channel with the bell mouth at the basolateral side. This Zn2+-induced channel may be similar to the tapered nanopores, which allow asymmetric ion transport.49
Then, to further explore the Zn2+-induced preferential direction of solute diffusion, bilateral flux assays were conducted under conditions mimicking dynamic flux. In the BBB, the apical side of cerebral capillary endothelial cells faces the blood while the basolateral side faces the cerebral interstitial fluid. According to the Bernoulli principle,50 a faster blood flow leads to a lower pressure in vivo on the apical side. As shown in Fig. 2C, upon Zn2+ treatment, the bilateral permeability of the probes increased significantly, especially the rate of outgoing flux (efflux). Compared with the small Eu–DTPA probe, the efflux/influx ratio of FD4 (MW ∼ 4000) was increased greatly to c.a. 4.6, suggesting that the barrier favours efflux of macromolecules.
Zn2+ induces TJ opening via the GSK3β/snail-mediated signal transduction
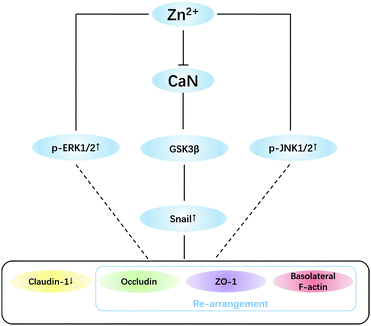
The effect of elevating the intracellular Zn2+ level (Fig. 4) suggested that Zn2+ may exert effects either inside or outside the cells. However, Zn2+ treatment did not change the activity of MMPs in MDCK cells (Fig. 5A), ruling out the involvement of MMPs in Zn2+-induced TJ opening.Among the signal transduction modulating TJ proteins, the level of snail, a zinc finger (ZnF) transcription factor, was herein observed to be up-regulated significantly by Zn2+ in both a time- and dose-dependent manner (Fig. 5B and C). It has been revealed that the increase of snail not only suppresses expression of claudin-1, but also causes F-actin re-arrangement.51–53 The nuclear localization of snail is dependent on both the ZnF domain and the Zn transporter ZIP6,54 while the level of snail is regulated by GSK3β.55 Snail can be phosphorylated by GSK3β, leading to nuclear exportation of snail protein and subsequent degradation. Conversely, phosphorylation inhibition of GSK3β causes elevation of snail expression.55,56 As the snail expression upon Zn2+ treatment synchronized well with the increase of phosphorylated GSK3β (p-GSK3β) and decease of claudin-1, it can be concluded that the Zn2+-induced TJ opening is activated through the GSK3β/snail-mediated pathway.
The GSK3β phosphorylation is known to be primarily up-regulated by Akt57,58 and down-regulated by calcineurin (CaN).59,60 CaN is a calmodulin-dependent serine/threonine protein phosphatase and Zn2+ is a potent inhibitor of CaN.61,62 Meanwhile, in TJ-related pathways, Akt is regulated by integrin linked kinase (ILK).63,64 We have found that inhibition of ILK caused Akt inactivation but did not affect p-GSK3β (data not shown) in MDCK cells. Therefore, phosphorylation of GSK3β was elevated due to inhibition of CaN (Fig. 5D).
In addition to the snail pathway, ERK1/2 and JNK1/2 are also reported to down-regulate claudin-1 expression,65–68 but the mechanisms are not clear yet. The results (Fig. 5B) suggested that activation of ERK1/2 and JNK1/2 by Zn2+ should be involved in TJ opening, which needs to be further investigated. To conclude, a putative mechanism for Zn2+-induced TJ opening is summarized in Fig. 6.
Why Zn2+ affected TJ only from the basolateral side remains a mystery for further research. Nevertheless, it was observed (Fig. 4) that the sum amount of Zn2+ uptake from either the apical or the basolateral side individually almost equalled that from both sides combined. This may suggest that Zn2+ taken from the basolateral side largely remains in different cellular compartments compared with Zn2+ taken from the apical side. This is possibly because the differentiated MDCK cells exhibit an apicobasal polarization characterized by the polarization of the microtubule network in cytoplasm and polarized distribution of enzymes and transport systems to the two ends of the plasma membrane.69 Thus, Zn2+ taken from the basolateral side could inhibit CaN activity and trigger downstream events, but Zn2+ from the other side could not.
Speculation on the potential role of Zn2+ in brain clearance of metabolites through the glymphatic system
To date, the physiological/pathological significance of high concentration synaptic Zn2+ release is poorly understood. It was postulated that Zn2+ release during ischemia may exert either neuroprotective or neurotoxic effects.70 In contrast to intracellular accumulation, the extracellular actions of Zn2+ after accumulation during ischemia may serve to limit neuronal injury by inhibiting NMDA channels and acid-sensing ion channels.71–73 The present results may indicate a new role of Zn2+ to accelerate brain clearance of toxins/wastes through the glymphatic system during pathological conditions such as ischemia (or even possibly in certain physiological processes) by regulating BBB TJ opening.The glymphatic system is a recently discovered macroscopic waste clearance system in the brain.74–76 It has been proposed that primarily during sleep, the gaps between vascular endothelial cells and astroglial cells are opened and a unique system of perivascular channels is formed. The cerebrospinal fluid (CSF) and interstitial fluid (ISF) flow through the perivascular channels and wash out various kinds of metabolic wastes, such as Aβ and its soluble oligmers.77 The failure of glymphatic function might contribute to pathology in neurodegenerative disorders, traumatic brain injury and stroke.78–80 In fact, borneol was proposed to play an important role in treatment of cerebral ischemia through opening the BBB intercellular tight junctions.81,82 Inspired by all the above, we speculate that opening of the perivascular gaps might be regulated by the high-concentration synaptic Zn2+ release. Moreover, the Zn2+-induced flared TJ channels in the vascular endothelial cells would facilitate the excretion of pathological products, particularly macromolecular toxins, out of the brain. The high Zn2+ concentration needed to effectively open the intercellular junction may provide a hint for why the brain needs to keep such a high level zinc deposit. Overall, the complex roles of Zn2+ in neural systems, particularly in glymphatic function, could be a fascinating new issue for future research in bioinorganic chemistry.
Conclusion
In summary, the present work studied the effects of Zn2+ on TJ structures and functions in the MDCK cell monolayer model. Our results demonstrated that high concentrations (>200 μM) of Zn2+ can reversibly open TJ pore paths, favouring efflux of macromolecules. Further investigation revealed that Zn2+ uptake from the basolateral side caused inhibition of CaN activity, resulting in phosphorylation inactivation of GSK3β, which in turn elevated the level of snail transcription suppressors. Consequently, via the snail-mediated signal transduction, Zn2+ caused the rearrangement of basolateral F-actin and down-regulation of claudin-1 expression, leading to the formation of asymmetric paracellular channels. The present work may indicate new roles of Zn2+ in regulating the BBB function, further suggesting a potential role of Zn2+ in brain clearance of metabolites through the glymphatic system.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Project (No. 21571006 and 21271012) supported by NNSFC. The National Key Research and Development Program of China (2016YFC0103605 and 2016YFC0103600).References
- S. R. Lee, S. J. Noh, J. R. Pronto, Y. J. Jeong, H. K. Kim, I. S. Song, Z. L. Xu, H. Y. Kwon, S. C. Kang, E. H. Sohn, K. S. Ko, B. D. Rhee, N. Kim and J. Han, The Critical Roles of Zinc: Beyond Impact on Myocardial Signaling, Korean J. Physiol. Pharmacol., 2015, 19, 389–399 CrossRef CAS PubMed.
- C. J. Frederickson, L. J. Giblin, A. Krezel, D. J. McAdoo, R. N. Mueller, Y. Zeng, R. V. Balaji, R. Masalha, R. B. Thompson, C. A. Fierke, J. M. Sarvey, M. de Valdenebro, D. S. Prough and M. H. Zornow, Concentrations of extracellular free zinc (pZn)e in the central nervous system during simple anesthetization, ischemia and reperfusion, Exp. Neurol., 2006, 198, 285–293 CrossRef CAS PubMed.
- A. R. Kay, Evidence for chelatable zinc in the extracellular space of the hippocampus, but little evidence for synaptic release of Zn, J. Neurosci., 2003, 23, 6847–6855 CAS.
- A. R. Kay, Imaging synaptic zinc: promises and perils, Trends Neurosci., 2006, 29, 200–206 CrossRef CAS PubMed.
- B. B. Zhang, M. Q. Ren, F. S. Sheu, F. Watt and A. Routtenberg, Quantitative analysis of zinc in rat hippocampal mossy fibers by nuclear microscopy, Neurosci. Res., 2012, 74, 17–24 CrossRef CAS PubMed.
- A. Takeda, Movement of zinc and its functional significance in the brain, Brain Res. Rev., 2000, 34, 137–148 CrossRef CAS PubMed.
- J. H. Weiss, S. L. Sensi and J. Y. Koh, Zn2+: a novel ionic mediator of neural injury in brain disease, Trends Pharmacol. Sci., 2000, 21, 395–401 CrossRef CAS PubMed.
- C. J. Frederickson, M. A. Klitenick, W. I. Manton and J. B. Kirkpatrick, Cytoarchitectonic distribution of zinc in the hippocampus of man and the rat, Brain Res., 1983, 273, 335–339 CrossRef CAS PubMed.
- S. Y. Assaf and S. H. Chung, Release of endogenous Zn2+ from brain-tissue during activity, Nature, 1984, 308, 734–736 CrossRef CAS PubMed.
- G. Wei, C. J. Hough, Y. Li and J. M. Sarvey, Characterization of extracellular accumulation of Zn2+ during ischemia and reperfusion of hippocampus slices in rat, Neuroscience, 2004, 125, 867–877 CrossRef CAS PubMed.
- A. Prakash, K. Bharti and A. B. A. Majeed, Zinc: indications in brain disorders, Fundam. Clin. Pharmacol., 2015, 29, 131–149 CrossRef CAS PubMed.
- J. J. Miner and M. S. Diamond, Mechanisms of restriction of viral neuroinvasion at the blood–brain barrier, Curr. Opin. Immunol., 2016, 38, 18–23 CrossRef CAS PubMed.
- B. W. Chow and C. H. Gu, The Molecular Constituents of the Blood-Brain Barrier, Trends Neurosci., 2015, 38, 598–608 CrossRef CAS PubMed.
- C. Zihni, C. Mills, K. Matter and M. S. Balda, Tight junctions: from simple barriers to multifunctional molecular gates, Nat. Rev. Mol. Cell Biol., 2016, 17, 564–580 CrossRef CAS PubMed.
- A. Hartsock and W. J. Nelson, Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton, Biochim. Biophys. Acta, Biomembr., 2008, 1778, 660–669 CrossRef CAS PubMed.
- R. Cabezas, M. Avila, J. Gonzalez, R. S. El-Bacha, E. Baez, L. Miguel Garcia-Segura, J. C. Jurado Coronel, F. Capani, G. Patricia Cardona-Gomez and G. E. Barreto, Astrocytic modulation of blood brain barrier: perspectives on parkinson's disease, Front. Cell. Neurosci., 2014, 8, 211 Search PubMed.
- J. Landy, E. Ronde, N. English, S. K. Clark, A. L. Hart, S. C. Knight, P. J. Ciclitira and H. O. Al-Hassi, Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer, World J. Gastroenterol., 2016, 22, 3117–3126 CrossRef CAS PubMed.
- J. L. Reyes, E. Molina-Jijon, R. Rodriguez-Munoz, P. Bautista-Garcia, Y. Debray-Garcia and M. D. C. Namorado, Tight junction proteins and oxidative stress in heavy metals-induced nephrotoxicity, BioMed Res. Int., 2013, 2013, 730789 Search PubMed.
- X. F. Cao, H. X. Lin, L. Muskhelishvili, J. Latendresse, P. Richter and R. H. Heflich, Tight junction disruption by cadmium in an in vitro human airway tissue model, Respir. Res., 2015, 16, 30 CrossRef PubMed.
- L. G. Navarro-Moreno, M. A. Quintanar-Escorza, S. Gonzalez, R. Mondragon, J. Cerbon-Solorzano, J. Valdes and J. V. Calderon-Salinas, Effects of lead intoxication on intercellular junctions and biochemical alterations of the renal proximal tubule cells, Toxicol. In Vitro, 2009, 23, 1298–1304 CrossRef CAS PubMed.
- Y. Zhang, X. D. Yang, K. Wang and D. C. Crans, The permeability and cytotoxicity of insulin-mimetic vanadium(III,IV,V)–dipicolinate complexes, J. Inorg. Biochem., 2006, 100, 80–87 CrossRef CAS PubMed.
- Z. Xu, X. Wang, R. Xiao and X. Yang, Combination of vitamin C and zinc gluconate prevented vanadium-induced tight junction leakage of MDCK cell monolayer, J. Chin. Pharm. Sci., 2013, 22, 403–408 CAS.
- Z. F. Qi, J. Liang, R. Pan, W. Dong, J. G. Shen, Y. R. Yang, Y. M. Zhao, W. J. Shi, Y. M. Luo, X. M. Ji and K. J. Liu, Zinc contributes to acute cerebral ischemia-induced blood-brain barrier disruption, Neurobiol. Dis., 2016, 95, 12–21 CrossRef CAS PubMed.
- Y. X. Shao, P. G. Wolf, S. S. Guo, Y. M. Guo, H. R. Gaskins and B. K. Zhang, Zinc enhances intestinal epithelial barrier function through the PI3K/AKT/mTOR signaling pathway in Caco-2 cells, J. Nutr. Biochem., 2017, 43, 18–26 CrossRef CAS PubMed.
- X. X. Wang, M. C. Valenzano, J. M. Mercado, E. P. Zurbach, C. J. Flounders and J. M. Mullin, Zinc enhancement of LLC-PK1 renal epithelial barrier function, Clin. Nutr., 2014, 33, 280–286 CrossRef CAS PubMed.
- M. C. Valenzano, K. DiGuilio, J. Mercado, M. Teter, J. To, B. Ferraro, B. Mixson, I. Manley, V. Baker, B. A. Moore, J. Wertheimer and J. M. Mullin, Remodeling of Tight Junctions and Enhancement of Barrier Integrity of the CACO-2 Intestinal Epithelial Cell Layer by Micronutrients, PLoS One, 2015, 10, 22 Search PubMed.
- M. Cereijido, J. Ehrenfeld, I. Meza and A. Martinezpalomo, Structural and functional membrane polarity in cultured monolayers of MDCK cells, J. Membr. Biol., 1980, 52, 147–159 CrossRef CAS PubMed.
- M. Cereijido, E. S. Robbins, W. J. Dolan, C. A. Rotunno and D. D. Sabatini, Polarized monolayers formed by epithelial-cells on a permeable and translucent support, J. Cell Biol., 1978, 77, 853–880 CrossRef CAS PubMed.
- C. Yeaman, K. K. Grindstaff and W. J. Nelson, New perspectives on mechanisms involved in generating epithelial cell polarity, Physiol. Rev., 1999, 79, 73–98 CrossRef CAS PubMed.
- E. Rodriguez-Boulan and I. G. Macara, Organization and execution of the epithelial polarity programme, Nat. Rev. Mol. Cell Biol., 2014, 15, 225–242 CrossRef CAS PubMed.
- Z. H. Xu, C. Y. Zhang, Y. Zhang and X. D. Yang, Europium Complexes as Novel Indicators of Paracellular Diffusion, Chem. Biodiversity, 2012, 9, 1916–1922 CAS.
- X. Y. Wang, N. Wang, L. Yuan, N. Li, J. X. Wang and X. D. Yang, Exploring tight junction alteration using double fluorescent probe combination of lanthanide complex with gold nanoclusters, Sci. Rep., 2016, 6, 32218 CrossRef CAS PubMed.
- C. A. Bates and W. Zheng, Brain disposition of alpha-Synuclein: roles of brain barrier systems and implications for Parkinson's disease, Fluids Barriers CNS, 2014, 11, 1–9 Search PubMed.
- H. Gu, Z. Zhong, W. Jiang, E. Du, R. Dodel, J. Liu, M. R. Farlow, W. Zheng and Y. Du, The role of choroid plexus in ivig-induced beta-amyloid clearance, Neuroscience, 2014, 270, 168–176 CrossRef CAS PubMed.
- L. Shen, C. R. Weber, D. R. Raleigh, D. Yu and J. R. Tumer, Tight Junction Pore and Leak Pathways: A Dynamic Duo, Annu. Rev. Physiol., 2011, 73, 283–309 CrossRef CAS PubMed.
- F. Qian, C. L. Zhang, Y. M. Zhang, W. J. He, X. Gao, P. Hu and Z. J. Guo, Visible Light Excitable Zn2+ Fluorescent Sensor Derived from an Intramolecular Charge Transfer Fluorophore and Its in Vitro and in Vivo Application, J. Am. Chem. Soc., 2009, 131, 1460–1468 CrossRef CAS PubMed.
- C. M. Van Itallie and J. M. Anderson, Architecture of tight junctions and principles of molecular composition, Semin. Cell Dev. Biol., 2014, 36, 157–165 CrossRef CAS PubMed.
- B. J. Saeedi, D. J. Kao, D. A. Kitzenberg, E. Dobrinskikh, K. D. Schwisow, J. C. Masterson, A. A. Kendrick, C. J. Kelly, A. J. Bayless, D. J. Kominsky, E. L. Campbell, K. A. Kuhn, G. T. Furuta, S. P. Colgan and L. E. Glover, HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity, Mol. Biol. Cell, 2015, 26, 2252–2262 CrossRef CAS PubMed.
- P. Loma, A. Guzman-Aranguez, M. J. P. de Lara and J. Pintor, Diadenosine tetraphosphate induces tight junction disassembly thus increasing corneal epithelial permeability, Br. J. Pharmacol., 2015, 172, 1045–1058 CrossRef CAS PubMed.
- A. Garcia-Ponce, A. Francisco Citalan-Madrid, M. Velazquez-Avila, H. Vargas-Robles and M. Schnoor, The role of actin-binding proteins in the control of endothelial barrier integrity, Thromb. Haemostasis, 2015, 113, 20–36 CrossRef PubMed.
- B. T. Hawkins, T. F. Lundeen, K. M. Norwood, H. L. Brooks and R. D. Egleton, Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases, Diabetologia, 2007, 50, 202–211 CrossRef CAS PubMed.
- M. Skowronska, M. Zielinska, L. Wojcik-Stanaszek, J. Ruszkiewicz, D. Milatovic, M. Aschner and J. Albrecht, Ammonia increases paracellular permeability of rat brain endothelial cells by a mechanism encompassing oxidative/nitrosative stress and activation of matrix metalloproteinases, J. Neurochem., 2012, 121, 125–134 CrossRef CAS PubMed.
- S. Yamashita, C. Miyagi, T. Fukada, N. Kagara, Y. S. Che and T. Hirano, Zinc transporter LIVI controls epithelial-mesenchymal transition in zebrafish gastrula organizer, Nature, 2004, 429, 298–302 CrossRef CAS PubMed.
- C. Hogstrand, P. Kille, M. L. Ackland, S. Hiscox and K. M. Taylor, A mechanism for epithelial-mesenchymal transition and anoikis resistance in breast cancer triggered by zinc channel ZIP6 and STAT3 (signal transducer and activator of transcription 3), Biochem. J., 2013, 455, 229–237 CrossRef CAS PubMed.
- K. M. Taylor, I. A. Muraina, D. Brethour, G. Schmitt-Ulms, T. Nimmanon, S. Ziliotto, P. Kille and C. Hogstrand, Zinc transporter ZIP10 forms a heteromer with ZIP6 which regulates embryonic development and cell migration, Biochem. J., 2016, 473, 2531–2544 CrossRef CAS PubMed.
- A. Cano, M. A. Perez-Moreno, I. Rodrigo, A. Locascio, M. J. Blanco, M. G. del Barrio, F. Portillo and M. A. Nieto, The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression, Nat. Cell Biol., 2000, 2, 76–83 CrossRef CAS PubMed.
- J. Ikenouchi, M. Matsuda, M. Furuse and S. Tsukita, Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by snail, J. Cell Sci., 2003, 116, 1959–1967 CrossRef CAS PubMed.
- T. Ohkubo and M. Ozawa, The transcription factor snail downregulates the tight junction components independently of E-cadherin downregulation, J. Cell Sci., 2004, 117, 1675–1685 CrossRef CAS PubMed.
- W. Guo, Y. Tian and L. Jiang, Asymmetric Ion Transport through Ion-Channel-Mimetic Solid-State Nanopores, Acc. Chem. Res., 2013, 46, 2834–2846 CrossRef CAS PubMed.
- R. Resnick, D. Halliday and J. Walker, Fundamentals of Physics, 2013, ch. 14, pp. 401–405 Search PubMed.
- B. De Craene, B. Gilbert, C. Stove, E. Bruyneel, F. van Roy and G. Berx, The transcription factor snail induces tumor cell invasion through modulation of the epithelial cell differentiation program, Cancer Res., 2005, 65, 6237–6244 CrossRef CAS PubMed.
- S. Berzal, M. Alique, M. Ruiz-Ortega, J. Egido, A. Ortiz and A. M. Ramos, GSK3, snail, and Adhesion Molecule Regulation by Cyclosporine A in Renal Tubular Cells, Toxicol. Sci., 2012, 127, 425–437 CrossRef CAS PubMed.
- O. M. Martinez-Estrada, S. Culleres, F. X. Soriano, H. Peinado, V. Bolos, F. O. Martinez, M. Reina, A. Cano, M. Fabre and S. Vilaro, The transcription factors Slug and snail act as repressors of Claudin-1 expression in epithelial cells, Biochem. J., 2006, 394, 449–457 CrossRef CAS PubMed.
- M. Murakami and T. Hirano, Intracellular zinc homeostasis and zinc signaling, Cancer Sci., 2008, 99, 1515–1522 CrossRef CAS PubMed.
- B. H. P. Zhou, J. Deng, W. Y. Xia, J. H. Xu, Y. M. Li, M. Gunduz and M. C. Hung, Dual regulation of snail by GSK-3 beta-mediated phosphorylation in control of epithelial-mesenchymal transition, Nat. Cell Biol., 2004, 6, 931–940 CrossRef CAS PubMed.
- R. E. Bachelder, S. O. Yoon, C. Franci, A. G. de Herreros and A. M. Mercurio, Glycogen synthase kinase-3 is an endogenous inhibitor of snail transcription: implications for the epithelial-mesenchymal transition, J. Cell Biol., 2005, 168, 29–33 CrossRef CAS PubMed.
- D. A. E. Cross, D. R. Alessi, P. Cohen, M. Andjelkovich and B. A. Hemmings, Inhibition of glycogen-synthase kinase-3 by insulin-mediated by protein-kinase-b, Nature, 1995, 378, 785–789 CrossRef CAS PubMed.
- B. D. Manning and A. Toker, AKT/PKB Signaling: Navigating the Network, Cell, 2017, 169, 381–405 CrossRef CAS PubMed.
- Y. I. Lee, M. Seo, Y. Kim, S. Y. Kim, U. G. Kang, Y. S. Kim and Y. S. Juhnn, Membrane depolarization induces the undulating phosphorylation/dephosphorylation of glycogen synthase kinase 3 beta and this dephosphorylation involves protein phosphatases 2A and 2B in SH-SY5Y human neuroblastoma cells, J. Biol. Chem., 2005, 280, 22044–22052 CrossRef CAS PubMed.
- Y. Kim, Y. I. Lee, M. Seo, S. Y. Kim, J. E. Lee, H. D. Youn, Y. S. Kim and Y. S. Juhnn, Calcineurin dephosphorylates glycogen synthase kinase-3 beta at serine-9 in neuroblast-derived cells, J. Neurochem., 2009, 111, 344–354 CrossRef CAS PubMed.
- K. Takahashi, E. Akaishi, Y. Abe, R. Ishikawa, S. Tanaka, K. Hosaka and Y. Kubohara, Zinc inhibits calcineurin activity in vitro by competing with nickel, Biochem. Biophys. Res. Commun., 2003, 307, 64–68 CrossRef CAS PubMed.
- S. Tanaka, E. Akaishi, K. Hosaka, S. Okamura and Y. Kubohara, Zinc ions suppress mitogen-activated interleukin-2 production in Jurkat cells, Biochem. Biophys. Res. Commun., 2005, 335, 162–167 CrossRef CAS PubMed.
- S. Persad, S. Attwell, V. Gray, M. Delcommenne, A. Troussard, J. Sanghera and S. Dedhar, Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 3207–3212 CrossRef CAS.
- Y. Qian, X. S. Zhong, D. C. Flynn, J. Z. Zheng, M. Qiao, C. Y. Wu, S. Dedhar, X. L. Shi and B. H. Jiang, ILK mediates actinfilament rearrangements and cell migration and invasion through PI3K/Akt/Rac1 signaling, Oncogene, 2005, 24, 3154–3165 CrossRef CAS PubMed.
- W. F. Huang, H. J. Zhao, H. M. Dong, Y. Wu, L. H. Yao, F. Zou and S. X. Cai, High-mobility group box 1 impairs airway epithelial barrier function through the activation of the RAGE/ERK pathway, Int. J. Mol. Med., 2016, 37, 1189–1198 CrossRef CAS PubMed.
- K. Nomura, K. Obata, T. Keira, R. Miyata, S. Hirakawa, K.-i. Takano, T. Kohno, N. Sawada, T. Himi and T. Kojima, Pseudomonas aeruginosa elastase causes transient disruption of tight junctions and downregulation of PAR-2 in human nasal epithelial cells, Respir. Res., 2014, 15, 21 CrossRef PubMed.
- D. Hackel, S. M. Krug, R.-S. Sauer, S. A. Mousa, A. Boecker, D. Pfluecke, E.-J. Wrede, K. Kistner, T. Hoffmann, B. Niedermirtl, C. Sommer, L. Bloch, O. Huber, I. E. Blasig, S. Amasheh, P. W. Reeh, M. Fromm, A. Brack and H. L. Rittner, Transient opening of the perineurial barrier for analgesic drug delivery, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 2018–2027 CrossRef PubMed.
- I. Larre, A. Lazaro, R. G. Contreras, M. S. Balda, K. Matter, C. Flores-Maldonado, A. Ponce, D. Flores-Benitez, R. Rincon-Heredia, T. Padilla-Benavides, A. Castillo, L. Shoshani and M. Cereijido, Ouabain modulates epithelial cell tight junction, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 11387–11392 CrossRef CAS PubMed.
- M. C. Gibson and N. Perrimon, Apicobasal polarization: epithelial form and function, Curr. Opin. Cell Biol., 2003, 15, 747–752 CrossRef CAS PubMed.
- S. L. Galasso and R. H. Dyck, The role of zinc in cerebral ischemia, Mol. Med., 2007, 13, 380–387 CAS.
- P. Paoletti, A. M. Vergnano, B. Barbour and M. Casado, Zinc at glutamatergic synapses, Neuroscience, 2009, 158, 126–136 CrossRef CAS PubMed.
- J. G. Hey, X. P. Chu, J. Seeds, R. P. Simon and Z. G. Xiong, Extracellular zinc protects against acidosis-induced injury of cells expressing Ca2+-permeable acid-sensing ion channels, Stroke, 2007, 38, 670–673 CrossRef CAS PubMed.
- Z. G. Xiong, X. M. Zhu, X. P. Chu, M. Minami, J. Hey, W. L. Wei, J. F. MacDonald, J. A. Wemmie, M. P. Price, M. J. Welsh and R. P. Simon, Neuroprotection in ischemia: Blocking calcium-permeable acid-sensing ion channels, Cell, 2004, 118, 687–698 CrossRef CAS PubMed.
- N. A. Jessen, A. S. F. Munk, I. Lundgaard and M. Nedergaard, The Glymphatic System: A Beginner's Guide, Neurochem. Res., 2015, 40, 2583–2599 CrossRef CAS PubMed.
- M. Nedergaard, Garbage Truck of the Brain, Science, 2013, 340, 1529–1530 CrossRef CAS PubMed.
- M. Nedergaard and S. A. Goldman, Brain drain, Sci. Am., 2016, 314, 44–49 CrossRef CAS PubMed.
- L. L. Xie, H. Y. Kang, Q. W. Xu, M. J. Chen, Y. H. Liao, M. Thiyagarajan, J. O'Donnell, D. J. Christensen, C. Nicholson, J. J. Iliff, T. Takano, R. Deane and M. Nedergaard, Sleep Drives Metabolite Clearance from the Adult Brain, Science, 2013, 342, 373–377 CrossRef CAS PubMed.
- J. M. Tarasoff-Conway, R. O. Carare, R. S. Osorio, L. Glodzik, T. Butler, E. Fieremans, L. Axel, H. Rusinek, C. Nicholson, B. V. Zlokovic, B. Frangione, K. Blennow, J. Menard, H. Zetterberg, T. Wisniewski and M. J. de Leon, Clearance systems in the brain-implications for Alzheimer disease, Nat. Rev. Neurol., 2015, 11, 457–470 CrossRef CAS PubMed.
- Z. G. Ren, J. J. Iliff, L. J. Yang, J. K. Yang, X. L. Chen, M. J. Chen, R. N. Giese, B. Z. Wang, X. F. Shi and M. Nedergaard, 'Hit & Run' model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation, J. Cereb. Blood Flow Metab., 2013, 33, 834–845 CrossRef CAS PubMed.
- J. J. Iliff and M. Nedergaard, Is There a Cerebral Lymphatic System?, Stroke, 2013, 44, S93–S95 CrossRef PubMed.
- B. Yu, M. Ruan, Z. N. Zhang, H. B. Cheng and X. C. Shen, Synergic Effect of Borneol and Ligustrazine on the Neuroprotection in Global Cerebral Ischemia/Reperfusion Injury: A Region-Specificity Study, J. Evidence-Based Complementary Altern. Med., 2016, 8, DOI:10.1155/2016/4072809.
- H. Y. Wu, Y. Tang, L. Y. Gao, W. X. Sun, Y. Hua, S. B. Yang, Z. P. Zhang, G. Y. Liao, Q. G. Zhou, C. X. Luo and D. Y. Zhu, The synergetic effect of edaravone and borneol in the rat model of ischemic stroke, Eur. J. Pharmacol., 2014, 740, 522–531 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |