Chondrocyte burst promotes space for mineral expansion†
Emilio Satoshi
Hara
 a,
Masahiro
Okada
a,
Masahiro
Okada
 a,
Noriyuki
Nagaoka
a,
Noriyuki
Nagaoka
 b,
Takako
Hattori
b,
Takako
Hattori
 c,
Letycia Mary
Iida
c,
Letycia Mary
Iida
 a,
Takuo
Kuboki
a,
Takuo
Kuboki
 d,
Takayoshi
Nakano
d,
Takayoshi
Nakano
 e and
Takuya
Matsumoto
e and
Takuya
Matsumoto
 *a
*a
aDepartment of Biomaterials, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, 2-5-1 Shikata-cho, Kita-ku, Okayama-shi, Okayama-ken, 700-8525, Okayama, Japan. E-mail: haraemilio@okayama-u.ac.jp; m_okada@cc.okayama-u.ac.jp; letycia.iida@gmail.com; tmatsu@md.okayama-u.ac.jp; Fax: +81-86-235-6669; Tel: +81-86-235-6666 Tel: +81-86-235-6667 Tel: +81-86-235-6665
bAdvanced Research Center for Oral & Craniofacial Sciences, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan. E-mail: nagaoka@okayama-u.ac.jp; Fax: +81-86-235-6669; Tel: +81-86-235-6734
cDepartment of Oral Biochemistry and Molecular Dentistry, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan. E-mail: hattorit@cc.okayama-u.ac.jp; Fax: +81-86-235-6669; Tel: +81-86-235-6646
dDepartment of Oral Rehabilitation and Regenerative Medicine, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan. E-mail: kuboki@md.okayama-u.ac.jp; Fax: +81-86-235-6680; Tel: +81-86-235-6682
eDivision of Materials and Manufacturing Science, Graduate School of Engineering, Osaka University, Osaka, Japan. E-mail: nakano@mat.eng.osaka-u.ac.jp; Fax: +81-6-6879-7512; Tel: +81-6-6879-7512
First published on 15th January 2018
Abstract
Analysis of tissue development from multidisciplinary approaches can result in more integrative biological findings, and can eventually allow the development of more effective bioengineering methods. In this study, we analyzed the initial steps of mineral formation during secondary ossification of mouse femur based on biological and bioengineering approaches. We first found that some chondrocytes burst near the mineralized area. External factors that could trigger chondrocyte burst were then investigated. Chondrocyte burst was shown to be modulated by mechanical and osmotic pressure. A hypotonic solution, as well as mechanical stress, significantly induced chondrocyte burst. We further hypothesized that chondrocyte burst could be associated with space-making for mineral expansion. In fact, ex vivo culture of femur epiphysis in hypotonic conditions, or under mechanical pressure, enhanced mineral formation, compared to normal culture conditions. Additionally, the effect of mechanical pressure on bone formation in vivo was investigated by immobilization of mouse lower limbs to decrease the body pressure onto the joints. The results showed that limb immobilization suppressed bone formation. Together, these results suggest chondrocyte burst as a novel fate of chondrocytes, and that manipulation of chondrocyte burst with external mechano-chemical stimuli could be an additional approach for cartilage and bone tissue engineering.
Insight, innovation, integrationAnalysis of tissue development from multidisciplinary approaches can result in more integrative biological findings, and can eventually allow the development of more effective bioengineering methods. In this study, we first found that chondrocytes burst near the mineralized area in mouse femur epiphysis. Chondrocyte burst was shown to be modulated by osmotic and mechanical pressure. We further hypothesized that chondrocyte burst could be associated with space-making for mineral expansion. In fact, ex vivo culture of femur epiphysis in hypotonic conditions or under mechanical pressure enhanced mineral formation. Together, these results suggest chondrocyte burst as a novel fate of chondrocytes, and that manipulation of chondrocyte burst with external mechano-chemical stimuli could be an additional approach for cartilage and bone tissue engineering. |
Introduction
Cartilage is an avascular tissue mainly composed of a dense collagenous extracellular matrix, and is an essential organ for joint movement and other vital functions, such as breathing. Cartilage tissue is also known to have a scaffold-like function for subsequent development of bones, such as in the case of primary and secondary ossification centers in long bones.1 In this context of endochondral ossification, chondrocytes secrete several matrix proteins (e.g., type II collagen) and mineralization factors that optimize the environment for mineralization. Chondrocytes eventually become hypertrophic and die (e.g., apoptosis), and promote space for mineral expansion.Throughout the last few decades, numerous studies have investigated the cellular mechanisms of chondrogenesis, chondrocyte maturation and hypertrophy and mineral formation.2–5 Additionally, bioengineering techniques have been developed for manipulation of cells and tissues for cartilage synthesis and reconstruction.6,7 Of note, these studies have indicated that mineralization occurs inside the bioengineered cartilaginous pellets, mainly due to chondrocyte death (e.g., apoptosis) caused by hypoxic levels in the inner region of the cell aggregate (pellet). Therefore, bioengineered cartilaginous pellets have been used for analysis of chondrogenesis, as well as chondrocyte death and bone formation. Nevertheless, highly tunable manipulation of cartilage tissue-associated mineralization still remains a major difficulty. Understanding the physico-chemical and biological factors associated with or determining chondrocyte death and subsequent mineral formation is crucial for further improvement in bioengineering techniques for cartilage tissue synthesis and reconstruction, as well as for control of bone formation or even further bone marrow fabrication.
In this study, we first looked at the initial process of bone formation in the secondary ossification center of mouse femur epiphysis, in an attempt to understand the very initial events associated with chondrocyte fate and mineral formation. We first identified the initial minerals at post-natal day 5.5 (P5.5) by histological staining. Next, using femur epiphysis explants, we found that chondrocytes had burst near the mineralized area. We hypothesized that chondrocyte burst could be associated with mineral formation and expansion; thus, we used chemico-mechanical stimuli to induce chondrocyte burst, and to manipulate bone tissue formation inside the femur epiphysis in ex vivo experiments.
Materials and methods
Animals
New-born Balb/c mice were used in the experiments according to the Guidelines for Animal Research of Okayama University, under the approval of the Animal Care and Use Committee of Okayama University (OKU-2014283 and OKU-2015542). For analysis of bone formation labeled with calcein (Sigma-Aldrich, St. Louis, MO, USA), mice were injected calcein (20 mg kg−1) intra-peritoneally at least one day before euthanasia. For lower limb immobilization experiments, P3 mice had the left lower limb fixed with a plastic tube (4.5 mm diameter × 21 mm length) and a fast-acting cyanoacrylate adhesive (Aron alpha, Toa Gosei, Tokyo, Japan). The contra-lateral limb was used as the control group. The animals were kept in plastic cages, with free access to water, and received standard diet.Time-lapse imaging and chondrocyte burst analysis
For time-lapse imaging, P6 knee joints were collected, and the dissected femur epiphysis samples were immediately cut fronto-distally in approximately 100 μm-thick slices, and kept in direct contact with the glass part of glass-bottom 35 mm dishes for approximately 30 sec, at 37 °C, to allow the tissue to attach onto the dish surface. Tissues were maintained in DMEM/F12 (Wako Pure Chemical Industries, Osaka, Japan) containing 1% antibiotics (penicillin and streptomycin, Sigma), and were observed with a 40× optical zoom lens under a microscope (Nikon Eclipse Ti, Tokyo, Japan), with 5% CO2 in air, at 37 °C. Time-lapse images were then processed to generate a movie using ImageJ software. Mounted videos were used for qualitative and quantitative measurements of chondrocyte burst per 0.35 × 105 μm2. Chondrocyte burst was defined as a change in cellular membrane conformation followed by a decrease in cell size. If there was no visible change in cell size, the cells were not considered as burst cells.Effect of osmotic pressure on chondrocyte burst and mineral formation
To analyze the effect of osmotic pressure on chondrocyte burst, hypotonic (0.5×), normal [isotonic (1×)] and hypertonic (2×) media were obtained by serial dilution of 10× concentrated DMEM (Sigma), with the pH adjusted to 7.5. No change in pH was observed during the serial dilution steps. Epiphysis cartilage slices were then incubated in each medium type, with 5% CO2 in air, at 37 °C, under a time-lapse microscope (Nikon Eclipse Ti) for 60 min. Time-lapsed images were then processed to generate a movie using ImageJ software, and then analyzed for the number of chondrocyte burst.For analysis of the effect of osmotic pressure on mineral formation after induction of chondrocyte burst, isolated P6 femur epiphyses were incubated in PBS solutions with different osmotic pressure (hypertonic, isotonic (normal) and hypotonic) for 24 h, and then in the mineralization-inducing medium (DMEM/F12 supplemented with β-glycerophosphate) for an additional 2 days. PBS with 1.37 M NaCl, 2.7 mM KCl, 10 mM Na2HPO4·12H2O and 1.8 mM KH2PO4 was used as normal (isotonic) solution. PBS with no NaCl and 2.74 M NaCl were used as hypotonic and hypertonic solution, respectively. Since mineralization is highly sensitive to the amount of mineralization associated ions (i.e., calcium and phosphorus), DMEM with different concentrations was not used in the mineralization-inducing experiments in order to maintain the concentrations of mineralization-associated ions equal in the tissue. After incubation in the mineralization-inducing medium, samples were fixed with 4% PFA, and submitted to bone volume analysis by microCT. Micro-CT images of the collected epiphysis were obtained using a SkyScan 1174 compact micro-CT (SkyScan, Aartselaar, Belgium), at a resolution of 6.4 μm. Micro-CT sections were reconstructed to produce the final 3D images using Nrecon and CTVol SkyScan software. Bone volume in all epiphyses was analyzed under the same parameters using CTAn SkyScan software.
Analysis of the effect of external mechanical pressure on chondrocyte burst and mineral formation
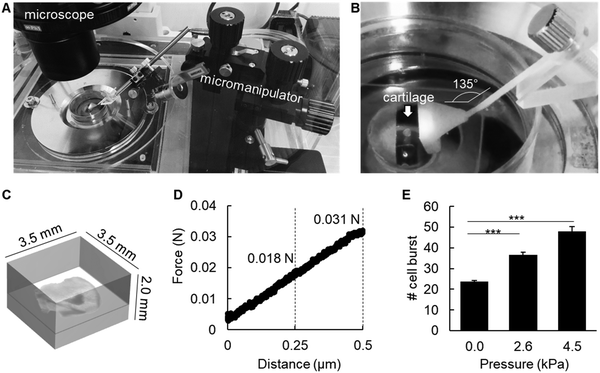
First, cartilage slices approximately 100 μm in thickness were embedded in 2% agarose gel (SeaPlaque GTG agarose, Lonza, Basel, Switzerland), as previously described.8 Mechanical stimulation of cartilage tissue explants embedded in agarose gel was performed by inducing a deformation in the gel of 250 μm and 500 μm from the gel surface with a micromanipulator (Narishige, Tokyo, Japan), which was fixed on the microscope stage (Nikon Eclipse Ti). The three arms of the micromanipulator were used to adjust the position of a 3D-printed probe for application of horizontal pressure to the gel. The 3D printed probe was designed with Clay Tools software (Geomagic, NC, USA), and printed in a desktop 3D printer (Objet 24, Stratasys, Eden Prairie, MN, USA). A rigid and opaque white polymer (VeroWhitePlus RGD835; Stratasys) containing an acrylic monomer and an acrylate oligomer was used as the raw material. The support material consisted of 1,2-propylene glycol, polyethylene glycol, an acrylic monomer, and a photo-initiator (FullCure 705, Stratasys).9 Mechanical pressure was applied for exact 60 min, with pictures taken every second. Time-lapsed images were then processed to generate a movie using ImageJ software, and then analyzed for the number of chondrocyte burst (Fig. S1, ESI†).For analysis of bone formation upon mechanical pressure-induced chondrocyte burst, a rectangle plastic dish was used as a mold for agarose gel fabrication. The agarose sol was poured into the mold, and before complete gelation, the entire epiphyses were embedded in the gel. After complete gelation, plastic blocks 1 mm in thickness were inserted in between the plastic dish wall and the agarose gel, to induce pressure onto the epiphysis. Epiphyses were maintained under pressure for 3 h, and then removed from the gel and cultured in DMEM/F12 supplemented with β-glycerophosphate for an additional 48 h. Bone volume was further analyzed by micro-CT, under the same conditions described above.
Finite element analysis (FEA)
A P5 toluidine blue-stained tissue cross section was used to capture the 2D image data (Fig. S2A, ESI†). The photograph was trimmed (70 × 54 pixels) and preserved as an 8-bit BMP file with Photoshop CS5 (Adobe, San Jose, CA, USA), and the BMP file was imported into an image processing application programmed by the authors with FORTRAN 95 format to construct a 2D quadrilateral model (2785 nodes and 2667 elements). The hypertrophic region was extracted based on the brightness of each pixel (Fig. S2B, ESI†). The compressive pressure was applied and divided on each node of the bottom surface, and zero boundary conditions were assigned at each node of the upper surface. FEA was conducted to solve the linear equation, which was obtained by using the traditional virtual work principle approach10 for the 2D quadrilateral model under plate strain deformation, by using a method of conjugated gradients11 programmed by the authors with FORTRAN 95 format under the assumption that the structures were isotropic and linearly elastic. Young's moduli of the normal cell region and hypertrophic cell region were assigned to 1 kPa and 10 kPa, respectively. The von Mises stress12 distribution calculated by FEA was visualized as shown in Fig. S2B and C (ESI†) with ParaView 4.2.0-RC1.13Statistical analysis
Analysis of the differences between groups was performed with unpaired Student's t-test, or one-way ANOVA followed by a Tukey post hoc correction test, when appropriate. GraphPad Prism 5 software was used for the analyses.Results
Identification of the initial mineralization site and analysis of time and stage-specific changes in femur epiphysis
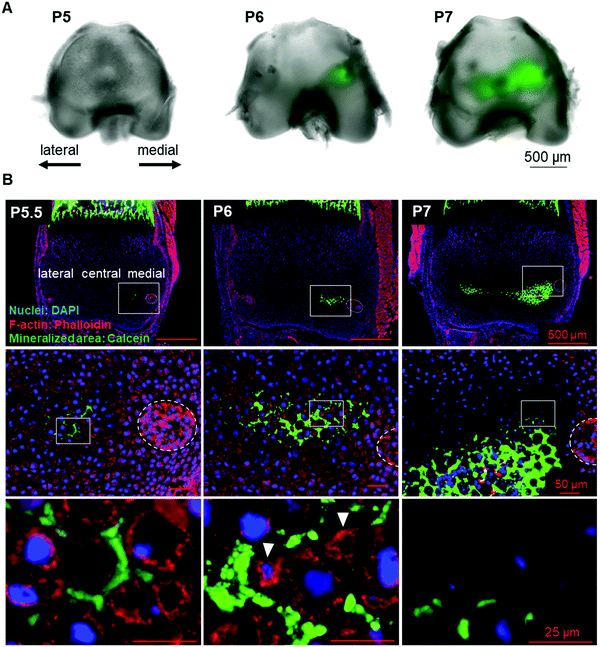
To identify the initial mineral deposition in the cartilage epiphysis of mouse femur, we performed histological analysis of calcein-injected mouse femur epiphysis. The results showed that the initial minerals were formed in the extracellular matrix (ECM) region of the medial side of P5.5 epiphysis (Fig. 1A and B, left panel). Subsequently, minerals expanded towards the lateral side from P6 to P7 (Fig. 1A and B). Concomitant with these observations in the inorganic material formation, we could observe morphological changes in chondrocytes in a site-specific manner. From P5, chondrocytes became hypertrophic and were arranged in close contact with each other, as depicted in ESI,† Fig. 2A (medial side). Importantly, as shown in Fig. 1B (left panels), there were no other cells involved in the initial mineralization steps in femur epiphysis, indicating that the early steps of mineralization (until P6) were not based on osteoblast activity, but mainly on that of chondrocytes.Chondrocyte burst
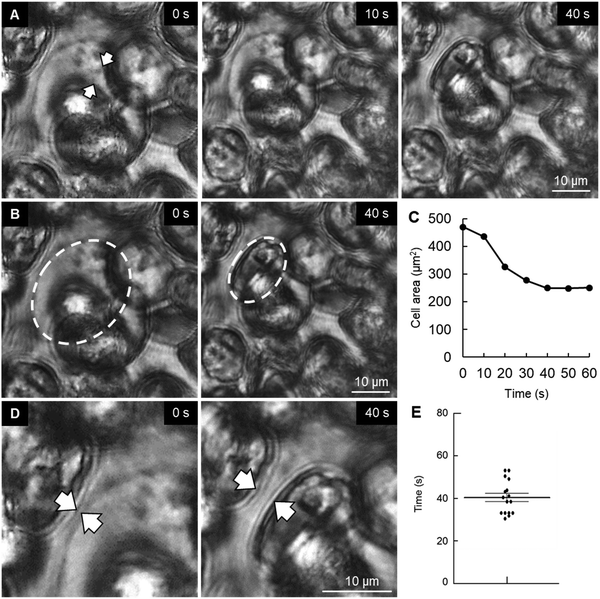
In an attempt to understand the process of space making for mineral formation and expansion, we performed a time-lapse imaging of P6 cartilage samples. Surprisingly, we found that chondrocytes in the vicinity of the mineralized area had slowly burst (Movie S1, ESI†). We could observe that the chondrocyte membrane collapsed and subsequently the cell shrank during the following seconds, with a decrease in half of total cell area (Fig. 2A–C). Analysis of cell burst images showed an average bursting time of 41 s (Fig. 2E). Of note, time-lapse observation of osteoblasts from mouse embryonic calvaria, which are involved in intramembranous ossification, did not show any cell burst under the same conditions (data not shown).Another observation from the time-lapse imaging was the extracellular space formed after chondrocyte burst (Fig. 2D), which was identical to the shape of the minerals observed in histological sections (Fig. 1B, arrowheads in the middle lower panel). Therefore, since hypertrophic chondrocytes were originally in close contact with each other, a decrease in chondrocyte volume due to chondrocyte death (burst or apoptosis14) could promote space for mineral expansion.
Osmotic pressure triggers chondrocyte burst and enhances mineral expansion in vitro
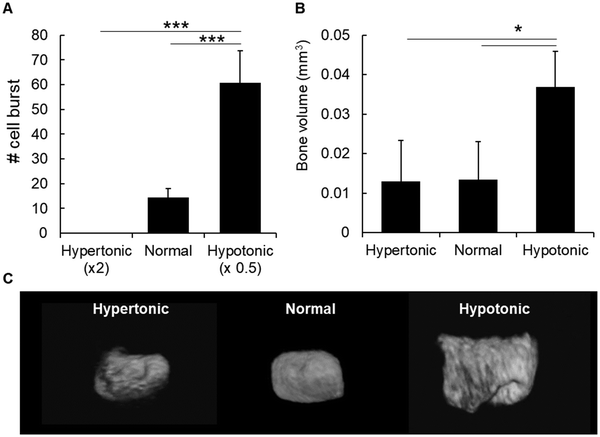
In order to understand the factors that could induce chondrocyte burst and mineral formation, we first analyzed the effect of osmotic pressure because previous reports have shown that a hypotonic condition induces the burst of erythrocytes,15 due to water influx into the cells. Likewise, we maintained slices of cartilage epiphysis in hypotonic media for 1 h and observed chondrocyte burst by time-lapse. As shown in Fig. 3A and Movies S2–S4 (ESI†), a hypotonic environment strongly induced chondrocyte burst, most likely due to water influx into the cells.15 On the other hand, we observed no chondrocyte burst in a hypertonic condition. In normal culture medium, there was only a few number of chondrocyte burst (Fig. 3A).Next, in order to investigate the effect of osmotic pressure on chondrocyte burst-associated bone formation, the entire P6 epiphyses were cultured temporarily (24 h) in PBS with different osmotic pressure. As shown in Fig. 3B and C, there was a significantly higher amount of bone formation in the samples maintained in the hypotonic condition, compared to those in the normal or hypertonic condition.
External mechanical force triggers chondrocyte burst and modulates endochondral ossification
Secondary ossification is a process that occurs in post-natal days and consequently under a dynamic biomechanical condition due to the body weight pressure onto the joints under movement. Therefore, we hypothesized that an external mechanical force could also induce chondrocyte burst, and consequently affect initial mineralization in the femur epiphysis. First, we performed simulations using FEA in an attempt to understand the pressure possibly applied onto the joints. Based on previous studies evaluating the mechanical stiffness of chondrocytes and the pericellular matrix, we assigned the Young's moduli of the normal cell region and the hypertrophic cell region to 1 kPa and 10 kPa, respectively.16–19 FEA could then predict that the medial side is the region where the external force is concentrated, due to its longer anatomical shape, compared to the lateral side (Fig. S1B and C, ESI†). These data support the notion that mechanical loading could be an important factor associated with initial bone formation at the medial side of femur epiphysis.Note that the aim for using FEA was not to calculate the accurate pressure distribution in the cartilage, but rather to analyze whether the pressure could concentrate in the region rich in hypertrophic cells (medial side). Therefore, a simple elastic 2D model was used in this study. It is also worth noting that we cannot justify even non-elastic 3D models in microscopic resolution due to the lack of parameters such as Young's modulus, diffusion coefficient and viscosity-related parameters of organic matrices, cell membranes and cytoplasms of normal and hypertrophic cells.
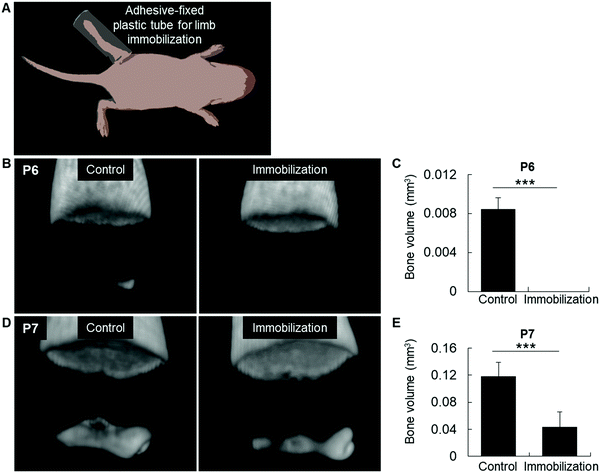
We then performed an in vivo experiment to reduce the body pressure onto the joints by immobilizing the lower limb with a plastic crutch fixed with a fast-acting cyanoacrylate adhesive at P3, before mineralization (Fig. 4A). Strikingly, we found no bone formation in the immobilized limb at P6, while bone development was normal in the contra-lateral control limb (Fig. 4B and C). Accordingly, at P7, bone volume was also decreased in the epiphysis of immobilized limbs, confirming a direct association between mechanical pressure and initial bone mineralization (Fig. 4D and E).
To analyze whether mechanical pressure could also induce chondrocyte burst, we used ex vivo slices of P6 epiphysis approximately 100 μm in thickness, and embedded the tissue in 2% agarose gel (Fig. 5A–C). The embedded cartilage samples were then submitted to different levels of mechanical pressure for 1 h, using an originally designed pressure device with a 3D-printed probe (Fig. 5A–C). For calculation of the pressure to be applied onto the epiphysis samples, the following values were considered. The body weight of a new born mouse is approximately 2 g (2 × 10−3 kg). Considering the gravitational field as 9.8 m s−2 and the joint area as 0.2 cm2, the pressure onto each joint would be (2 × 10−3 × 9.8 ÷ 0.2 ÷ 4 joints = 0.0245 kgf cm−2 = 2.4 kPa). The force applied onto the epiphysis-containing gel was 0.017 N (≈ 2.6 kPa) and 0.031 N (≈ 4.5 kPa) upon a compression of 250 μm and 500 μm, respectively. The authors used the distance in micrometers as a way to better control the pressure applied onto the epiphysis samples by using the micromanipulator. The agarose gel presented a linear elastic property under these experimental settings (Fig. 5D). As shown in Fig. 5E, the number of chondrocyte burst increased in a pressure-dependent manner, indicating a direct association of mechanical stress as a trigger for chondrocyte burst.
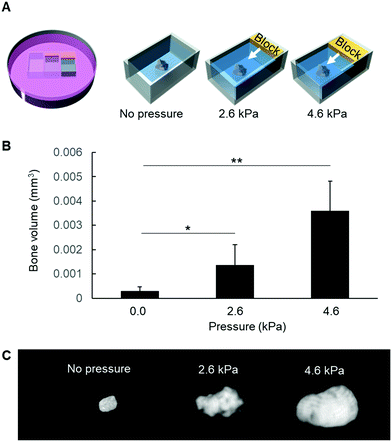
Finally, in an attempt to confirm whether mechanical pressure could be associated with mineral formation, the entire epiphyses were embedded in agarose and submitted to different mechanical pressures for 3 h in vitro (Fig. 6A). Afterwards, the epiphyses were removed from the agarose gel and cultured in β-glycerophosphate-supplemented DMEM/F12. As shown in Fig. 6B and C, mechanical pressure significantly induced mineral formation inside the epiphysis, in a pressure-dependent manner.
Taken together, these results indicate that mechanical stress is an important factor for chondrocyte burst and for in vivo mineral formation, suggesting that initial mineralization in the medial side of the femur epiphysis could be associated with mechanical stress-triggered chondrocyte burst.
Discussion
Secondary ossification of femur epiphysis in mice initiates during the first postnatal week, and it enables detailed observation of the dynamic changes in organic and inorganic materials in a time- and stage-specific manner. Additionally, it is unique in that it allows the analysis of external mechanical stress of the body weight onto the epiphysis at the very initial mineralization stages. In other words, it enables the investigation of the effect of biomechanical (biophysical) forces on initial bone mineralization associated with biochemical and morphological changes in chondrocytes, in the absence of osteoblasts.A time-lapse imaging of ex vivo cartilage epiphysis samples showed that chondrocytes go through a bursting process. Here, we defined cell burst not as a complete destruction of the cells, but as a shrinkage of their volume. Previous studies have reported the chondrocyte bursting phenomenon, using monolayer cells20,21 or intact ex vivo cultured cartilage tissue.22 When chondrocytes were maintained in a hypotonic environment, there was a marked increase in chondrocyte volume without changes in cellular dry mass, and subsequently, the cells burst, with marked loss in dry mass.20,21 Accordingly, we herein demonstrated that a hypotonic condition markedly induced chondrocyte burst. Moreover, we showed that a hypotonic condition enhanced in vitro mineral formation inside the epiphyses.
To the authors’ knowledge, there is no study that has measured directly the osmotic pressure of tissues during bone development in vivo. Osmotic pressure in vivo can be supposed to be changed by several factors, such as by the invasion of negatively charged blood plasma proteins (e.g., albumin) in the extracellular region. Additionally, changes in the concentration of specific ions [e.g., calcium, phosphorus, hydrogen (pH)] could also be factors facilitating chondrocyte burst. It is known that cells make use of ATP-dependent ion pumps to control their osmolality in relation to the extracellular environment.15 Therefore, the activity of ATP or ion pump levels could be impaired or their expression levels decreased, in comparison to other cell types. In fact, loss of activity of ATP transporters, which affect intracellular ion concentration, is known to affect osmotic pressure and induce cell swelling.23 Subsequently, cell swelling could be an important phenomenon facilitating chondrocyte burst. Interestingly, a previous study using a single-cell photolysis experimental model showed that intracellular calcium rose prior to cellular membrane lysis induced by 632 nm laser light, and that this early rise in intracellular calcium was necessary for cellular membrane rupture.24 Treatment of cells with the channel blockers was effective in either decreasing or eliminating cell burst, even under lethal doses of photosensitizer and irradiation.24 Based on this report, we assumed that calcium could be one strong candidate factor facilitating chondrocyte burst. Accordingly, previous studies have demonstrated that fluid flow-induced shear stress or mechanical stress induced an increase in intracellular calcium possibly through mechanosensitive channels, and that these signals could be propagated to adjacent cells through gap-junctions.25–27 On the other hand, in vitro experiments have shown that high phosphate levels induce an increase in the expression of the hypertrophic chondrocyte marker, collagen type X, and also induce chondrocyte death, when associated with calcium.28 Thus, the dynamic changes in osmotic pressure, in particular after blood vessel invasion into the cartilage in femur epiphysis, after alkaline phosphatase activity inside chondrocytes (which would increase the intracellular phosphorus levels), and after initial calcium-phosphate formation in the extracellular matrix, could be altogether affecting intra- and extracellular ion concentrations and eventually could be directly or indirectly associated with chondrocyte burst and subsequent mineral formation.
Hypertrophic chondrocytes, which were first observed in the medial side of P4 and P5 epiphysis, present a 5-time increase in cell volume and also marked changes in their stiffness.16 A previous study reported that chondrocytes maintained in hypotonic conditions also present a significant increase in their volume, but a marked decrease in their viscoelastic properties associated with dissociation or redistribution of the F-actin cytoskeleton.21 In other words, osmotic pressure could be one potential factor associated with chondrocyte hypertrophy, and could facilitate chondrocyte burst by decreasing its viscoelastic properties. We then assumed that mechanical pressure could be another important factor inducing chondrocyte burst. In fact, mechanical pressure could significantly induce chondrocyte burst, and could also enhance mineral formation in the epiphysis. The pressure onto the joint (epiphysis) could induce a concentration of force in the medial side of the epiphysis, as simulated by FEA, and this pressure could then induce chondrocyte burst. Consequently, following the burst of chondrocytes, there could be permanent micro-deformation of the cartilage, which could in turn further enhance the concentration of mechanical pressure on this site. Of note, not only the anatomical morphology of the epiphysis (medial side being longer than the lateral side), but also biochemical modifications in hypertrophic chondrocytes, including the formation of intracellular vesicles1 (Fig. S3, ESI†), could be mechanically facilitating factors for cell burst in the medial side. Additionally, due to the highly charged and hydrated nature of the cartilage extracellular matrix, mechanical loading induces exudation of the interstitial water, resulting in dynamic changes in the interstitial osmolarity.20 Therefore, both mechanical and osmotic stress could be affecting simultaneously the chondrocytes and induce cell burst.
The present observations of chondrocyte burst may have an impact not only on developmental biology, but also on tissue engineering and bioengineering fields. External mechanical forces have been pointed out as important factors for in vitro synthesis of cartilage tissue, as these forces are known to induce the synthesis of ECM components by chondrocytes and strengthen the mechanical properties of the synthesized tissue.29 Nevertheless, application of intense mechanical stress to chondrocytes may potentially induce cell burst, and a deeper knowledge of the parameters and thresholds of the mechano-properties of chondrocytes, such as stiffness according to their differentiation stage (proliferative vs. hypertrophic chondrocytes), may direct novel methods for cartilage tissue engineering. On the other hand, mechanical stress has also been demonstrated to be an important factor regulating osteoblast and osteocyte activity, consequently affecting overall bone turnover. Previous studies have also shown that the stiffness of the surrounding microenvironment can control bone formation during primary endochondral ossification of mouse femur.2,3,8 Therefore, it may be possible that chondrocyte burst could be one intermediate event in these models of cartilage-to-bone formation, and manipulation of external mechanical properties of the surrounding environment could affect not only chondrocytes but also osteoblasts and osteocytes.
These results suggest that chondrocyte burst could be an event to promote space making for mineral expansion. More interestingly, chondrocyte burst could give origin to chondrocyte membrane nanofragments, which were shown to be nucleation sites for mineralization.30 In other words, chondrocyte death (e.g., apoptosis, burst) could not simply be a process for space-making, but could allow cellular components (e.g., phosphoproteins) to extravasate throughout the extracellular matrix, and could give origin to cell membrane nanofragments, which could become materials that promote mineral formation in the initial steps of osteoblast-independent mineralization in the femur epiphysis.30
In summary, we introduce the concept of chondrocyte burst as a novel fate of chondrocytes associated with initial mineral formation in femur epiphysis, and demonstrate that manipulation of chondrocyte burst with external mechano-chemical stimuli could be an additional approach for cartilage and bone tissue engineering.
Authors contributions
E. S. H. designed the study, performed the experiments, collected and analyzed all data, prepared all figures and wrote the manuscript. M. O. performed Finite Elemental Analysis, analyzed data and wrote part of the manuscript. N. N. helped in designing part of the experiments. T. H. performed part of the experiments and supplied materials. T. K. analyzed data and supplied materials. T. N. supplied materials. T. M. designed the study, analyzed data and wrote the manuscript.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by JSPS Postdoctoral Fellowship for Foreign Researchers (to E. S. H.), as well as by JSPS KAKENHI Grant Number (JP16H06990, JP16H05533, JP25220912, JP25293402 and JP26106718). The authors also thank Bjorn R. Olsen for his valuable suggestions on the manuscript.References
- Bones and Cartilage: Developmental and Evolutionary Skeletal Biology, ed. B. K. Hall, Academic Press, Cambridge, 2015 Search PubMed.
- E. S. Hara, M. Ono, H. T. Pham, W. Sonoyama, S. Kubota, M. Takigawa, T. Matsumoto, M. F. Young, B. R. Olsen and T. Kuboki, J. Bone Miner. Res., 2015, 30, 1585–1596 CrossRef CAS PubMed.
- J. Sasaki, T. Matsumoto, H. Egusa, M. Matsusaki, A. Nishiguchi, T. Nakano, M. Akashi, S. Imazato and H. Yatani, Integr. Biol., 2012, 4, 1207–1214 RSC.
- E. S. Hara, M. Ono, Y. Yoshioka, J. Ueda, Y. Hazehara, H. T. Pham, T. Matsumoto and T. Kuboki, Cells Tissues Organs, 2016, 201, 88–96 CrossRef PubMed.
- K. Johnson, S. Zhu, M. S. Tremblay, J. N. Payette, J. Wang, L. C. Bouchez, S. Meeusen, A. Althage, C. Y. Cho, X. Wu and P. G. Schultz, Science, 2012, 336, 717–721 CrossRef CAS PubMed.
- C. H. Lee, J. L. Cook, A. Mendelson, E. K. Moioli, H. Yao and J. J. Mao, Lancet, 2010, 376, 440–448 CrossRef CAS.
- E. S. Hara, M. Ono, S. Kubota, W. Sonoyama, Y. Oida, T. Hattori, T. Nishida, T. Furumatsu, T. Ozaki, M. Takigawa and T. Kuboki, Biochimie, 2013, 95, 374–381 CrossRef CAS PubMed.
- G. A. Sathi, K. Kenmizaki, S. Yamaguchi, H. Nagatsuka, Y. Yoshida, A. Matsugaki, T. Ishimoto, S. Imazato, T. Nakano and T. Matsumoto, Tissue Eng., Part C, 2015, 21, 567–575 CrossRef CAS PubMed.
- K. A. Rahman, G. A. Sathi, H. Taketa, M. Farahat, M. Okada, Y. Torii and T. Matsumoto, 3D Printing and Additive Manufacturing, 2015, 2, 5–11 CrossRef.
- Programming the Finite Element Method, ed. I. M. Smith, D. V. Griffiths and L. Margetts, Wiley, Hoboken, 5th edn, 2013 Search PubMed.
- H. R. Schwarz, J. Appl. Math. Phys., 1979, 30, 343 Search PubMed.
- R. v. Mises, Göttin. Nachr. Math. Phys, 1913, 1, 582 Search PubMed.
- Inc. Kitware, ParaView – Open source scientific visualization, http://paraview.org.
- D. Bortner and J. A. Cidlowski, Cell Death Differ., 2002, 9, 1307–1310 CrossRef PubMed.
- Molecular Cell Biology, ed. A. Berk, H. Lodish, S. L. Zipursky, P. Matsudaira, D. Baltimore and J. Darnell, W.H. Freeman Publishers, New York, 2000 Search PubMed.
- C. C. van Donkelaar and W. Wilson, Biomech. Model. Mechanobiol., 2012, 11, 655–664 CrossRef CAS PubMed.
- J. Gao, E. Roan and J. L. Williams, PLoS One, 2015, 10, e0124862 Search PubMed.
- J. Sanchez-Adams and K. A. Athanasiou, Biomech. Model. Mechanobiol., 2012, 11, 1047–1056 CrossRef PubMed.
- P. Radhakrishnan, N. T. Lewis and J. J. Mao, Ann. Biomed. Eng., 2004, 32, 284–291 CrossRef PubMed.
- N. T. Shaked, J. D. Finan, F. Guilak and A. Wax, J. Biomed. Opt., 2010, 15, 010505 Search PubMed.
- F. Guilak, G. R. Erickson and H. P. Ting-Beall, Biophys. J., 2002, 82, 720–727 CrossRef CAS PubMed.
- Y. Li, V. Trivedi, T. V. Truong, D. S. Koos, R. Lansford, C. M. Chuong, D. Warburton, R. A. Moats and S. E. Fraser, Nat. Commun., 2015, 6, 6798 CrossRef CAS PubMed.
- Pathophysiology: The Biologic Basis for Disease in Adults and Children ed. K. L. MacCance and S. E. Huether, Mosby, Saint Louis, 2015 Search PubMed.
- N. A. Busch, S. R. Reiken, M. Toner and M. L. Yarmush, J. Biomech. Eng., 1998, 120, 570–578 CrossRef CAS PubMed.
- C. E. Yellowley, C. R. Jacobs, Z. Li, Z. Zhou and H. J. Donahue, Am. J. Physiol., 1997, 273, C30–36 CrossRef CAS PubMed.
- P. D'Andrea and F. Vittur, FEBS Lett., 1997, 400, 58–64 CrossRef.
- P. D'Andrea, A. Calabrese, I. Capozzi, M. Grandolfo, R. Tonon and F. Vittur, Biorheology, 2000, 37, 75–83 Search PubMed.
- D. Magne, G. Bluteau, C. Faucheux, G. Palmer, C. Vignes-Colombeix, P. Pilet, T. Rouillon, J. Caverzasio, P. Weiss, G. Daculsi and J. Guicheux, J. Bone Miner. Res., 2003, 18, 1430–1442 CrossRef CAS PubMed.
- A. C. Shieh and K. A. Athanasiou, Ann. Biomed. Eng., 2003, 31, 1–11 CrossRef PubMed.
- E. S. Hara, M. Okada, N. Nagaoka, T. Hattori, T. Kuboki, T. Nakano and T. Matsumoto, ACS Biomater. Sci. Eng., 2017 DOI:10.1021/acsbiomaterials.7b00962.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ib00130d |
| This journal is © The Royal Society of Chemistry 2018 |