A feasibility study of “range-extended” EXAFS measurement at the Pt L3-edge of Pt/Al2O3 in the presence of Au2O3†
Hiroyuki
Asakura
 *ab,
Naomi
Kawamura
*ab,
Naomi
Kawamura
 c,
Masaichiro
Mizumaki
c,
Kiyofumi
Nitta
c,
Kenji
Ishii
c,
Masaichiro
Mizumaki
c,
Kiyofumi
Nitta
c,
Kenji
Ishii
 d,
Saburo
Hosokawa
d,
Saburo
Hosokawa
 ab,
Kentaro
Teramura
ab,
Kentaro
Teramura
 ab and
Tsunehiro
Tanaka
ab and
Tsunehiro
Tanaka
 *ab
*ab
aDepartment of Molecular Engineering, Graduate School of Engineering, Kyoto University, Kyotodaigaku Katsura, Nishikyo-ku, Kyoto 615-8510, Japan. E-mail: asakura@moleng.kyoto-u.ac.jp; tanakat@moleng.kyoto-u.ac.jp
bElements Strategy Initiative for Catalysts & Batteries (ESICB), Kyoto University, 1-30 Goryo-Ohara, Nishikyo-ku, Kyoto 615-8245, Japan
cJapan Synchrotron Radiation Research Institute, SPring-8, 1-1-1 Kouto, Sayo, Hyogo 679-5198, Japan
dSynchrotron Radiation Research Center, National Institutes for Quantum and Radiological Science and Technology, 1-1-1 Kouto, Sayo, Hyogo 679-5148, Japan
First published on 27th October 2017
Abstract
A feasibility study of a “range-extended” EXAFS measurement was conducted on the Pt L3-edge of Pt/Al2O3 in the presence of Au2O3. The Pt L3-edge EXAFS spectrum of the Pt/Al2O3 sample was successfully obtained without any interference at the Au L3-edge by means of high energy resolution fluorescence detection (HERFD) of the Pt Lα1 line with a Bragg type X-ray emission analyser crystal. A newly observed significant enhancement of the EXAFS oscillation amplitude was discussed on the points of the core-hole lifetime width and X-ray energy width. We also successfully obtained reasonable physical parameters by a conventional EXAFS curve fitting on the HERFD-EXAFS data. The HERFD-EXAFS technique can broaden the scope of application of EXAFS spectroscopy for complicated samples with many different elements.
Introduction
X-ray absorption spectroscopy (XAS) is one of the core level spectroscopy techniques, which enables us to obtain information on the electronic and local geometric structures around an X-ray absorbing atom. Thus, XAS is an element-specific technique. The element specificity is one of the advantages of XAS. However, the X-ray absorption fine structure (XAFS) of the target element sometimes experiences interference from another element, which has its absorption edge near that of the target element. To overcome this intrinsic limitation, several groups have pursued essentially element specific EXAFS measurement methods by applying approaches that are more sophisticated for their own purposes. For example, Cramer et al. reported a “range-extended” EXAFS approach for the refinement of the nickel site structure in Desulfovibrio gigas (D. gigas) hydrogenase.1 They simply removed the Cu K-edge (9.0 keV) XAFS feature of the coexisting Cu contaminant by subtracting the Cu K-edge XAFS of a certain Cu model compound from the raw Ni K-edge (8.3 keV) XAFS spectrum of D. gigas hydrogenase with a plausible scaling factor. This approach is indeed interesting; however, the Cu K-edge XAFS spectrum used for the subtraction is not clear and the validity is still ambiguous, even though this simple subtraction strategy is verified for the Ni K-edge EXAFS spectra of Ni[(S2C2)Ph2]2 and NiS2(NH2)2Ph2 in the presence and absence of CuF2 and CuCl2. In 2005, Glatzel et al. introduced a novel concept for the application of the high energy resolution fluorescence detection (HERFD) method2 to EXAFS measurement, which can potentially overcome the intrinsic limitation of the EXAFS analysis by the overlapping of absorption edges in a narrow range.3 They also named the technique “range-extended” EXAFS. They measured an EXAFS spectrum of LaOCl at the La L2-edge (5.9 keV) without the La L1-edge (6.3 keV) XAFS feature using the HERFD method with spherically bent Ge analyser crystals in the Bragg geometry at a high flux undulator beamline 18ID BioCAT at the Advanced Photon Source (Argonne National Laboratory, USA). They showed La L2-edge range-extended EXAFS spectra of a LaOCl sample as well as ones diluted with BN to 50% and 10%. As the concentration of LaOCl decreased, the La L1-edge feature got smaller. However, the La L1-edge feature is still not negligible in their k3-weighted EXAFS spectra. They also pointed out the possibility of the versatile application of their “range-extended” EXAFS technique as a more practical element specific one for mixed samples with multiple X-ray absorption edges. Yano et al. applied this technique to the structural investigation of the Mn4Ca cluster in the oxygen evolving complex of photosystem II.4 They used the range-extended EXAFS technique to remove the Fe K-edge XAFS feature derived from the Fe contaminant of the oxygen evolving complex sample and successfully narrowed the possible structure of the Mn4Ca centre to several models. Even though the range-extended EXAFS technique actually broadens the scope of application of EXAFS spectroscopy, the technique is not very common5 because of the limitation of the sample conditions.6,7In this paper, we evaluate the feasibility of the range-extended EXAFS technique to obtain a Pt L3-edge EXAFS spectrum of Pt/Al2O3 in the presence of Au, which has the L3-edge X-ray absorption edge (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 919 eV) close to the Pt L3-edge (11
919 eV) close to the Pt L3-edge (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 564 eV). For example, Kaito et al. reported a mixed material of Pt and Au for the oxygen reduction reaction of a fuel cell. Because of the apparent interruption by the Au L3-edge feature in the Pt L3-edge EXAFS, they used a Pt K-edge and Au K-edge XAS measurement to investigate the local structure.8 However, such high-energy X-rays are only available at a limited number of synchrotron radiation facilities.
564 eV). For example, Kaito et al. reported a mixed material of Pt and Au for the oxygen reduction reaction of a fuel cell. Because of the apparent interruption by the Au L3-edge feature in the Pt L3-edge EXAFS, they used a Pt K-edge and Au K-edge XAS measurement to investigate the local structure.8 However, such high-energy X-rays are only available at a limited number of synchrotron radiation facilities.
Our initial interest in the application of “range-extended” EXAFS measurement is analyses on the local structure of Pt species of dilute Pt catalysts in the presence of a W element and the local structure of Pt and Au atoms of Pt–Au clusters. In such cases, high energy XAS measurement at the Pt K-edge (78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 395 eV), W K-edge (69
395 eV), W K-edge (69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 525 eV), and Au K-edge (80
525 eV), and Au K-edge (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 725 eV) must be very difficult because of very weak X-ray fluorescence intensity. In addition, common Pt L3-edge (11
725 eV) must be very difficult because of very weak X-ray fluorescence intensity. In addition, common Pt L3-edge (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 564 eV) measurement suffers from the existence of the W L2-edge (11
564 eV) measurement suffers from the existence of the W L2-edge (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 544 eV) or Au L3-edge (11
544 eV) or Au L3-edge (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 919 eV). Thus, we first conducted a feasibility study of the “range-extended” EXAFS measurement on a Pt–Au mixture and examined the applicability of the conventional XAS analysis technique including EXAFS curve fitting analysis.
919 eV). Thus, we first conducted a feasibility study of the “range-extended” EXAFS measurement on a Pt–Au mixture and examined the applicability of the conventional XAS analysis technique including EXAFS curve fitting analysis.
Experimental
Preparation of Pt/Al2O3 samples
0.1, 0.5, and 1.0 wt% Pt/Al2O3 were synthesized by a common impregnation method. For example, 0.1 wt% Pt/Al2O3 was prepared by the following procedure. Pt(NH3)2(NO2)2 stock solution (0.0418 g, 46.4 wt% as metal basis, Furuya Metal Co., Ltd) was diluted with pure water (1.2 mL) in an evaporating dish. A portion of γ-Al2O3 (JRC-ALO-7, Catalysis Society of Japan) was added to the dish. The mixture was heated using a water bath to 80 °C and continuously stirred with a glass rod until dry. The dried sample was calcined in air at 500 °C for 3 h. The other Pt/Al2O3 samples were prepared in a similar manner. To prepare an XAS sample with Pt L3 and Au L3 absorption edges to perform a feasibility study of range-extended EXAFS spectroscopy, a small amount of Au2O3 was added to the 0.1 wt% Pt/Al2O3 powder. The Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au ratio was determined with inductively coupled plasma optical emission spectrometry using an iCAP7000 (Thermo Fisher Scientific Inc.) to be Pt
Au ratio was determined with inductively coupled plasma optical emission spectrometry using an iCAP7000 (Thermo Fisher Scientific Inc.) to be Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au = 1
Au = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Thus, the mixed sample was approximately 0.1 wt% Pt/0.2 wt% Au/Al2O3.
2. Thus, the mixed sample was approximately 0.1 wt% Pt/0.2 wt% Au/Al2O3.
XAS measurements
Pt L3-edge XAS spectra of Pt/Al2O3 samples in the transmission mode, total fluorescence mode with a Pilatus 100/K detector,9 partial fluorescence mode with a one-element silicon drift detector (SDD) (Amptek), or high-energy-resolution fluorescence detection mode with an analyser crystal and the Pilatus detector were measured at a QST (National Institute for Quantum and Radiological Science and Technology, Japan) beamline BL11XU10 for a preliminary experiment and a public beamline BL39XU at SPring-8 (Japan Synchrotron Radiation Research Institute, Japan). The energy resolution of the SDD was about 100 eV. At the BL11XU, the incident beam was monochromatized by a Si(111) double-crystal monochromator. The beam size was about 0.05 (horizontal) × 1.0 (vertical) mm2. The scattered X-rays were analyzed by using a Si(733) spherically bent analyser (XRSTECH LLC., USA). The analyser crystal and the Pilatus detector were kept on a horizontal Rowland circle of 2 m diameter. The X-ray emission and scattering spectra were measured by rotating the analyser in the Bragg geometry. At the BL39XU, the incident beam was monochromatized by a Si(220) double-crystal monochromator. The beam size was restrained with a slit opening of 0.2 (horizontal) × 0.2 (vertical) mm2. The emitted X-rays were analyzed using a Ge(660) spherically bent analyser crystal (Saint-Gobain SA, France). The analyser crystal and the Pilatus detector were kept on a horizontal Rowland circle of 0.8 m diameter. Both the X-ray emission and scattering spectra were measured by rotating the analyser in the Bragg geometry. The X-ray energy resolution was ca. 1.5 eV at 9442 eV (Pt Lα1 line). The Pt L3-edge XAS spectra in the partial fluorescence yield mode was measured at 9442 eV (Pt Lα1 line) by the HERFD method. Thus, the HERFD method is one of the partial fluorescence yield (PFY) methods.Hereafter, for clarity in this paper, we call the fluorescence mode measurement with a Pilatus detector as the total fluorescence yield (TFY) mode, the fluorescence mode measurement with an SDD as the partial fluorescence yield (PFY) mode, and the fluorescence mode measurement with an analyser crystal using a Pilatus detector as the HERFD mode. Data reduction was carried out with the Athena and Artemis ver. 0.9.25 and FEFF 6L,11 which is freely distributed and included in the Demeter package.12 The back-scattering amplitude or phase shift parameters were simulated with FEFF 6L and used to perform the curve fitting procedure on the obtained EXAFS spectra, as will be discussed in the following section.
Results and discussion
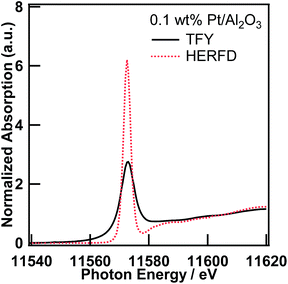
Pt L3-edge XAS spectra of 0.1 wt% Pt/Al2O3 in the TFY and fluorescence modes
First, we measured the Pt L3-edge XAS spectra of 0.5 wt% Pt/Al2O3 in both transmission and TFY modes, and confirmed that self-absorption effects are negligible. Thus, the Pt/Al2O3 samples, whose Pt content are lower than 0.5 wt%, are suitable for the XAS measurement in the fluorescence mode (Fig. S1†).Fig. 1 shows the Pt L3-edge XANES spectra of 0.1 wt% Pt/Al2O3 in the TFY and HERFD modes. The energy resolution of the Pt L3-edge XANES spectrum measured in the HERFD mode is astonishingly improved. To the best of our knowledge, this phenomenon was first reported by Hämäläinen et al. in 1991 for a few XANES spectra of Dy compounds14 based on the discussion by Tulkki and Aberg.2 This impressive improvement in the energy resolution can be understood because of the difference in the overall energy resolution in the TFY and HERFD modes. In general, the energy resolution of the XAS spectra is determined by the light source properties, instrumental properties (e.g., stability, aperture of the slits, and perfection and parallelism of the double crystal monochromator), and inherit physical phenomena (e.g., lifetime broadening effect15). In the present case, the energy resolution of the incident X-ray at the BL39XU was estimated from the full width half maximum of the X-ray elastic scattering spectrum at around 9.5 keV and found to be ca. 0.8 eV. The energy bandwidth accepted by the Pilatus detector is much wider than the natural energy width of both the L3-edge and the M5-edge. However, the analyser crystal used in the HERFD mode enables us to separate the Pt Lα1 (L3–M5, 9442 eV) fluorescence X-ray from the Lα2 line (L3–M4, 9362 eV). Based on the discussion of the energy resolution of the Pb L3-edge HERFD XANES by Swarbrick et al.,15 the overall energy resolution of the Pt L3-edge HERFD XAS can also be estimated to be 2.9 eV, which is much smaller than that in the TFY mode (5.2 eV), from the core-hole lifetime of the initial/intermediate and final states, along with the energy bandwidth of the incident X-ray and emitted X-ray, that is, the Pt L3 state (5.1 eV) and the M5 state (2.4 eV) for the Lα1 line (L3–M5), 0.8 eV (incident X-ray) and 1.5 eV (emitted X-ray), respectively, with the convolution formula given by de Groot et al.16 and Olivero et al.17 Therefore, as also discussed by Hämäläinen et al.,14 the energy resolution in the TFY mode was restrained to the core-hole lifetime of the L3 state; however, in the HERFD mode, it was only restrained to one narrower than that of the M5 state, but not the L3 state.
The high-resolution XANES measurement has already been utilized in various scientific fields, such as environmental science,15 biochemistry,18 and the fundamental science of the molecular orbitals of Os19 and La.20 This is a very interesting topic, but in this paper, we focus on the EXAFS region of the XAS spectra measured in the HERFD mode as follows.
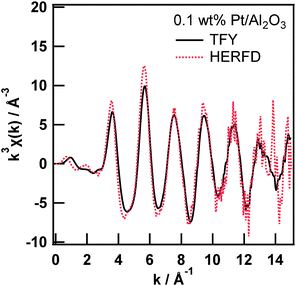
The comparison between the Pt L3-edge EXAFS oscillations of 0.1 wt% Pt/Al2O3 measured in the TFY and HERFD modes is shown in Fig. 2. Even though the X-ray flux density at the sample position is considerably high (i.e., 1012 photons per s), with access to a highly brilliant X-ray undulator at SPring-8, a small solid angle and imperfection of the analyser crystal decreased the total fluorescence X-ray reaching the X-ray detector. The EXAFS oscillation is extracted from the averaged XAS spectra from six measurements (ca. 1 h per spectrum). The two EXAFS oscillations are fundamentally equal. However, the amplitude of the EXAFS oscillation measured in the HERFD mode is significantly larger than that in the TFY mode. The amplitude enhancement of the EXAFS oscillation might be ascribed to the difference in the overall energy resolution, as discussed above regarding the high-resolution XANES spectra. Thus, the enhancement of the EXAFS oscillation amplitude can be attributed to the relatively narrow natural width at the M5 state.
Then, we tried to perform quantitative analysis on the EXAFS spectra by using a curve fitting technique from a practical point of view. First, we obtained an amplitude reduction factor (S02) from EXAFS curve fitting analysis of the first shell of the Pt foil and found it to be 0.821.
Next, we performed a curve fitting analysis on the first shell of the 0.1 wt% Pt/Al2O3 EXAFS spectrum measured in the TFY mode, as summarized in Table 1. The coordination number and atomic distance of the Pt–O shell were found to be 6.4 ± 1.4 and 2.00 ± 0.01 Å, respectively, which are reasonable for the generation of β-PtO2-like species on Al2O3. This result implies two facts: the XAS measurements are performed well and the amplitude reduction factor is chemically transferable. Third, we moved to the 0.1 wt% Pt/Al2O3 EXAFS spectrum measured in the HERFD mode. The first shell of the Fourier transformed EXAFS spectrum looks significantly higher than that of the one measured in the TFY mode (Fig. S2†). Then, we conducted EXAFS curve fitting analysis on the 0.1 wt% Pt/Al2O3 HERFD EXAFS spectrum with a fixed coordination number of the Pt–O first shell equal to 6.4. Finally, we found the amplitude reduction factor for the HERFD mode to be 1.00. The fitting result is provided in the ESI (Fig. S3†). All the fitting procedures had an R factor below 0.003, indicating the goodness of the fit.
| CN | R/Å | DW/Å2 | ΔE/eV | |
|---|---|---|---|---|
| a CN: coordination number, R: atomic distance, DW: XAFS Debye–Waller factor, and ΔE: energy correction during the fitting procedure. | ||||
| Pt–O | 6.4 ± 1.4 | 2.00 ± 0.01 | 0.0024 ± 0.0017 | 11.1 ± 2.9 |
For example, the k3-weighted EXAFS spectra at the Mn K-edge of PS II samples in the conventional PFY and HERFD modes reported by Yano et al. appear to be almost identical to each other in the k range up to the Fe K-edge derived from the Fe concomitant.4 This result can be explained in the same manner. The natural widths of the Mn K-edge and L2-edge for the Kα2 (K–L2) line, and the L3-edge for the Kα1 (K–L3) line are 1.2, 1.5, and 0.42 eV, respectively. Based on the aforementioned Swarbrick paper,15 the overall lifetime broadening effect on the Mn K-edge HERFD EXAFS measurement was estimated to be 1.3 eV, which is close to the energy resolution of the overall energy bandwidth of the X-ray. Therefore, the amplitudes of the EXAFS oscillation, in both conventional PFY and HERFD modes, were identical to each other, which differs from our case. In our case, the natural widths of the Pt L3-edge and M4-edge for the Lα2 (L3–M4) line, and the M5-edge for the Lα1 (L3–M5) line are 5.1, 3.1, and 2.4 eV, respectively. Then, the overall lifetime broadening effect on the Pt L3-edge HERFD EXAFS measurement was estimated to be 2.9 eV, which is significantly smaller than that of the transmission mode (5.2 eV). At present, we assume that the difference in the amplitude reduction factors between the TFY and HERFD modes comes from the total energy resolution and is mainly dependent on the overall core-hole lifetime broadening discussed above. Indeed, the HERFD EXAFS spectrum of the 0.1 wt% Pt/Al2O3 convoluted using a lifetime broadening Lorentz function (γ = 5.0 eV) is identical to the EXAFS spectrum measured in TFY mode (Fig. S4†). However, the difference might arise from other factors, such as the difference in the many-body effect on the second-order optical process in the HERFD method.
Pt L3-edge EXAFS analysis of 0.1 wt% Pt/Al2O3 in the presence of Au2O3 with range-extended EXAFS
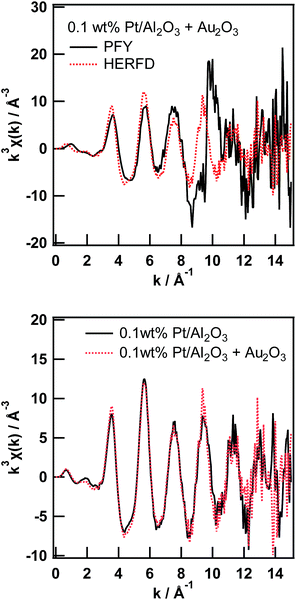
As already pointed out by Glatzel et al.,3 the range-extended EXAFS measurement must be performed only on samples suitable for fluorescence XAS measurement. Thus, the amount of the target element has to be sufficiently diluted to fulfill the requirement for the fluorescence measurement,13 and the coexisting elements must be sufficiently diluted to not interfere with the EXAFS oscillation of interest. We would like to point out that the prerequisite for the coexisting elements is slightly laxer than that for the target element. Thus, the total amount of coexisting elements must be sufficiently small to not interfere with the XAFS of the target element; however, the elements can consist of relatively large particles, which is not suitable for fluorescence measurement. Therefore, to perform a feasibility study of the range-extended EXAFS technique, we just added a small amount of Au2O3 powder to the Pt/Al2O3 sample and measured the Pt L3-edge EXAFS spectra in the conventional PFY mode (with an SDD) and in the HERFD mode.Pt L3-edge XAS spectra of 0.1 wt% Pt/Al2O3 in the presence of Au2O3 in the PFY mode and in the HERFD mode as well as their magnified view around the Au L3-edge are shown in Fig. 3. As clearly seen in the magnified view (Fig. 3 (bottom)), the Au L3-edge absorption edge of Au2O3 was found at around 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 920 eV in the EXAFS region of the Pt L3-edge of 0.1 wt% Pt/Al2O3. Then, the extracted Pt L3-edge EXAFS oscillation measured in the conventional PFY mode with an SDD is severely distorted by the interference of the Au L3-edge (k = 9.6 Å−1), as shown in Fig. 4 (top), because of the relatively low energy resolution of the SDD and the spectral tails of Au L lines. In contrast, there is no interference from the Au L3-edge on the EXAFS oscillation measured in the HERFD mode. This result demonstrates that the HERFD method enables us to perform EXAFS analysis on a target element even if some other absorption edges are in the EXAFS region of interest. Indeed, the Pt L3-edge EXAFS oscillations of both 0.1 wt% Pt/Al2O3 and its mixture with Au2O3 look essentially identical to each other, as shown in Fig. 4 (bottom).
920 eV in the EXAFS region of the Pt L3-edge of 0.1 wt% Pt/Al2O3. Then, the extracted Pt L3-edge EXAFS oscillation measured in the conventional PFY mode with an SDD is severely distorted by the interference of the Au L3-edge (k = 9.6 Å−1), as shown in Fig. 4 (top), because of the relatively low energy resolution of the SDD and the spectral tails of Au L lines. In contrast, there is no interference from the Au L3-edge on the EXAFS oscillation measured in the HERFD mode. This result demonstrates that the HERFD method enables us to perform EXAFS analysis on a target element even if some other absorption edges are in the EXAFS region of interest. Indeed, the Pt L3-edge EXAFS oscillations of both 0.1 wt% Pt/Al2O3 and its mixture with Au2O3 look essentially identical to each other, as shown in Fig. 4 (bottom).
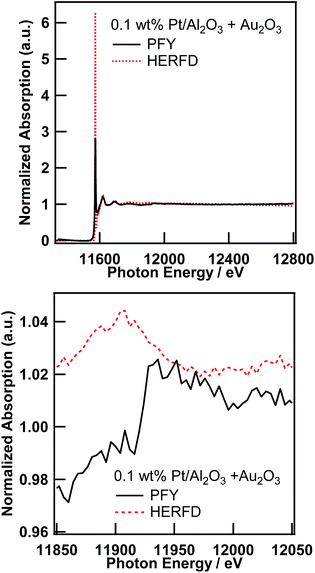 | ||
| Fig. 3 Pt L3-edge XAS spectra of 0.1 wt% Pt/Al2O3 in the presence of Au2O3 in the conventional PFY mode and in the HERFD mode (top), and their magnified view around the Au L3-edge (bottom). | ||
Dependence on X-ray emission energy
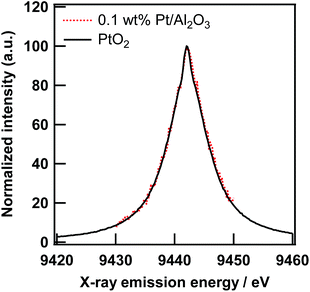
In the field of XAS measurement based on X-ray emission spectroscopy, multiple groups have already demonstrated several sophisticated state-selective X-ray absorption spectroscopies. For example, Peng et al. reported a pioneering study on the high-resolution X-ray emission spectra (XES) and XAS spectra of several Mn compounds with various ligands. The HERFD method enabled them to measure high resolution Kβ1,3 and its satellite, and find their sensitivity to oxidation and a spin state of the Mn element.21 Izumi et al. have reported site-selective XAS measurements based on the HERFD method for Cu catalysts.22,23 Hayashi et al. reported spin and valence-selective XAS measurements of EuS and a mixture of Eu(II)S and Eu2(III)O3, respectively, by utilizing the chemical sensitivity of X-ray emission spectra around the Euγ4 line.24 In the previous section, the enhancement of the EXAFS amplitude was ascribed to the improvement of the overall energy resolution in the HERFD mode. However, it can be derived from an unintentional state-selectivity, such as valence-sensitive XES of the Pt/Al2O3 samples. If so, the scope of the application of HERFD-EXAFS is very narrow.The X-ray emission spectra of the Pt Lα1 line of PtO2 and 0.1 wt% Pt/Al2O3 are shown in Fig. 5. The electronic structure of the Pt atom in these samples can be different because of the smallness of the PtO2-like species on Al2O3. However, both XES are identical to each other, which means that the Pt Lα1 line is not sensitive to the chemical state of the Pt atoms. In addition, the FWHM of the Pt Lα1 line was about 7.3 eV. As mentioned before, the energy resolution of the incident X-ray and crystal analyser are 0.8 eV and 1.5 eV, respectively. Thus, the XAS measurement in the HERFD mode can be made versatile using chemically insensitive X-ray emission lines (at least in the Pt L3-edge study).
Conclusions
In this study, we successfully demonstrated the feasibility of HERFD-EXAFS measurements. We utilized one of the range-extended EXAFS approaches at the Pt L3-edge of Pt/Al2O3 in the presence of Au2O3 and its quantitative analysis by using a conventional EXAFS curve fitting technique. We found that the Pt L3-edge HERFD-EXAFS spectrum was significantly enhanced against the corresponding spectrum measured in total fluorescence yield mode. This new finding can be explained by the difference in the overall energy resolution in HERFD and TFY modes.The HERFD-EXAFS method realizes EXAFS analysis on the arbitrary target element in the presence of other elements, which have their absorption edges close to that of the target element. We have already measured a Pt L3-edge EXAFS spectrum of some catalysts in the presence of W, which was our initial interest in the application of EXAFS spectroscopy to a mixture of several elements with their X-ray absorption edges close to each other. This result will be discussed in a separate paper.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
XAS experiments were performed at a QST beamline BL11XU at SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2016A3567 and 2016B3564) and at a public beamline BL39XU at SPring-8 with the approval of the JASRI (Proposal No. 2015A1470, 2015B1260, 2016A1159 and 2016A1837). H. A. appreciates discussion with Prof. Masao Tabuchi (Nagoya University, Japan) on the interpretation of HERFD-EXAFS spectra. This work was performed under the management of “Elements Strategy Initiative for Catalysts & Batteries (ESICB)” supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, program “Elements Strategy Initiative to Form Core Research Center.” A part of this work was performed under the Shared Use Program of the National Institutes for Quantum and Radiological Science and Technology (QST) Facilities (Proposal No. 2016A-E07 and 2016B-H04) supported by the QST Advanced Characterization Nanotechnology Platform as a program of “Nano-technology Platform” of the MEXT (Proposal No. A-16-QS-0006). This study was partially supported by Grants-in-Aid for Scientific Research (C) (Grant No. 15K05195) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.Notes and references
- W. Gu, L. Jacquamet, D. S. Patil, H. X. Wang, D. J. Evans, M. C. Smith, M. Millar, S. Koch, D. M. Eichhorn, M. Latimer and S. P. Cramer, J. Inorg. Biochem., 2003, 93, 41–51 CrossRef CAS PubMed.
- J. Tulkki and T. Aberg, J. Phys. B: At. Mol. Phys., 1982, 15, L435 CrossRef CAS.
- P. Glatzel, F. M. F. de Groot, O. Manoilova, D. Grandjean, B. M. Weckhuysen, U. Bergmann and R. Barrea, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 014117 CrossRef.
- J. Yano, Y. Pushkar, P. Glatzel, A. Lewis, K. Sauer, J. Messinger, U. Bergmann and V. Yachandra, J. Am. Chem. Soc., 2005, 127, 14974–14975 CrossRef CAS PubMed.
- S. Lafuerza, J. García, G. Subías, J. Blasco and P. Glatzel, Phys. Rev. B, 2016, 93, 205108 CrossRef.
- M. Bianchini and P. Glatzel, J. Synchrotron Radiat., 2012, 19, 911–919 CrossRef CAS PubMed.
- P. Glatzel, T.-C. Weng, K. Kvashnina, J. Swarbrick, M. Sikora, E. Gallo, N. Smolentsev and R. A. Mori, J. Electron Spectrosc. Relat. Phenom., 2013, 188, 17–25 CrossRef CAS.
- T. Kaito, H. Mitsumoto, S. Sugawara, K. Shinohara, H. Uehara, H. Ariga, S. Takakusagi, Y. Hatakeyama, K. Nishikawa and K. Asakura, J. Phys. Chem. C, 2014, 118, 8481–8490 CAS.
- E. F. Eikenberry, C. Brönnimann, G. Hülsen, H. Toyokawa, R. Horisberger, B. Schmitt, C. Schulze-Briese and T. Tomizaki, Nucl. Instrum. Methods Phys. Res., Sect. A, 2003, 501, 260–266 CrossRef CAS.
- T. Inami, T. Fukuda, J. Mizuki, H. Nakao, T. Matsumura, Y. Murakami, K. Hirota and Y. Endoh, Nucl. Instrum. Methods Phys. Res., Sect. A, 2001, 467–468, 1081–1083 CrossRef CAS.
- S. I. Zabinsky, J. J. Rehr, A. Ankudinov, R. C. Albers and M. J. Eller, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 52, 2995–3009 CrossRef CAS.
- B. Ravel and M. Newville, J. Synchrotron Radiat., 2005, 12, 537–541 CrossRef CAS PubMed.
- J. Jaklevic, J. A. Kirby, M. P. Klein, A. S. Robertson, G. S. Brown and P. Eisenberger, Solid State Commun., 1977, 23, 679–682 CrossRef CAS.
- K. Hämäläinen, D. P. Siddons, J. B. Hastings and L. E. Berman, Phys. Rev. Lett., 1991, 67, 2850–2853 CrossRef PubMed.
- J. C. Swarbrick, U. Skyllberg, T. Karlsson and P. Glatzel, Inorg. Chem., 2009, 48, 10748–10756 CrossRef CAS PubMed.
- F. M. F. de Groot, M. H. Krisch and J. Vogel, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 195112 CrossRef.
- J. J. Olivero and R. L. Longbothum, J. Quant. Spectrosc. Radiat. Transfer, 1977, 17, 233–236 CrossRef.
- A. Mijovilovich, H. Hayashi, N. Kawamura, H. Osawa, P. C. A. Bruijnincx, R. J. M. Klein Gebbink, F. M. F. de Groot and B. M. Weckhuysen, Eur. J. Inorg. Chem., 2012, 2012, 1589–1597 CrossRef CAS.
- K. A. Lomachenko, C. Garino, E. Gallo, D. Gianolio, R. Gobetto, P. Glatzel, N. Smolentsev, G. Smolentsev, A. V. Soldatov, C. Lamberti and L. Salassa, Phys. Chem. Chem. Phys., 2013, 15, 16152–16159 RSC.
- O. Hirsch, K. O. Kvashnina, L. Luo, M. J. Süess, P. Glatzel and D. Koziej, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 15803–15808 CrossRef CAS PubMed.
- G. Peng, F. M. F. deGroot, K. Hämäläinen, J. A. Moore, X. Wang, M. M. Grush, J. B. Hastings, D. P. Siddons and W. H. Armstrong, J. Am. Chem. Soc., 1994, 116, 2914–2920 CrossRef CAS.
- Y. Izumi, H. Oyanagi and H. Nagamori, Bull. Chem. Soc. Jpn., 2000, 73, 2017–2023 CrossRef CAS.
- Y. Izumi and H. Nagamori, Bull. Chem. Soc. Jpn., 2000, 73, 1581–1587 CrossRef CAS.
- H. Hayashi, N. Kawamura, M. Mizumaki and T. Takabatake, Anal. Chem., 2009, 81, 1522–1528 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Pt L3-edge XAS spectra of 0.1 or 0.5 wt% Pt/Al2O3 in the transmission, TFY, or HERFD modes and a curve fitting result. See DOI: 10.1039/c7ja00309a |
| This journal is © The Royal Society of Chemistry 2018 |