Molecular dynamics study of mesophase transitions upon annealing of imidazolium-based ionic liquids with long-alkyl chains
Abstract
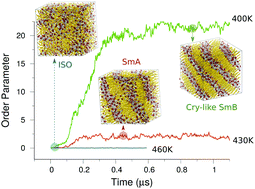
Molecular dynamics simulations are performed on a 1-dodecyl-3-methylimidazolium hexafluorophosphate ([C12mim][PF6]) ionic liquid using a united-atom model. The ionic liquid exhibits second step relaxation at temperatures below a crossover point, where the diffusion coefficient shows an Arrhenius to non-Arrhenius transition. Annealing below this crossover temperature makes an isotropic to mesophase transition, where the smectic A (SmA) phase or crystal-like smectic B (SmB) phase forms. Hundreds of nanoseconds are required for completing these transitions. A normal diffusion process is found for anions along the layer-normal and -lateral directions in the SmA phase, but only in the lateral directions in the SmB phase. We find a preserved orientational order for the imidazolium-ring rotational and the alkyl-chain reorientational dynamics in both of the smectic phases.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...