Synthesis, characterization and cytotoxicity of arene–ruthenium(ii) complexes with acylpyrazolones functionalized with aromatic groups in the acyl moiety†
Abstract
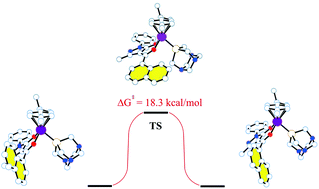
A series of neutral ruthenium(II)–arene complexes, [(arene)Ru(QR)Cl] (arene = p-cymene or hexamethylbenzene), containing 4-acyl-5-pyrazolonate (QR) ligands with aromatic substituents in the acyl moiety (a phenyl in QPh and a 1-naphthyl in Qnaph) and related ionic complexes [(arene)Ru(QR)(PTA)][PF6] (PTA = 1,3,5-triaza-7-phosphaadamantane) have been synthesized and characterized by IR, 1H, 13C and 31P NMR spectroscopy, elemental analysis and ESI mass spectrometry. The structures of five of these compounds were also determined by X-ray crystallography. DFT studies have been performed on all complexes and, in the case of two cationic [(arene)Ru(Qnaph)(PTA)][PF6], the existence of two conformers with a different relative orientation of the naphthyl group in the Qnaph ligand has been assessed, showing that they possess similar energies, in agreement with the experimentally observed NMR spectra in solution. The cytotoxicity of the 4-acyl-5-pyrazolonate proligands (HQR) and complexes was evaluated in vitro against human ovarian carcinoma cells (A2780 and A2780cisR) and non-tumorous human embryonic kidney (HEK293) cells. In general, each complex is about equally cytotoxic to all three cell lines and the PTA derivatives with the naphthyl-modified QR ligands are the most active of the series.


 Please wait while we load your content...
Please wait while we load your content...