Role of lattice oxygen and oxygen vacancy sites in platinum group metal catalysts supported on Sr3Fe2O7−δ for NO-selective reduction†
Kosuke
Beppu
 a,
Saburo
Hosokawa
a,
Saburo
Hosokawa
 *ab,
Hiroyuki
Asakura
*ab,
Hiroyuki
Asakura
 ab,
Kentaro
Teramura
ab,
Kentaro
Teramura
 ab and
Tsunehiro
Tanaka
ab and
Tsunehiro
Tanaka
 *ab
*ab
aDepartment of Molecular Engineering, Graduate School of Engineering, Kyoto University, Kyotodaigaku Katsura, Nishikyo-ku, Kyoto 615-8510, Japan. E-mail: hosokawa@moleng.kyoto-u.ac.jp; tanakat@moleng.kyoto-u.ac.jp
bElements Strategy Initiative for Catalysts & Batteries (ESICB), Kyoto University, Kyotodaigaku Katsura, Nishikyo-ku, Kyoto 615-8245, Japan
First published on 13th November 2017
Abstract
This study demonstrates that NO-selective reduction with C3H6 and CO proceeds over platinum group metal (PGM; Pd, Rh, or Pt) catalysts supported on Sr3Fe2O7−δ with a layered perovskite-type oxide. Among the examined catalysts, Pd-loaded Sr3Fe2O7−δ having high oxygen storage capacity shows the highest catalytic activity at stoichiometric oxygen concentration because the Pd catalyst can release the lattice oxygen of Sr3Fe2O7−δ at lower temperature compared to the Rh- or Pt-loaded catalyst. When the NO-selective reduction at 773 K is carried out at various oxygen concentrations, PGM/Sr3Fe2O7−δ shows superior catalytic activity over a wide range of oxygen concentrations to PGM/Al2O3, which does not have oxygen storage capacity. The oxygen vacancy sites in Sr3Fe2O7−δ, which are generated by the oxidation of C3H6 and CO over PGM/Sr3Fe2O7−δ, are revealed to receive oxygen ions formed by NO reduction on the PGM species; as a result, the PGM species maintain their metal state and act as active sites for NO reduction. Namely, Sr3Fe2O7−δ plays the role of an “oxygen buffer” in inhibiting the PGM-to-oxide transformation. Pt-loaded Sr3Fe2O7−δ can utilize the oxygen vacancy sites more effectively for the catalytic reaction than the Pd and Rh catalysts. To the best of our knowledge, this is the first report on the effective application of perovskite materials with oxygen storage capacity as catalyst supports for NO-selective reduction. The study results are expected to provide valuable information for designing a novel catalyst based on oxygen storage materials.
Introduction
CeO2 and CeO2-based oxides having excellent redox properties can release and store oxygen molecules depending on the reductive or oxidative atmosphere.1 Based on this unique redox function, CeO2-based oxides have been applied as useful materials in various fields such as organic synthesis,2,3 oxide ion conductors,4,5 soot oxidation,6,7 and automotive catalysts.8–11 The redox properties of CeO2 have been improved by heterogeneous metal doping,12–14 morphology control,6,15etc. Zr-doped CeO2, i.e., CeO2–ZrO2 solid solution, is a representative material because it has high oxygen storage capacity12,16,17 and is widely used as a catalyst support for the purification of automotive exhaust gas.8,18 Furthermore, since CeO2-based materials are non-stoichiometric oxygen compounds, many studies on oxygen vacancy sites have been reported.3,8,11,18 For example, the oxygen vacancy sites over CeO2 have been found to be effective for the formation of N2O or N2 in the NO–CO reaction.8,11,18Recently, because of their structural diversity, perovskite-type oxides and perovskite-derived oxides showing oxygen storage performance have gained much attention as alternatives to CeO2-based materials.19–23 Perovskite-derived materials such as Ca0.8Sr0.2MnO3 (ref. 23) and BaYMn2O5+δ (ref. 20 and 21) have been reported to deliver high performance as oxygen storage materials. However, these materials have hardly been applied as supports for automotive catalysts.
We have also reported that Sr3Fe2O7−δ having a layered perovskite structure shows high oxygen storage capacity and high structural stability under severe reductive conditions.24 Pd loading on Sr3Fe2O7−δ has been found to drastically lower the operating temperature for oxygen storage and improve the oxygen release rate.25 It is noteworthy that Sr3Fe2O7−δ can release and store oxygen through topotactic transition, which is interpreted as a transition with retention of cation ordering.26–28 When considering the phase transition of simple transition metal oxides such as α-Fe2O3, the oxygen ions in the lattice of FeO, obtained by the reductive treatment of α-Fe2O3, assume a close-packed structure by rearrangement of the cation and oxygen ions in α-Fe2O3.29,30 Therefore, it is difficult to obtain oxygen vacancy sites in simple transition metal oxides. On the other hand, Sr3Fe2O7−δ, which releases lattice oxygen via topotactic transition, can form a large number of oxygen vacancy sites in the layered perovskite structure. The oxygen storage performance and oxygen vacancy sites in Sr3Fe2O7−δ are expected to play an effective role in various catalytic reactions.
With this background, the present study demonstrates NO-selective reduction over platinum group metal (PGM = Rh, Pd, Pt) catalysts supported on Sr3Fe2O7−δ. The contribution of the lattice oxygen and oxygen vacancy sites of Sr3Fe2O7−δ to NO-selective reduction is investigated. To the best of our knowledge, this is the first example of the purification of automotive exhaust gas utilizing lattice oxygen and oxygen vacancy sites in a perovskite-type oxide having oxygen storage capacity.
Experimental
Preparation of PGM catalysts
Sr3Fe2O7−δ was synthesized by a polymerized complex method, as mentioned in our previous report.25 PGM catalysts were synthesized by an impregnation method. The following PGM precursors were used as source materials: Pt(NH3)2(NO2)2 (HNO3 solution) (4.64 wt%, FURUYA METAL Co., Ltd.), Rh acetylacetonate (97%, Sigma-Aldrich), and Pd acetate (99.9%, Sigma-Aldrich). Rh acetylacetonate or Pd acetate was dissolved in ethyl acetate (99.5%, Wako Pure Chemicals), and Pt(NH3)2(NO2)2 in water. Then, the support (Sr3Fe2O7−δ or γ-Al2O3 (JRC-ALO-7)) was added, and the solution was evaporated at 353 K. The powder obtained after drying the solution containing the PGM precursor and Sr3Fe2O7−δ was calcined at 1073 K for 5 h. The loading amount of the PGM species was 1.0 wt% on a metal basis.NO-selective reduction
The catalytic reaction was carried out using a fixed bed flow reactor at atmospheric pressure. In a tubular reactor, 200 mg of the catalyst sample (25/50 mesh) was placed. The total gas flow rate was 100 mL min−1. The catalyst was pretreated by heating to 773 K under He flow (30 mL min−1) and held for 1 h. The gas concentration was analysed using a gas chromatograph (Shimadzu GC8A) with MS-5A and Porapak-Q columns. The temperature-programmed reaction was carried out by increasing the temperature from 373 K in a stepwise manner (50 K intervals) under stoichiometric conditions: NO, 1000 ppm; CO, 1000 ppm; C3H6, 250 ppm; O2, 1125 ppm. The catalytic activity for various oxygen concentrations was evaluated at 773 K under the following reaction atmosphere: NO, 1000 ppm; CO, 1000 ppm; C3H6, 250 ppm; O2, various concentrations (675–1462.5 ppm); He as balance gas. The λ value was defined as follows: the number of oxygen atoms in the reaction system ([NO] + [CO] + [O2] × 2) was divided by the number of oxygen atoms under stoichiometric conditions ([NO] + [CO] + [O2] × 2 = 4250 ppm). The catalytic reaction was commenced under lean conditions (O2, 1462.5 ppm) and the oxygen concentration was changed to 625 ppm (rich conditions) in a stepwise manner while maintaining the NO and CO concentrations. Subsequently, the oxygen concentration was varied to achieve lean conditions. The reaction gas was held for 30 min at each oxygen concentration. The lean–rich cycle repeatability test for NO-selective reduction was carried out with switching the atmosphere between lean (NO, 1000 ppm; CO, 1000 ppm; C3H6, 250 ppm; O2, 1462.5 ppm) and rich (NO, 1000 ppm; CO, 1000 ppm; C3H6, 250 ppm; O2, 675 ppm) conditions. The catalyst was heated to 773 K under lean conditions and held for 30 min. Then, the reaction conditions were changed between lean and rich conditions every 1 h. The outlet gases were detected by using a micro gas chromatograph (Agilent 490 Micro GC) with MS-5A and PoraPLOT Q columns.H2-TPR
Temperature-programmed reduction with H2 (H2-TPR) was carried out on a flow-type reactor, as outlined in a previous report.24,25 H2 (2 vol% in Ar; 30 ml min−1) was passed through the reactor charged with a sample (0.05 g) under atmospheric pressure. The reactor was heated to 1223 K in an electric furnace at a heating rate of 5 K min−1, and the amount of H2 consumed was monitored with a thermal conductivity detector (TCD) (Shimadzu GC8A).NO pulse
NO pulse experiments were carried out with a flow-type reactor (MicrotracBEL BELCAT-B). The samples (25 mg) were pretreated at 773 K by switching the atmosphere between H2 and 1 vol% O2/He (30 mL min−1) every 20 min, and finally under a H2 atmosphere. Then, the samples were heated to 773 K under He flow (30 mL min−1). The NO pulse (0.997 cm3, 1 vol% in He = 0.434 μmolNO per pulse) was injected into the reactor. The individual injection of pulse was purged with He. The outlet gas was analyzed by means of a TCD-gas chromatograph with an MS-5A or a Porapak Q column. The number of NO pulses was 40.OSC measurement
Oxygen storage capacity (OSC) measurement was carried out with a thermogravimeter (Rigaku Thermoplus), as mentioned in our previous report.24 The catalysts (100 mg) were heated to 773 K in 5% O2/Ar and held until constant weights were achieved. Then, the weight changes of the samples upon switching the atmosphere every 20 min between 5% O2/Ar and 5% H2/Ar were recorded at 773 K.Characterization
X-ray diffraction (XRD) patterns were recorded on a Rigaku Ultima IV apparatus using CuKα radiation. X-ray photoelectron spectroscopy (XPS) measurement was conducted on a Shimadzu ESCA-3400 using MgKα radiation. Each spectrum was calibrated by C1s at 284.6 eV. The specific surface area was evaluated from the N2 adsorption isotherm using the Brunauer–Emmett–Teller method. N2 adsorption was carried out on a MicrotracBEL BELSORP-mini II at 77 K.Results and discussion
Fig. 1 shows the results of NO-selective reduction at stoichiometric oxygen concentration over PGM catalysts supported on Sr3Fe2O7−δ. The oxidation activity over Sr3Fe2O7−δ was observed at 623 K and it reached 60% at 773 K. NO reduction activity was not observed even at 773 K. These catalytic activities were drastically enhanced by PGM loading, although Sr3Fe2O7−δ had a very low surface area (<10 m2 g−1) as a catalyst support. Pd/Sr3Fe2O7−δ showed higher catalytic activity than Pt/Sr3Fe2O7−δ and Rh/Sr3Fe2O7−δ; CO and C3H6 oxidation commenced at 473 K and NO reduction proceeded from 523 K. Before NO conversion to N2 reached 100%, a small amount of N2O as a by-product was observed in all catalysts (Fig. S1†), indicating that N2O species might be an intermediate species in NO-selective reduction over PGM catalysts. The oxidation activity of Pt/Sr3Fe2O7−δ became higher than that of Rh/Sr3Fe2O7−δ in the high temperature region. The CO and C3H6 conversions over the PGM catalysts are displayed in Fig. S1.† Pd and Pt species enhanced the oxidation of both CO and C3H6. However, Rh species promoted the CO oxidation well, but moderately enhanced the C3H6 oxidation. Then, the activity inversion between Pt- and Rh-loaded catalysts is attributed to the different oxidation characteristics of PGM species for the oxidation reaction. In addition, the reduction of NO proceeded after the oxidation of C3H6 and CO in all catalysts.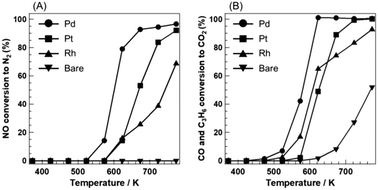 | ||
| Fig. 1 (A) NO conversion to N2 and (B) CO and C3H6 conversion to CO2 (λ = 1) over PGM catalysts supported on Sr3Fe2O7−δ. | ||
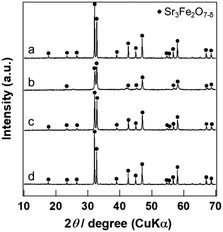
The XRD patterns of PGM/Sr3Fe2O7−δ are displayed in Fig. 2. The XRD patterns of PGM/Sr3Fe2O7−δ were similar to that of the bare catalyst. Furthermore, peaks due to the PGM species were not observed in the XRD patterns of PGM/Sr3Fe2O7−δ. Previously, the Pd species in Pd/Sr3Fe2O7−δ has been revealed to be highly dispersed as Pd2+, whose coordination state is different from that in the PdO structure.25 Similar to Pd/Sr3Fe2O7−δ, a highly dispersed PGM species must be stabilized on Sr3Fe2O7−δ in Rh- and Pt-loaded catalysts.
The peaks of Pt/Sr3Fe2O7−δ were broader than those of the other catalysts. This might be due to the formation of a hydrate phase by water used as an impregnation solvent. Since Sr3Fe2O7−δ has been reported to be hydrated under the existence of excess water,31 the crystal structure of Sr3Fe2O7−δ must be changed into the hydrate phase during the preparation of Pt/Sr3Fe2O7−δ. The hydrate phase was mostly recovered from the crystal structure of Sr3Fe2O7−δ by calcination at 1073 K. Although the relative intensity of the peaks in the XRD pattern of Pt/Sr3Fe2O7−δ was slightly different from that of Sr3Fe2O7−δ, the peak positions in the XRD pattern of Pt/Sr3Fe2O7−δ were almost the same as those of Sr3Fe2O7−δ. Therefore, the crystal structure of Sr3Fe2O7−δ in the Pt catalyst is essentially identical to those in the other catalysts. In addition, the specific surface area (6 m2 g−1) of Pt/Sr3Fe2O7−δ was comparable to those (4–5 m2 g−1) of Rh/Sr3Fe2O7−δ and Pd/Sr3Fe2O7−δ. From these results, the peak broadening of the Pt catalyst was not thought to affect the results of the reaction.
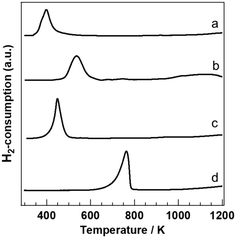
To clarify the reduction behaviour of PGM/Sr3Fe2O7−δ, H2-TPR profiles were investigated (Fig. 3). Each PGM catalyst showed one reduction peak. The reduction peaks of PGM/Sr3Fe2O7−δ were drastically shifted to lower temperature compared to those of Sr3Fe2O7−δ. The oxygen release amounts up to 773 K were 657 μmolO2 gcat−1 and 693 μmolO2 gcat−1 in the Pt and Rh catalysts, respectively. We have reported that the oxygen release amounts of the Pd and bare catalysts are 665 μmolO2 gcat−1 and 618 μmolO2 gcat−1, respectively, and that the reduction peak of Pd/Sr3Fe2O7−δ is attributed to the reduction from Pd2+ and Fe4+ to Pd0 and Fe3+.24,25 Since the oxygen release amounts of Pt/Sr3Fe2O7−δ and Rh/Sr3Fe2O7−δ were comparable to that of Pd/Sr3Fe2O7−δ, each reduction peak of PGM/Sr3Fe2O7−δ was also attributed to the reduction of Fe and PGM species. This result indicates that the oxygen release properties of Sr3Fe2O7−δ are promoted by Pt and Rh loading, as in the case of Pd loading on Sr3Fe2O7−δ. The order of the effect for lowering the oxygen release temperature was Pd > Rh > Pt, corresponding to the order of the light-off temperature for CO and C3H6 oxidation, especially CO oxidation, in each PGM catalyst.
It is known that the oxidation reaction over metal oxide catalysts proceeds by the Mars–van Krevelen mechanism, which is interpreted as follows: a reactant such as CO or C3H6 is oxidized by utilizing the lattice oxygen of a catalyst, and the lattice oxygen is recovered by the oxidant in a reaction system.32–36 Therefore, the activity in an oxidation reaction via the Mars–van Krevelen mechanism is known to correlate with the mobility of the lattice oxygen, which can be evaluated by H2-TPR analysis.34–36 For example, Scirè et al. reported that the oxidation activity for the combustion of volatile organic compounds (VOCs) over Au/CeO2 was well correlated with the mobility of the lattice oxygen in CeO2.34 Therefore, the Au species loaded over CeO2 enhances the release of lattice oxygen, thus improving the oxidation activity via the Mars–van Krevelen mechanism. From the previous reports32–36 and the results of H2-TPR, highly dispersed PGM species enhance the lattice oxygen release of Sr3Fe2O7−δ, resulting in improvement of CO and C3H6 oxidation via the Mars–van Krevelen mechanism. Generally, oxygen vacancy sites, which are generated by the release of lattice oxygen during the oxidation reaction, are filled by molecular oxygen as in the case of the combustion of VOCs. In NO-selective reduction, NO or molecular oxygen can be utilized as an oxidant. That is, NO would fill the oxygen vacancy sites to form N2 molecules. We will describe the details of NO reduction later.
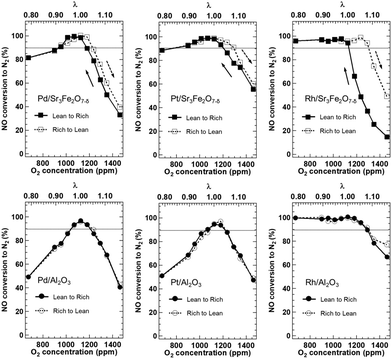
PGM/Sr3Fe2O7−δ having oxygen storage capacity may be able to adjust the oxygen concentration in NO-selective reduction. NO-selective reduction at various oxygen concentrations over PGM/Sr3Fe2O7−δ was carried out. PGM/Al2O3, which does not have oxygen storage capacity, was used for comparison. CO and C3H6 oxidation activity exhibited a similar trend in all catalysts (Fig. S2†): high oxidation activity was observed at high oxygen concentrations (lean conditions, λ > 1), and the activity decreased with decreasing oxygen concentration. On the other hand, the catalysts showed different NO reduction behavior from one another (Fig. 4). In the case of all the catalysts, when the reaction conditions were changed from lean to stoichiometric (λ = 1), the NO reduction activity improved with decreasing oxygen concentration, reaching 100% near the stoichiometric conditions (λ = 1). Under rich conditions, the NO reduction activities over Pd/Sr3Fe2O7−δ and Pt/Sr3Fe2O7−δ decreased slightly but Rh/Sr3Fe2O7−δ maintained high NO reduction activity. When the reaction conditions were changed from rich to stoichiometric, the NO reduction activity improved with increasing oxygen concentration, reaching 100% around the stoichiometric conditions in Pd/Sr3Fe2O7−δ and Pt/Sr3Fe2O7−δ. Interestingly, the high NO reduction activity over PGM/Sr3Fe2O7−δ was maintained even when the reaction conditions were changed from stoichiometric to lean. The order of maintenance of the high NO reduction activity under the lean conditions was Pt/Sr3Fe2O7−δ ≈ Rh/Sr3Fe2O7−δ ≫ Pd/Sr3Fe2O7−δ. PGM/Al2O3 did not show any improvement in NO reduction activity under the lean conditions. These results suggest that Sr3Fe2O7−δ contributes to the enhancement of NO reduction activity over a wide range of oxygen concentrations. When the repeatability test of Pt/Sr3Fe2O7−δ, which showed the highest NO reduction activity under slightly lean conditions in the NO-selective reduction, was carried out, the catalyst was used repeatedly at least three times without deactivation during lean–rich cycles (Fig. S4†).
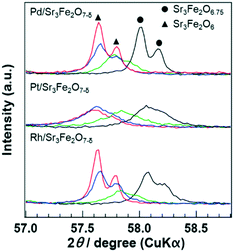
XRD analysis of the catalyst recovered at each point during the reaction was performed to evaluate the lattice oxygen content in PGM/Sr3Fe2O7−δ. The as-synthesized sample, sample treated under the rich conditions (λ = 0.79), and sample after the reaction (i.e., the samples were exposed to rich-to-lean conditions) were compared. In addition, the sample reduced at 773 K under a H2 atmosphere was investigated. The XRD pattern of each catalyst in a wide range is displayed in Fig. S3.† All the patterns were essentially similar to those of Sr3Fe2O6.75 or Sr3Fe2O6 without additional peaks due to by-products, indicating that PGM/Sr3Fe2O7−δ maintained its crystal structure during the reaction. A magnified view of the XRD patterns of PGM/Sr3Fe2O7−δ during the reaction is shown in Fig. 5. The XRD peaks of the samples treated under the rich conditions shifted to lower angles as compared to those of the as-synthesized samples. The peak positions of the samples treated under the rich conditions corresponded to those of the samples reduced at 773 K under a H2 atmosphere, indicating that the amounts of lattice oxygen released from both samples were similar. Considering the fact that the Fe4+ species in Sr3Fe2O6.75 is completely reduced to Fe3+ under a H2 atmosphere at 773 K, PGM/Sr3Fe2O6.75 is reduced to PGM/Sr3Fe2O6 under the rich conditions. This result implies that lattice oxygen is used for the oxidation of CO or C3H6 according to the Mars–van Krevelen mechanism. The peaks for each catalyst after the reaction did not return to those for the as-synthesized catalysts but shifted to a higher angle than those for the samples treated under the rich conditions. This phenomenon indicated that the lattice oxygen is partly recovered by NO or O2 after the lean/rich/lean transition.
The PGM/Sr3Fe2O7−δ catalysts, which showed the same oxygen release amount under the rich conditions, had different properties for maintaining the NO reduction activity under the lean conditions. To clarify the factors affecting the NO reduction activity of PGM/Sr3Fe2O7−δ, we conducted NO pulse experiments (Fig. 6(A)). No N2 formation was observed over Sr3Fe2O7−δ reduced at 773 K in a H2 atmosphere, and PGM loading drastically improved the N2 formation over Sr3Fe2O7−δ (Fig. 6(B)). PGM/Sr3Fe2O7−δ showed higher N2 formation than PGM/Al2O3 (Fig. 6(B) and S5†). When the formation amount of N2 decreased, N2O and NO were initially detected in the NO pulse experiment (Fig. S6†). The material balance for nitrogen species estimated from the total amount of N2, N2O and NO was almost 100%, indicating that NO species is hardly adsorbed or stored during the NO pulse experiments on Pt/Sr3Fe2O7−δ. Interestingly, O2 formation was not observed in all catalysts which exhibit N2 formation for the NO pulse experiment (Fig. S7†); that is, O species formed by NO dissociation must oxidize the PGM species or support. Then, the ideal N2 formation amount over a 1 wt% PGM catalyst is calculated from the following equations representing the reaction between PGM and NO:
| Pt + 2NO → N2 + PtO2 51.3 μmolN2 gcat−1 |
| Pd + NO → 1/2N2 + PdO 47.0 μmolN2 gcat−1 |
| Rh + 3/2NO → 3/4N2 + 1/2Rh2O3 72.9 μmolN2 gcat−1 |
The N2 formation amounts over PGM/Al2O3 were smaller than the ideal amounts, suggesting that all PGM species on Al2O3 could not be attributed to NO reduction. On the other hand, PGM/Sr3Fe2O7−δ generated a larger amount of N2 than the ideal amounts. These results suggest that a synergetic effect between the PGM species and Sr3Fe2O7−δ contributes to NO reduction.
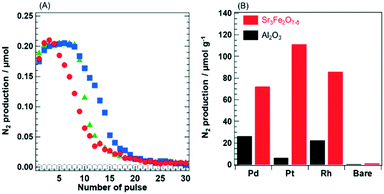
Since N2 formation was not detected in the case of PGM/Sr3Fe2O7−δ without H2 treatment, the oxygen vacancy sites in Sr3Fe2O7−δ generated by the pretreatment are considered to participate in N2 formation. If the oxygen vacancy sites receive oxygen ions generated from NO dissociation on PGM species, the amount of N2 must agree well with the amount of oxygen vacancy sites. The Pt catalyst, which showed the highest N2 formation amount among the examined catalysts, stored 111 μmolO2 gcat−1, while the value was much lower than the amount (657 μmolO2 gcat−1) of oxygen vacancy sites in Pt/Sr3Fe2O6 obtained by reduction in the H2 atmosphere. Furthermore, the ideal number of oxygen vacancy sites (22–33 μmolO2 gcat−1), calculated from the surface area of PGM/Sr3Fe2O7−δ and the area of the (001) facet of Sr3Fe2O6 (ref. 24) obtained by reduction under H2 flow, is much smaller than the amount of the oxygen species (72–111 μmolO2 gcat−1) estimated from the NO pulse experiment. Therefore, we conclude that PGM/Sr3Fe2O7−δ generate N2 by utilizing the oxygen vacancy sites at the surface and subsurface of the support.
Summarizing the results of NO pulse, the interpretations are as follows. NO reduction must proceed over the metal state of PGM species in PGM/Sr3Fe2O7−δ, as the metal state of PGM species has been well known to be the active site for NO reduction. Actually, when the valence state of Pt in Pt/Sr3Fe2O7−δ during the NO pulse experiment was evaluated by XPS analysis (Fig. S8†), the valence state of Pt remained mainly a metal state37 in Pt/Sr3Fe2O7−δ before decreasing the N2 production amount. However, the Pt species was oxidized by NO at the point where N2 formation was not observed. Since the Pt species maintaining its metal state for a long period can dissociate NO molecules, Pt/Sr3Fe2O7−δ retains a high N2 formation amount. On the other hand, O2 formed by NO dissociation was not detected. Considering the relationship between the N2 formation amount and oxygen storage amount, the dissociated oxygen species must be preferentially incorporated into the oxygen vacancy sites around the surface and subsurface of Sr3Fe2O7−δ, instead of oxidation of PGM species occurring. In other words, the vacancy site in Sr3Fe2O7−δ acts as an “oxygen buffer” for inhibiting the oxidation of the PGM species. In addition, NO reduction to N2 needed PGM loading and the reduction treatment of the catalyst; that is, the oxygen vacancy site around the PGM species plays an important role in NO reduction.
Although the Pd catalyst showed the lowest N2 production in the NO pulse experiment, this catalyst exhibited the highest activity for NO conversion among the PGM catalysts in NO-selective reduction (Fig. 1). In NO-selective reduction, the reductants for the catalysts are CO and C3H6, and the catalyst having high CO and C3H6 oxidation activity must be easily reduced during the reaction. Pd/Sr3Fe2O7−δ had the highest oxidation property and was reduced at the lowest temperature among the examined catalysts in H2-TPR analysis. Therefore, Pd/Sr3Fe2O7−δ, which could be easily reduced by CO and C3H6, showed the highest NO reduction activity in the NO-selective reduction (λ = 1).
Excess O2 in NO-selective reduction up to λ = 1.08 was not detected at all over PGM/Sr3Fe2O7−δ after a lean/rich transition, but was observed in PGM/Al2O3. That is, excess O2 must be stored into the vacancy site in the Sr3Fe2O7−δ support with maintaining the high NO conversion. Therefore, the oxygen vacancy sites receive excess O2 molecules as well as oxygen species formed by NO dissociation. Considering the oxygen storage capacities of PGM/Sr3Fe2O7−δ estimated from H2–O2 titration at 773 K (Fig. S9†), the oxygen storage amount of Sr3Fe2O7−δ itself is essentially identical to those of PGM/Sr3Fe2O7−δ. These results suggest that O2 molecules penetrated into particles inside irrespective of the PGM species. However, the NO reduction property evaluated from the NO pulse experiment is dramatically enhanced by PGM loading, indicating that the oxygen vacancy site around the PGM species mainly receives the oxygen species formed by NO dissociation over the PGM species. Since these results reveal that the amount of available oxygen vacancy sites for O2 molecules is much larger than that for NO molecules, NO-selective reduction over PGM/Sr3Fe2O7−δ seems to proceed even under slightly lean conditions.
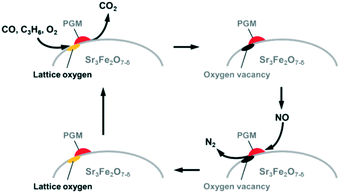
Based on all the results, a possible mechanism for NO-selective reduction over PGM/Sr3Fe2O7−δ is proposed (Fig. 7), when the reaction conditions were changed from rich to lean conditions. First, the oxidation of CO and C3H6 proceeds with the aid of lattice oxygen in Sr3Fe2O7−δ under rich conditions. The oxygen vacancy sites formed by the oxidation inhibit the oxidation of the PGM species even under slightly lean conditions; that is, the PGM species, which are the active sites for NO reduction, maintain their metal state by removing the excess oxygen species to the oxygen vacancy sites. Finally, the oxygen vacancy sites are recovered with the NO or O2 molecule.
Conclusions
In conclusion, this paper clarifies the following ambiguous facts concerning NO-selective reduction over PGM/Sr3Fe2O7−δ. PGM loading on Sr3Fe2O7−δ drastically improves CO and C3H6 oxidation activity and induces NO reduction. The Pd catalyst shows the highest activity among the examined samples. H2-TPR experiments show that the Pd catalyst can release lattice oxygen at lower temperature than that for the other catalysts. These results suggest that the oxygen release properties of Sr3Fe2O7−δ contribute to the catalytic activity. On the other hand, the Pt and Rh catalysts show higher NO reduction activity over a wider range of oxygen concentrations than the Pd catalyst. The NO pulse experiments reveal that oxygen ions generated by NO reduction over PGM on Sr3Fe2O7−δ flow into the oxygen vacancy sites of Sr3Fe2O7−δ, causing the PGM species to maintain their metal state as active species even for NO-selective reduction under slightly lean conditions. Pt/Sr3Fe2O7−δ utilizes the oxygen vacancy sites more efficiently than the Rh- and Pd-loaded catalysts. These results are first reported for NO-selective reduction using lattice oxygen and oxygen vacancy sites in perovskite-type oxides showing high oxygen storage performance. Moreover, the findings of our study serve as a useful guideline for the development of novel automotive catalysts loaded on a perovskite-type oxide support with oxygen storage capacity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the Program for Elements Strategy Initiative for Catalysts & Batteries (ESICB).Notes and references
- T. Montini, M. Melchionna, M. Monai and P. Fornasiero, Chem. Rev., 2016, 116, 5987–6041 CrossRef CAS PubMed.
- H. Miura, K. Wada, S. Hosokawa, M. Sai, T. Kondo and M. Inoue, Chem. Commun., 2009, 4112–4114 RSC.
- M. Tamura and K. Tomishige, Angew. Chem., Int. Ed., 2015, 54, 864–867 CrossRef CAS PubMed.
- H. Yahiro, K. Eguchi and H. Arai, Solid State Ionics, 1989, 36, 71–75 CrossRef CAS.
- J.-G. Li, T. Ikegami and T. Mori, Acta Mater., 2004, 52, 2221–2228 CrossRef CAS.
- E. Aneggi, D. Wiater, C. de Leitenburg, J. Llorca and A. Trovarelli, ACS Catal., 2014, 4, 172–181 CrossRef CAS.
- E. Aneggi, V. Rico-Perez, C. D. Leitenburg, S. Maschio, L. Soler, J. Llorca and A. Trovarelli, Angew. Chem., Int. Ed., 2015, 54, 14040–14043 CrossRef CAS PubMed.
- P. Fornasiero, G. R. Rao, J. Kašpar, F. L'Erario and M. Graziani, J. Catal., 1998, 175, 269–279 CrossRef CAS.
- J. Kašpar, P. Fornasiero and M. Graziani, Catal. Today, 1999, 50, 285–298 CrossRef.
- M. Sugiura, M. Ozawa, A. Suda, T. Suzuki and T. Kanazawa, Bull. Chem. Soc. Jpn., 2005, 78, 752–767 CrossRef CAS.
- C. Deng, B. Li, L. Dong, F. Zhang, M. Fan, G. Jin, J. Gao, L. Gao, F. Zhang and X. Zhou, Phys. Chem. Chem. Phys., 2015, 17, 16092–16109 RSC.
- M. Ozawa, M. Kimura and A. Isogai, J. Alloys Compd., 1993, 193, 73–75 CrossRef CAS.
- X. Wu, X. Wu, Q. Liang, J. Fan, D. Weng, Z. Xie and S. Wei, Solid State Sci., 2007, 9, 636–643 CrossRef CAS.
- K. Harada, T. Oishi, S. Hamamoto and T. Ishihara, Bull. Chem. Soc. Jpn., 2013, 86, 963–967 CrossRef CAS.
- S. K. Meher and G. R. Rao, J. Colloid Interface Sci., 2012, 373, 46–56 CrossRef CAS PubMed.
- P. Fornasiero, R. D. Monte, G. R. Rao, J. Kašpar, S. Meriani, A. Trovarelli and M. Graziani, J. Catal., 1995, 151, 168–177 CrossRef CAS.
- A. Suda, H. Sobukawa, T. Suzuki, T. Kandori, Y. Ukyo and M. Sugiura, J. Ceram. Soc. Jpn., 2001, 109, 177–180 CrossRef CAS.
- G. R. Rao, P. Fornasiero, R. D. Monte, J. Kašpar, G. Vlaic, G. Balducci, S. Meriani, G. Gubitosa, A. Cremona and M. Graziani, J. Catal., 1996, 162, 1–9 CrossRef CAS.
- T. Motohashi, Y. Hirano, Y. Masubuchi, K. Oshima, T. Setoyama and S. Kikkawa, Chem. Mater., 2013, 25, 372–377 CrossRef CAS.
- T. Motohashi, T. Ueda, Y. Masubuchi and S. Kikkawa, J. Phys. Chem. C, 2013, 117, 12560–12566 CAS.
- T. Motohashi, M. Kimura, Y. Masubuchi, S. Kikkawa, J. George and R. Dronskowski, Chem. Mater., 2016, 28, 4409–4414 CrossRef CAS.
- J. Vieten, B. Bulfin, F. Call, M. Lange, M. Schmücker, A. Francke, M. Roeb and C. Sattler, J. Mater. Chem. A, 2016, 4, 13652–13659 CAS.
- B. Bulfin, J. Vieten, D. E. Starr, A. Azarpira, C. Zachäus, M. Hävecker, K. Skorupska, M. Schmücker, M. Roeb and C. Sattler, J. Mater. Chem. A, 2017, 5, 7912–7919 CAS.
- K. Beppu, S. Hosokawa, K. Teramura and T. Tanaka, J. Mater. Chem. A, 2015, 3, 13540–13545 CAS.
- K. Beppu, S. Hosokawa, T. Shibano, A. Demizu, K. Kato, K. Wada, H. Asakura, K. Teramura and T. Tanaka, Phys. Chem. Chem. Phys., 2017, 19, 14107–14113 RSC.
- Y. Tsujimoto, C. Tassel, N. Hayashi, T. Watanabe, H. Kageyama, K. Yoshimura, M. Takano, M. Ceretti, C. Ritter and W. Paulus, Nature, 2007, 450, 1062–1065 CrossRef CAS PubMed.
- H. Kageyama, T. Watanabe, Y. Tsujimoto, A. Kitada, Y. Sumida, K. Kanamori, K. Yoshimura, N. Hayashi, S. Muranaka, M. Takano, M. Ceretti, W. Paulus, C. Ritter and G. Andre, Angew. Chem., Int. Ed., 2008, 47, 5740–5745 CrossRef CAS PubMed.
- S. N. Achary, S. K. Sali, N. K. Kulkarni, P. S. R. Krishna, A. B. Shinde and A. K. Tyagi, Chem. Mater., 2009, 21, 5848–5859 CrossRef CAS.
- A. H. Hill, F. Jiao, P. G. Bruce, A. Harrison, W. Kockelmann and C. Ritter, Chem. Mater., 2008, 20, 4891–4899 CrossRef CAS.
- O. Crisan and A. D. Crisan, J. Alloys Compd., 2011, 509, 6522–6527 CrossRef CAS.
- M. Matvejeff, M. Lehtimaki, A. Hirasa, Y.-H. Huang, H. Yamauchi and M. Karppinen, Chem. Mater., 2005, 17, 2775 CrossRef CAS.
- P. Mars and D. W. van Krevelen, Chem. Eng. Sci., 1954, 3, 41–59 CrossRef CAS.
- M. Baldi, E. Finocchio, F. Milella and G. Busca, Appl. Catal., B, 1998, 16, 43–51 CrossRef CAS.
- S. Scirè, S. Minicò, C. Crisafulli, C. Satriano and A. Pistone, Appl. Catal., B, 2003, 40, 43–49 CrossRef.
- M. Luo, J. Ma, J. Lu, Y. Song and Y. Wang, J. Catal., 2007, 246, 52–59 CrossRef CAS.
- S. Hosokawa, R. Tada, T. Shibano, S. Matsumoto, K. Teramura and T. Tanaka, Catal. Sci. Technol., 2016, 6, 7868–7874 CAS.
- P. Bera, A. Gayen, M. S. Hegde, N. P. Lalla, L. Spadaro, F. Frusteri and F. Arena, J. Phys. Chem. B, 2003, 107, 6122–6130 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cy01861d |
| This journal is © The Royal Society of Chemistry 2018 |