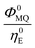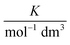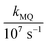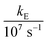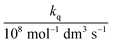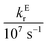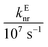Fluorescence quenching of the N-methylquinolinium cation by pairs of water or alcohol molecules†
Flor
Rodríguez-Prieto
 *ab,
Carlos Costa
Corbelle
b,
Berta
Fernández
*ab,
Carlos Costa
Corbelle
b,
Berta
Fernández
 b,
Jorge A.
Pedro
a,
M. Carmen
Ríos Rodríguez
b,
Jorge A.
Pedro
a,
M. Carmen
Ríos Rodríguez
 *ab and
Manuel
Mosquera
*ab and
Manuel
Mosquera
 *ab
*ab
aCentro Singular de Investigación en Química Biolóxica e Materiais Moleculares (CIQUS), Universidade de Santiago de Compostela, E-15782 Santiago de Compostela, Spain. E-mail: flor.rodriguez.prieto@usc.es; carmen.rios@usc.es; manuel.mosquera@usc.es
bDepartamento de Química Física, Facultade de Química, Universidade de Santiago de Compostela, E-15782 Santiago de Compostela, Spain
First published on 24th November 2017
Abstract
N-Methylquinolinium cation (MQ+) in its first-excited singlet state is a strong oxidant commonly used as a photosensitizer, whose fluorescence is therefore quenched by electron donors. Interestingly, the fluorescence of MQ+ is also quenched by hydroxy compounds such as water and alcohols, more difficult to oxidize. We investigated the quenching mechanism of MQ+ fluorescence by small amounts of water and alcohols in acetonitrile solution. The fluorescence intensities and lifetimes exhibited a nonlinear dependence on the quencher concentration. We found evidence that emissive exciplexes MQ+*-ROH are formed between the excited quinolinium and the hydroxy compounds. An accurate quantitative description of the results was obtained with a model in which the exciplex reacts with a second molecule of the hydroxy compound, which quenches the fluorescence. The rate constant of this process increased as the quencher ionization energy decreased. We showed also that a low basicity of the hydroxy compound inhibits the quenching process. These results are consistent with the existence of a concerted photoinduced proton-coupled electron transfer (PCET) involving an intermediate complex of the excited quinolinium with a H-bonded molecular pair of the hydroxy compounds. In these pairs, a water or an alcohol molecule is able to donate an electron to the photoexcited quinolinium cation and a proton to the second H-bonded hydroxy molecule, showing an enhanced reducing power in comparison with the isolated molecule. The structure of the intermediate complex was investigated using high-level quantum mechanical calculations. At high water concentrations in acetonitrile/water mixtures, the quenching process is slowed down, indicating that higher water aggregates are less effective for a PCET process. The results obtained may be relevant to the study of water oxidation and electron transfer in biological systems.
Introduction
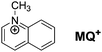
N-Methylquinolinium (Chart 1) belongs to the family of quinolinium cations, which upon photoexcitation behave as potent oxidants and are commonly used as photocatalysts.1,2 Due to their strong electron-accepting character in the first-excited singlet state, the fluorescence of quinolinium compounds is quenched by electron transfer from a variety of electron donors, at a diffusion-controlled rate in the most favourable cases.3–8 This feature has been used for physiological anion-sensing applications and for building highly sensitive fluorescent sensors.7–12 Surprisingly, fluorescence quenching by harder-to-oxidize species like methanol and ethanol was also observed.13 Additionally, quenching by water was also proposed to explain the fluorescence enhancement observed upon addition of ClO4− and HSO4− to aqueous solutions of quinolinium cations, through the protective effect of adduct formation.13 However, no conclusive explanation was found about the nature of the process by which water and alcohols quench the fluorescence of quinolinium compounds, as an electron transfer from these hard-to-oxidize species seems unlikely. With the aim of unravelling the quenching mechanism, we studied the fluorescence quenching of MQ+ by water and aliphatic alcohols in acetonitrile solution. We chose this solvent because it is polar enough to dissolve the quinolinium salt and has a high resistance to oxidation. We report here the finding that a pair of water or alcohol molecules quench the excited cation, whereas a single molecule is unable to do so. We propose that a proton-coupled electron transfer process enhances the electron donating capability of the hydroxy compounds and brings about an electron-transfer process that is impossible for a single water or aliphatic alcohol molecule.Electron transfer is frequently made possible by the rate increase brought about by photoexcitation of the reactants, through coupling proton and electron transfer, or other enhancement mechanisms.14–18 Electronic excitation brings about a strong increase of molecular electron donor and acceptor abilities. Photoexcitation is therefore a method of choice to promote electron transfer processes, as it is also the coupling of electron and proton transfer, which catalyses redox processes by avoiding high-energy intermediates. PCET processes are at the heart of the most important chemical and biochemical energy conversion and storage processes, such as photosynthesis, respiration, and solar fuels.16–23 The coordinated movement of electrons and protons is also implicated in other fundamental biological processes, like DNA mutation and repair, biological photoprotection mechanisms, and the function of proteins.15,17,21–25
Photoinduced PCET processes combine the two above-mentioned enhancement mechanisms and lead therefore to a large increase in the rate of electron transfer processes. This pattern is used by green plants in the photosynthetic process, and is also at the heart of many artificial photosynthesis schemes that pursue water splitting to form hydrogen and oxygen, or water reduction of CO2 to carbon-based fuels.16–23,26,27 Photoinduced PCET is therefore a cornerstone for the design of renewable energy-conversion systems, which, together with the need to understand the involvement of PCET in water and in biological systems, triggered increasing interest in the study of its detailed mechanism from both the experimental and the theoretical points of view.14–31
In this paper, we report an in-depth quantitative investigation of the fluorescence quenching mechanism of the N-methylquinolinium cation by water and aliphatic alcohols in acetonitrile. The results provide evidence for a novel photoinduced PCET quenching mechanism, which involves an electron transfer from a pair of molecules of the hydroxy compounds to the photoexcited quinolinium. This knowledge will contribute to the understanding of biological processes and energy-converting systems where PCET involving hydroxy compounds plays a central role.
Experimental
Materials
N-Methylquinolinium iodide was prepared by the dropwise addition of a dilute solution of CH3I in toluene over a solution of quinoline in toluene. After refluxing the mixture for 5 h, the solid obtained was filtered and washed with acetone. Solutions were made up in double-distilled water and in spectroscopy-grade solvents, and were not degassed. We employed N-methylquinolinium iodide solutions with concentrations of ∼10−4 mol dm−3 for absorption and fluorescence lifetimes measurements, and ∼10−5 mol dm−3 for steady-state fluorescence measurements. Chemicals were purchased from Sigma-Aldrich and used without further purification.Absorption and fluorescence measurements
UV-vis absorption spectra were recorded in a Varian Cary 3E spectrophotometer. Fluorescence excitation and emission spectra were obtained in a Jobin-Yvon Spex Fluoromax-2 spectrofluorometer, with correction for instrumental factors by means of a reference photodiode and correction files supplied by the manufacturer. Fluorescence quantum yields were measured using quinine sulfate (<3 × 10−5 mol dm−3) in aqueous HClO4 (1.0 mol dm−3) as standard (Φ = 0.546).32,33 To improve the accuracy of the quantum yield measurements, log-normal functions were fitted to the emission bands to evaluate the band areas.34 All the experiments were carried out at 20 °C.Fluorescence decays were measured using the time-correlated single-photon counting technique in an Edinburgh Instruments LifeSpec-ps time-resolved spectrometer equipped with a sub-nanosecond pulsed LED from PicoQuant as the excitation source (308 nm). The reconvolution analysis software supplied by the manufacturer was employed.
Data analyses and ab initio calculations
Theoretical equations were fitted to the experimental data by means of a nonlinear weighted least-squares routine based on the Marquardt algorithm.35 Principal-component global analyses were performed using a commercial software package (MATLAB for Windows, The MathWorks Inc., Natick, MA). The reported uncertainties correspond to the statistically estimated standard deviation of the measured quantities (type A standard uncertainty).36 They are presented in parentheses after the estimated values of the measurands, referred to the corresponding last digits of the quoted results.36MQ+ has an iodide ion as a counterion and this anion quenches its fluorescence at a diffusion-controlled rate.4 The effect of the constant iodide concentration on MQ+ fluorescence is entirely negligible for the steady-state fluorescence measurements due to the low concentration used for these experiments (∼10−5 mol dm−3), but has a small effect that must be considered in the time-resolved experiments because of the 10-times higher concentration of the N-methylquinolinium iodide employed for these measurements. This small effect must be taken into account for the global analysis of steady-state and time-resolved measurements. For this reason, we have introduced in the analysis the linear dependence of the deactivation rate constant of excited MQ+ on the iodide concentration in acetonitrile (the quenching rate constant is 5.22 × 1010 mol−1 dm3 s−1, measured for concentrations up to 2 × 10−4 mol dm−3).
The ab initio calculations were performed with the Gaussian 09 program package.37
Results and discussion
Kinetic analysis of the fluorescence quenching of MQ+ by hydroxy compounds
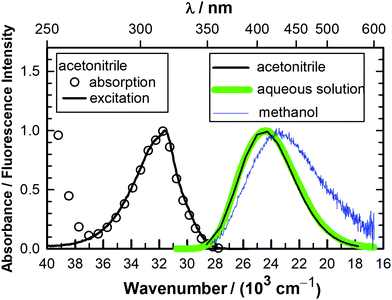
The absorption spectra of MQ+ are almost identical in acetonitrile, water and the complete series of small aliphatic alcohols used in this work (see later). The fluorescence excitation spectra of MQ+ in acetonitrile, water, methanol, and ethanol were also almost identical, showed emission wavelength independence, and matched the first absorption band (Fig. 1 and Fig. 1S–3S in the ESI†). The fluorescence emission spectra in the same solvents were independent of the excitation wavelength and were very similar in aqueous solution and in acetonitrile, but exhibited a small red shift in methanol and ethanol (Fig. 1 and Fig. 4S, ESI†). The intensity, nevertheless, considerably declined in the series. The fluorescence quantum yield of MQ+ decreased 2 times on going from acetonitrile to aqueous solution, and further diminished by a factor of 100 on going to methanol or ethanol solutions (Table 1). To get information on the fluorescence quenching mechanism, we studied the effect of small amounts of water and alcohols on the fluorescence of MQ+ in acetonitrile solutions.| Solvent | Φ |
|---|---|
| Acetonitrile | 0.422 |
| Water | 0.232 |
| Methanol | 0.00398 |
| Ethanol | 0.00221 |
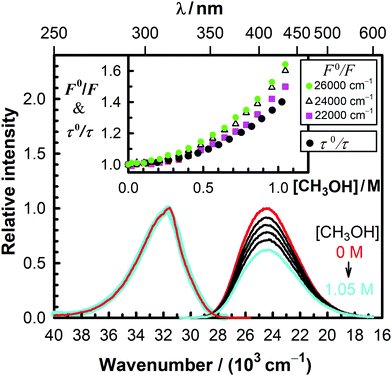
We investigated the decrease of the fluorescence intensity of MQ+ in acetonitrile upon addition of water, methanol, ethanol, 1-propanol, 1,2-ethanediol, and 1,3-propanediol. Plots of the fluorescence intensity ratio in the absence and presence of the quencher (F0/F) versus quencher concentration showed nonlinear Stern–Volmer relationships with an upward curvature in all cases (Fig. 2 and Fig. 5S–9S, ESI†). As can be seen from these plots, F0/F gradually changed with the emission wavenumber, which revealed that the shape of the emission spectra changed with the addition of quenchers. These results suggest that a second fluorescent species is formed. This species must exist only in the excited state, as the excitation spectra remained unchanged in all cases upon addition of quenchers (cf.Fig. 2 and Fig. 5S–9S, ESI†).
To examine the possible effect of hydrogen bonding on the quenching process, we studied the influence of 2,2,2-trifluoroethanol on the fluorescence of MQ+ solutions in acetonitrile. We chose this alcohol due to its extremely low hydrogen-bond basicity, originated by the strong electron-withdrawing effect of the fluorine atoms.38,39 We found that no fluorescence quenching at all was observed in the alcohol concentration range of 0 to 0.39 mol dm−3 (Fig. 10S, ESI†).
The fluorescence decay of MQ+* in acetonitrile in the absence of quenchers was monoexponential, with a lifetime of τ0 = 23.37(4) ns. It remained monoexponential upon addition of hydroxy compounds (some examples are shown in Fig. 11S, ESI†), but the lifetime τ decreased as their concentration increased. Nonlinear Stern–Volmer plots of τ0/τ versus quencher concentration were obtained in all cases, but their curvatures were significantly different from those obtained for the intensity ratio F0/F (cf.Fig. 2 and Fig. 5S–9S, ESI†). This behaviour, together with the change of the spectral shape observed upon addition of hydroxy compounds, revealed that a quenching mechanism more complex than static and/or dynamic quenching must be operating.
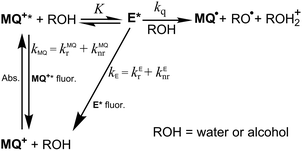
Principal-component analysis applied to the series of fluorescence emission spectra of MQ+ at different quencher concentrations showed that two independent spectral components are needed to reproduce the spectral series.40,41 This indicates that a new emissive species appears upon addition of quenchers, which we identify with an exciplex E* formed by the excited quinolinium cation and the quencher (see Scheme 1).
 | ||
| Scheme 1 Excitation and deactivation patterns proposed for MQ+ in acetonitrile in the presence of water or alcohols. | ||
To explain the complex fluorescence intensity and lifetime dependence on the quencher concentration (Fig. 2 and Fig. 5S–9S, ESI†), we propose that after excitation of MQ+ and formation of the exciplex E*, a second molecule of the hydroxy compound approaching E* induces its nonradiative deactivation. Furthermore, as the fluorescence decay of MQ+* was monoexponential, we assume that a fast equilibrium is established between MQ+* and E*. To test the proposed mechanism, a thorough data analysis was carried out, as explained below.
Any emission spectrum F of the series must be a linear combination of the spectra of the emissive species MQ+* and E*. In eqn (1), F0MQ and F0E represent the fluorescence spectra that would be obtained for MQ+* and E* if each absorbed photon formed an excited molecule of the respective species, and only the unimolecular deactivation of these excited species would be operative (see the kinetic model, ESI†). The coefficients CMQ and CE are the contributions of the MQ+* and E* spectra to the experimental spectrum, and their values depend on the quencher concentration.
| F = CMQF0MQ + CEF0E | (1) |
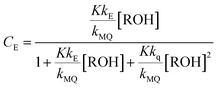
From the mechanism shown in Scheme 1, one can easily derive eqn (2)–(4), which show the predicted dependence on the quencher concentration of the emission coefficients CMQ and CE, and of the lifetime ratio τ0/τ (see the kinetic model in the ESI†).
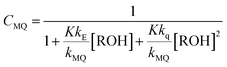 | (2) |
 | (3) |
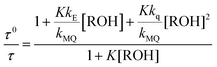 | (4) |
In these equations (see Scheme 1), kMQ and kE are the unimolecular deactivation rate constants of the excited quinolinium cation and the exciplex, respectively. They correspond to the sum of the radiative kr and the nonradiative knr deactivation constants of the respective species. kq represents the quenching rate constant of the exciplex by ROH, K is the exciplex formation equilibrium constant, and τ0 is the lifetime of MQ+* in the absence of quenchers.
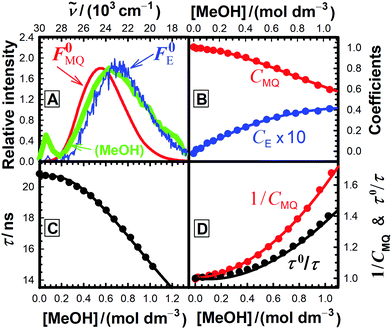
For each quencher, we analysed using Principal-Component Global Analysis (PCGA),40,41 with the set of eqn (1)–(4), the series of fluorescence spectra and lifetimes of MQ+ obtained at different quencher concentrations. From these analyses, we obtained the fluorescence spectra F0MQ and F0E, together with the optimized values of K, kMQ, kE, and kq, collected in Table 2. Fig. 3 shows graphically the fit results obtained for methanol: part (A) displays the optimized spectra F0MQ and F0E, and parts (B), (C) and (D) show the experimental fluorescence lifetimes τ and the coefficients CMQ and CE as a function of quencher concentration, together with plots of the fitted curves calculated with the single set of parameters shown in Table 2. It is seen that the model quantitatively reproduces the fluorescence intensity and lifetime data. The same goodness of fit was obtained for water and the rest of the alcohols investigated (Fig. 12S–16S, ESI†), which reflects the model consistency.
| Quencher | E i/eV | η 0E | |||||||
|---|---|---|---|---|---|---|---|---|---|
| a T = 293 K. b From ref. 42 c From ref. 43 | |||||||||
| Water | 12.62b | 0.83 | 0.51 | 0.217(5) | 4.41(3) | 2.81(5) | 0.250(3) | 1.4 | 1.4 |
| 2,2,2-Trifluoroethanol | 11.49b | — | — | — | — | — | — | — | — |
| Methanol | 10.84b | 0.56 | 0.75 | 0.112(3) | 4.35(1) | 2.6(1) | 1.90(4) | 2.0 | 0.6 |
| Ethanol | 10.48b | 1.9 | 0.22 | 0.123(5) | 4.33(2) | 8.1(3) | 6.5(2) | 1.8 | 6.3 |
| 1-Propanol | 10.22b | 2.4 | 0.18 | 0.115(6) | 4.35(1) | 10.4(6) | 9.0(4) | 1.8 | 8.6 |
| 1,2-Ethanediol | 10.16b | 5.8 | 0.073 | 0.28(2) | 4.34(1) | 24(1) | 6.2(3) | 1.7 | 22 |
| 1,3-Propanediol | 9.7c | 3.2 | 0.13 | 0.169(5) | 4.34(1) | 16.9(7) | 12.1(5) | 2.2 | 14.7 |
We show in Fig. 3(A) that the fluorescence spectrum recorded for MQ+ in neat methanol almost completely overlaps with the spectrum F0E obtained by PCGA for the exciplex MQ+*-methanol in acetonitrile (the same is also true for ethanol, see Fig. 13S, ESI†). We interpret this fact as an indication that the exciplex is the main emissive species in pure methanol, favoured by the high concentration of the solvent.
The integrated areas of the fluorescence spectra F0MQ and F0E obtained from PCGA contain information on the quantum yields of these species in the solvent used, acetonitrile. The ratio of the areas equals the quotient Φ0MQ/η0E, where Φ0MQ denotes the fluorescence quantum yield of MQ+ in acetonitrile in the absence of quenchers, and η0E represents the fluorescence quantum efficiency of E* if only the unimolecular photophysical deactivation of this excited species would be operative (η0E = kEr/kE, see details in the ESI†). The values of Φ0MQ/η0E for various quenchers are collected in Table 2. As Φ0MQ is the fluorescence quantum yield of MQ+ in neat acetonitrile (0.422), the values of η0E for the different exciplexes in this solvent can be calculated, together with their radiative and nonradiative deactivation constants (Table 2). For water and methanol, the η0E value is slightly greater than Φ0MQ, which means that for these species, the fluorescence quenching is entirely due to the bimolecular reaction process with a second molecule of the hydroxy compound. For the rest of the alcohols, η0E is somewhat lower than Φ0MQ, but the main quenching comes also from the bimolecular deactivation process with rate constant kq (compare for example the results obtained on going from water to 1,3-propanediol: a 4-fold decrease of η0E but almost a 50-fold increase in the bimolecular quenching rate constant kq). Therefore, we conclude that the main process responsible for the strong fluorescence quenching observed is not the formation of the exciplex, but its bimolecular quenching by a second molecule of the hydroxy compound. In the following, we discuss the nature of this quenching process.
Involvement of an electron transfer process in the quenching mechanism
Quinolinium cations are strong electron-acceptor photosensitizers, whose fluorescence has been shown to be quenched by low concentrations of anions through a dynamic electron-transfer mechanism.4,8 In this work, we show that water and aliphatic alcohols exhibit a much lower quenching efficiency of MQ+ fluorescence and a more complex mechanism compared to the anions. If an electron transfer is involved in this quenching process, its efficiency must increase with the ease of oxidation of the quencher. As the standard electrode potentials E0 are not known for all quenchers, we used the gas-phase ionization energies (Ei), linearly correlated with E0,44 as a measure of their ability to donate an electron. The data presented in Table 2 suggest that an electron transfer may be involved in the quenching process, as a trend of increasing quenching rate constant with decreasing ionization energy of the quencher is observed, and a linear correlation exists between log kq and Ei (Fig. 4). Nevertheless, the process is not a simple dynamic quenching as for the anions,4,8 as could be anticipated by the more difficult alcohol and water oxidation. Even for stronger oxidants like photoexcited methyl viologen (MV2+*), an endergonic process was predicted for electron transfer from water45 (reduction potentials E0(MV2+*/MV˙+) = 3.65 V vs. NHE,45 much higher than E0(MQ+*/MQ˙) = 2.9 V vs. NHE).3 This is corroborated by our results, as the reaction of MQ+* with water to form the exciplex does not quench the fluorescence of MQ+* (η0E = 0.51, slightly greater than Φ0MQ = 0.422), as expected for a very weak donor–acceptor interaction.46,47 The exciplexes formed by MQ+* with alcohols of lower Ei may have a higher degree of charge-transfer character, which would justify their lower fluorescence efficiency. This is supported by the values of the radiative and nonradiative deactivation constants of the exciplexes (Table 2): whereas kEr is almost constant for all exciplexes, a clear increase of kEnr is found for the quenchers with lower ionization energy. Nevertheless, the values of kEnr are sufficiently low to ascertain the weak charge transfer in the exciplexes even for the alcohols with stronger electron-donating capacity.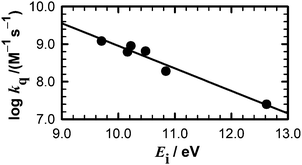 | ||
| Fig. 4 Dependence of the quenching constant kq on the ionization energy of the quenchers listed in Table 2. | ||
As we pointed out above, the main contribution to the quenching comes from the reaction of the exciplex with a second quencher molecule. The linear decrease of log kq with increasing Ei of the quencher (Fig. 4) strongly supports the hypothesis that the process involves an electron transfer from the quencher to the exciplex. However, an explanation is required for the fact that 2,2,2-trifluoroethanol does not quench the MQ+ fluorescence, despite having an intermediate Ei value between those of water and methanol (Table 2). Even the solvent acetonitrile has an Ei value (12.20 eV) lower than water,42 but nevertheless, the fluorescence quantum yield of MQ+ is higher in acetonitrile than in water (Table 1). What distinguishes 2,2,2-trifluoroethanol and acetonitrile from water and other alcohols is their different hydrogen-bond ability and acid–base character. This led us to think that, in addition to the electron transfer, a proton transfer may also have a role in the quenching process.
Quantum mechanical study of the MQ+–2H2O complex
In order to gain some insight into the quenching mechanism, we investigated using quantum mechanical methods the complex of MQ+* with two water molecules. We started by carrying out geometry optimizations for the MQ+ molecule in the ground and in the first-excited electronic state. We used density functional theory (DFT and TD-DFT),48,49 together with the B3LYP functional50,51 and the correlation consistent polarized valence triple zeta basis set of Dunning et al. (cc-pVTZ).52 Taking the MQ+ DFT(B3LYP)/cc-pVTZ geometry as the starting point, we optimized the ground-state geometry of the complex that resulted from the addition of two water molecules. For this, we considered five initial intermolecular configurations with the two water molecules located at different positions, i.e. interacting with each other and also above and below the MQ+ molecule ring plane. In the most stable configuration, the two water molecules were located on one side of the MQ+ ion, with one of the water oxygen atoms on the plane of the MQ+ rings and the second water molecule interacting with the first through a hydrogen bond. For this configuration, we optimized the ground- and the first-excited-state geometries using DFT and TD-DFT (B3LYP) and the cc-pVTZ and the aug-cc-pVTZ basis sets. The differences between the cc-pVTZ and the aug-cc-pVTZ intramolecular geometries were not significant, but this was not the case for the intermolecular parameters. Taking this into account, in the following we consider the aug-cc-pVTZ results. The corresponding ground- and excited-state geometries are reported in the ESI† (Fig. 17S and Tables 1S, 2S, ESI†). The lowest excitation has a DFT(B3LYP)/aug-cc-pVTZ energy of 3.82 eV and the responsible orbitals are mainly the HOMO and the LUMO of the MQ+ molecule.The optimized excited-state geometry of the MQ+*–2H2O complex (Fig. 5) shows a water dimer coordinated through an O atom to two H atoms of the quinolinium molecule. The main differences between the ground- and the excited-state geometries are due to the intermolecular interactions between the water dimer and the MQ+ molecule. In the excitation, the water molecule that is closest to the MQ+ unit suffers a displacement towards the closest hydrogen atom in the methyl group, and the distance between the two water molecules increases (the O–O distance varies from 2.801 Å to 2.822 Å). The distance between the water O and the methyl H (2.304 Å) is slightly larger than the distance between the O and the H atom of the quinolinium ring at position 2 (2.229 Å), and both are indicative of the existence of C–H⋯O hydrogen bonds.53 The values of the equilibrium constant K for exciplex formation are similar for water and alcohols (Table 2), indicating that the strength of the interaction of H2O or ROH with MQ+* is about the same in all cases studied. This result is in accordance with the similar hydrogen-bond basicity of water and these alcohols,54 so we presume the complexes of MQ+* with H2O or ROH to have an analogous structure.
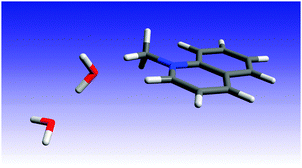 | ||
| Fig. 5 B3LYP/aug-cc-pVTZ optimized geometry of the MQ+*−2H2O complex in the first-excited singlet state. | ||
Proposal of a PCET quenching mechanism
We can take the structure of the complex formed by MQ+* with two water molecules in the gas phase as indicative of the more stable way of approaching the two water molecules to MQ+* in a non-protogenic solvent as acetonitrile. This structure is adequate for a PCET to take place. If this is true, electron transfer from the central water (or alcohol) molecule to MQ+* will occur in concert with proton transfer to the neighbouring water molecule through the pre-existing hydrogen bond. This is in agreement with the fact that the hypothetical radical cation intermediate of an initial electron transfer (ROH˙+) is highly acidic,55–57 and will transfer a proton to the second ROH molecule to form the more stable species RO˙ and ROH2+. The feasibility of the concerted proton transfer would allow the electron transfer to occur by providing a lower-energy path to the reaction products.The very low hydrogen-bond basicity of 2,2,2-trifluoroethanol38,39 would hinder the formation of the exciplex and the trimolecular reactive complex. Moreover, its proton basicity is also extremely low, as deduced from its very low gas basicity58 and its inability to accept the proton of relatively strong photoacids in liquid solution.59 These characteristics impede the PCET process for 2,2,2-trifluoroethanol. Acetonitrile has also a very low hydrogen-bond basicity and proton basicity in liquid solutions,38,39 and is unable to participate in a PCET reaction due to the lack of ionisable protons. These facts explain why acetonitrile and 2,2,2-trifluoroethanol do not quench the fluorescence of the N-methylquinolinium cation, in spite of being more easily oxidized than water.
With the quenching constants obtained for methanol and ethanol solutions in acetonitrile (Table 2), we can calculate an extrapolated value of the fluorescence quantum yield of MQ+ in pure alcohol solutions (24.70 mol dm−3 methanol and 17.13 mol dm−3 ethanol). The predicted values are Φ = 5.5 × 10−3 for methanol and Φ = 6.2 × 10−3 for ethanol. These results are in reasonable agreement with the experimental values (Table 1), taking into account the large extrapolation from dilute solutions and the change in the medium from acetonitrile to alcohol. Nevertheless, the fluorescence quantum yield of MQ+ predicted in the same way as that for pure water solution (Φ = 1.1 × 10−2) is much lower than the experimental value (Table 1). This led us to think that a change in the quenching mechanism must occur upon increasing the water concentration. To test this hypothesis, we measured the fluorescence intensities of MQ+ in the whole concentration range from pure acetonitrile to pure water. The fluorescence spectral shape hardly changes for these mixtures (cf.Fig. 1 and Fig. 5S(C), ESI†), but the fluorescence intensity decreases with increasing water content until a molar fraction of xH2O ≈ 0.5, increasing afterwards. Fig. 6 shows the influence of the water content on the fluorescence intensity quotient F0/F, with a maximum at equimolar amounts of water and acetonitrile.
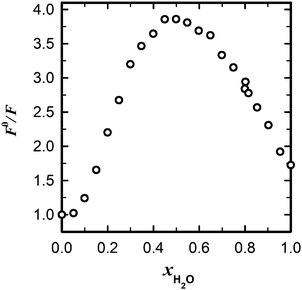 | ||
Fig. 6 Dependence of the relative fluorescence intensity of MQ+ on the molar fraction of water in mixtures acetonitrile/water (![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) exc = 31 exc = 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 cm−1, 650 cm−1, ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) em = 24 em = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 cm−1). 400 cm−1). | ||
The behaviour observed for MQ+ in acetonitrile/water mixtures is probably due to the microheterogeneity of these solutions, which has been demonstrated by a range of different techniques.60,61 At low water concentrations, the water molecules form small aggregates of a few molecules, also associated with the acetonitrile molecules. As the water content is increased, microscopic domains of self-associated water exist, as well as acetonitrile domains, until the water content is so high that an extensive hydrogen-bonded network is established.60 Our results (Fig. 6) point to the fact that the ability of the water molecules to cooperate via PCET to donate an electron is higher for small aggregates than for an extensive hydrogen-bonded network of water. This can be related to the higher ability of a water molecule that does not participate in the ordinary water structure to donate an electron pair toward a hydrogen bond than one that does.61
A close parallelism exists between the behaviour of MQ+ here described and that of the stronger oxidant methyl viologen. The fluorescence of MV2+ is also quenched by water, methanol and ethanol, but not by acetonitrile or 2,2,2-trifluoroethanol.45 Femtosecond transient absorption experiments in bulk methanol have shown that the radical cation of methyl viologen is produced at an ultrafast rate (<180 fs) as a result of the oxidation of a solvent molecule by the photoexcited MV2+.45 To explain the rapid quenching of the first-excited singlet state of MV2+ in aqueous solution, Kohler and co-workers proposed a concerted proton–electron transfer reaction.62 By using ultrafast spectroscopic techniques, they presented convincing evidence that the strongly oxidizing excited state of MV2+ triggers the proton-coupled oxidation of a water molecule, which transfers a proton to the bulk solvent and an electron to MV2+* to form the hydroxyl radical. Although the MV˙+/OH˙ radical pair was not detected probably due to fast back electron transfer, a photoproduct was identified as the charge-transfer complex formed between ground-state MV2+ and a hydroxide ion. We propose here that a similar mechanism can take place for the weaker oxidant MQ+* in acetonitrile solution, with a water or alcohol molecule in a H-bonded pair acting as an efficient electron-donating entity towards MQ+* through the concerted proton transfer to the second hydroxy molecule of the pair. This result is in accord with previous quantum-mechanical calculations, which show a strong decrease in Ei of water upon dimer formation.63 The concerted action of a pair of water or alcohol molecules in proton transfer reactions has also been demonstrated.64–67
Electron transfer from water or alcohols to other excited chromophores has also been established. Femtosecond experiments in bulk solvents showed that excited oxazine 750 is reduced by different aliphatic alcohols.68 Moreover, several quantum-mechanical calculations on excited chromophores clustered with water or alcohols predict that an electron transfer from the hydroxy compound to the chromophore can take place. Examples comprise oxazine 750 clustered with two ethanol molecules68 or 7H-adenine clustered with several water molecules.69 In some cases, the initial electron transfer is followed by a proton transfer from water to the excited chromophore (for example, in the complexes pyridine-H2O,70 benzoquinone-H2O,71 1-methylcytosine-(H2O)2,72 9H-adenine-(H2O)573 and acridine-H2O).74 The capacity of the triplet excited state of acridine orange to split the O–H bond of phenol derivatives through PCET has also been experimentally demonstrated.75
According to our proposal, the quenching process of MQ+* by small amounts of water and alcohols in acetonitrile solution can be described as a concerted multiple-site electron-proton transfer (MS-EPT),22 also called bidirectional PCET,18 in which the electron and proton transfers occur from a single donor (water or alcohol) to different acceptors. This type of PCET plays a major role in a variety of biological processes and in a wide range of chemical systems involving hydroxy compounds as electron and proton donors.18,22,62,76–81
Recognition of the relatively high efficiency of water pairs as electron donors can contribute to understanding the puzzling photorelaxation and electron-transfer mechanisms of biomolecules, where water molecules have been shown to play a fundamental role.82,83 Our results are also relevant to the issues of relaxation mechanisms of excited molecules in hydroxylic solvents, solar water splitting and solar fuel production.
Conclusions
The quantitative analysis of the fluorescence quenching of MQ+* by small amounts of water and alcohols in acetonitrile solution revealed that upon excitation, emissive exciplexes are formed between MQ+* and one molecule of water or alcohol. The fluorescence emission spectra of the exciplexes were resolved and their fluorescence efficiencies were determined. In all cases, the fluorescence of the exciplex was quenched by a second water or alcohol molecule, the quenching rate constant increases as the ionization energy of the hydroxy compound decreased. We propose that this deactivation pathway is due to a photoinduced PCET involving an intermediate H-bonded complex of the excited quinolinium with a pair of molecules of the hydroxy compounds. In this pair, the electron transfer from H2O/ROH to MQ+* is facilitated by concerted proton transfer to the second molecule of the hydroxy compound. In accord with this mechanism, a very low basicity of the hydroxy compound inhibits the electron transfer. We also showed that an extensive hydrogen-bonded network of water molecules is less effective than the smaller aggregates for the enhancement of electron transfer via PCET.Our findings support that water and alcohol dimers show a much stronger reducing power than the isolated molecules in acetonitrile. The results obtained may be relevant to the study of water oxidation and electron transfer in biological systems.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has received financial support from Gobierno de España, Ministerio de Economía y Competitividad (Grant CTQ2014-59020-R), Xunta de Galicia (Grants ED431B 2016/024, ED431D R2016/007, ED431C 2017/17 and Centro Singular de Investigación de Galicia Accreditation 2016–2019, ED431G/09), and the European Union (European Regional Development Fund – ERDF). Fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasil (J. A. P.) and Xunta de Galicia, Spain (C. C. C.) are gratefully acknowledged. We thank Carlos Carreira Blanco for performing some fluorescence measurements.References
- N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–10166 CrossRef CAS PubMed.
- T. Del Giacco, A. Faltoni and F. Elisei, Phys. Chem. Chem. Phys., 2008, 10, 200–210 RSC.
- U. C. Yoon, S. L. Quillen, P. S. Mariano, R. Swanson, J. L. Stavinoha and E. Bay, J. Am. Chem. Soc., 1983, 105, 1204–1218 CrossRef CAS.
- S. Jayaraman and A. S. Verkman, Biophys. Chem., 2000, 85, 49–57 CrossRef CAS PubMed.
- C. D. Geddes, K. Apperson, J. Karolin and D. J. S. Birch, Anal. Biochem., 2001, 293, 60–66 CrossRef CAS PubMed.
- R. Rautela, P. Arora, N. K. Joshi, S. Pant and H. C. Joshi, J. Mol. Liq., 2016, 218, 632–636 CrossRef CAS.
- J. G. Harangozo, Z. Miskolczy, L. Biczok, V. Wintgens and C. Lorthioir, J. Inclusion Phenom. Macrocyclic Chem., 2015, 81, 377–384 CrossRef CAS.
- Z. Miskolczy, J. G. Harangozo, L. Biczok, V. Wintgens, C. Lorthioir and C. Amiel, Photochem. Photobiol. Sci., 2014, 13, 499–508 CAS.
- C. D. Geddes, Meas. Sci. Technol., 2001, 12, R53–R88 CrossRef CAS.
- C. D. Geddes, Sens. Actuators, B, 2001, 72, 188–195 CrossRef CAS.
- I. J. Bazany-Rodriguez, D. Martinez-Otero, J. Barroso-Flores, A. K. Yatsimirsky and A. Dorazco-Gonzalez, Sens. Actuators, B, 2015, 221, 1348–1355 CrossRef CAS.
- R. X. Zhang, P. F. Li, W. J. Zhang, N. Li and N. Zhao, J. Mater. Chem. C, 2016, 4, 10479–10485 RSC.
- S. C. Chao, J. Tretzel and F. W. Schneider, J. Am. Chem. Soc., 1979, 101, 134–139 CrossRef CAS.
- N. Mataga, H. Chosrowjan and S. Taniguchi, J. Photochem. Photobiol., C, 2005, 6, 37–79 CrossRef CAS.
- Proton-Coupled Electron Transfer Themed Issue, Chem. Rev., 2010, 110, 6937–7100 CrossRef PubMed.
- C. J. Gagliardi, B. C. Westlake, C. A. Kent, J. J. Paul, J. M. Papanikolas and T. J. Meyer, Coord. Chem. Rev., 2010, 254, 2459–2471 CrossRef CAS.
- S. Hammes-Schiffer, J. Am. Chem. Soc., 2015, 137, 8860–8871 CrossRef CAS PubMed.
- J. C. Lennox, D. A. Kurtz, T. Huang and J. L. Dempsey, ACS Energy Lett., 2017, 2, 1246–1256 CrossRef.
- M. Hambourger, G. F. Moore, D. M. Kramer, D. Gust, A. L. Moore and T. A. Moore, Chem. Soc. Rev., 2009, 38, 25–35 RSC.
- D. G. Nocera, Acc. Chem. Res., 2012, 45, 767–776 CrossRef CAS PubMed.
- Proton-coupled Electron Transfer: A Carrefour for Chemical Reactivity Traditions, ed. S. Formosinho and M. Barroso, Royal Society of Chemistry, Cambridge, UK, 2012 Search PubMed.
- D. R. Weinberg, C. J. Gagliardi, J. F. Hull, C. F. Murphy, C. A. Kent, B. C. Westlake, A. Paul, D. H. Ess, D. G. McCafferty and T. J. Meyer, Chem. Rev., 2012, 112, 4016–4093 CrossRef CAS PubMed.
- A. Migliore, N. F. Polizzi, M. J. Therien and D. N. Beratan, Chem. Rev., 2014, 114, 3381–3465 CrossRef CAS PubMed.
- Y. Zhang, X.-B. Li, A. M. Fleming, J. Dood, A. A. Beckstead, A. M. Orendt, C. J. Burrows and B. Kohler, J. Am. Chem. Soc., 2016, 138, 7395–7401 CrossRef CAS PubMed.
- Y. Zhang, K. de La Harpe, A. A. Beckstead, R. Improta and B. Kohler, J. Am. Chem. Soc., 2015, 137, 7059–7062 CrossRef CAS PubMed.
- O. S. Wenger, Acc. Chem. Res., 2013, 46, 1517–1526 CrossRef CAS PubMed.
- C. J. Gagliardi, L. Wang, P. Dongare, M. K. Brennaman, J. M. Papanikolas, T. J. Meyer and D. W. Thompson, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 11106–11109 CrossRef CAS PubMed.
- C. Costentin, M. Robert, J.-M. Saveant and C. Tard, Acc. Chem. Res., 2014, 47, 271–280 CrossRef CAS PubMed.
- J. J. Warren and J. M. Mayer, Biochemistry, 2015, 54, 1863–1878 CrossRef CAS PubMed.
- J. Soetbeer, P. Dongare and L. Hammarström, Chem. Sci., 2016, 7, 4607–4612 RSC.
- B. H. Solis, A. G. Maher, D. K. Dogutan, D. G. Nocera and S. Hammes-Schiffer, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 485–492 CrossRef CAS PubMed.
- G. A. Crosby and J. N. Demas, J. Phys. Chem., 1971, 75, 991–1024 CrossRef CAS.
- W. H. Melhuish, J. Phys. Chem., 1961, 65, 229–235 CrossRef CAS.
- D. B. Siano and D. E. Metzler, J. Chem. Phys., 1969, 51, 1856–1861 CrossRef CAS.
- D. W. Marquardt, J. Soc. Ind. Appl. Math., 1963, 11, 431–441 CrossRef.
- Joint Committee for Guides in Metrology (JCGM), Evaluation of Measurement Data – Guide to the Expression of Uncertainty in Measurement, JCGM, Paris, 2008.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford, CT, USA, 2009 Search PubMed.
- C. Laurence, K. A. Brameld, J. Graton, J.-Y. Le Questel and E. Renault, J. Med. Chem., 2009, 52, 4073–4086 CrossRef CAS PubMed.
- C. Laurence, J. Legros, P. Nicolet, D. Vuluga, A. Chantzis and D. Jacquemin, J. Phys. Chem. B, 2014, 118, 7594–7608 CrossRef CAS PubMed.
- W. Al-Soufi, M. Novo and M. Mosquera, Appl. Spectrosc., 2001, 55, 630–636 CrossRef CAS.
- W. Al-Soufi, M. Novo, M. Mosquera and F. Rodríguez Prieto, in Reviews in Fluorescence 2009, ed. C. D. Geddes, Springer, New York, 2011, pp. 23–45 Search PubMed.
- S. G. Lias, J. E. Bartmess, J. F. Liebman, J. L. Holmes, R. D. Levin and W. G. Mallard, in NIST Chemistry WebBook, NIST Standard Reference Database Number 69, ed. P. J. Linstrom and W. G. Mallard, National Institute of Standards and Technology, Gaithersburg MD, 20899, DOI:10.18434/T4D303, accessed November 2017.
- J. Fossey, P. Mourgues, R. Thissen and H. E. Audier, Int. J. Mass Spectrom., 2003, 227, 373–380 CrossRef CAS.
- Y. Fu, L. Liu, H. Z. Yu, Y. M. Wang and Q. X. Guo, J. Am. Chem. Soc., 2005, 127, 7227–7234 CrossRef CAS PubMed.
- J. Peon, X. Tan, J. D. Hoerner, C. G. Xia, Y. F. Luk and B. Kohler, J. Phys. Chem. A, 2001, 105, 5768–5777 CrossRef CAS.
- A. Rosspeintner and E. Vauthey, Phys. Chem. Chem. Phys., 2014, 16, 25741–25754 RSC.
- B. Dereka, M. Koch and E. Vauthey, Acc. Chem. Res., 2017, 50, 426–434 CrossRef CAS PubMed.
- P. Hohenberg and W. Kohn, Phys. Rev., 1964, 136, B864–B871 CrossRef.
- W. Kohn and L. J. Sham, Phys. Rev., 1965, 140, A1133–A1138 CrossRef.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. T. Lee, W. T. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- T. H. Dunning, J. Chem. Phys., 1989, 90, 1007–1023 CrossRef CAS.
- G. Gilli and P. Gilli, The Nature of the Hydrogen Bond: Outline of a Comprehensive Hydrogen Bond Theory, Oxford University Press, Oxford, UK, 2013 Search PubMed.
- C. Laurence, M. Berthelot, M. Helbert and K. Sraidi, J. Phys. Chem., 1989, 93, 3799–3802 CrossRef CAS.
- R. G. Pearson, J. Am. Chem. Soc., 1986, 108, 6109–6114 CrossRef CAS.
- F. G. Bordwell and J. P. Cheng, J. Am. Chem. Soc., 1989, 111, 1792–1795 CrossRef CAS.
- X. Cai, M. Sakamoto, M. Fujitsuka and T. Majima, J. Phys. Chem. A, 2007, 111, 1788–1791 CrossRef CAS PubMed.
- E. P. L. Hunter and S. G. Lias, J. Phys. Chem. Ref. Data, 1998, 27, 413–656 CrossRef CAS.
- A. Brenlla, M. Veiga Gutierrez, M. C. Rios Rodriguez, F. Rodriguez-Prieto, M. Mosquera and J. L. Perez Lustres, J. Phys. Chem. Lett., 2014, 5, 989–994 CrossRef CAS PubMed.
- Y. Marcus, J. Phys. Org. Chem., 2012, 25, 1072–1085 CrossRef CAS.
- Y. Marcus and Y. Migron, J. Phys. Chem., 1991, 95, 400–406 CrossRef CAS.
- J. D. Henrich, S. Suchyta and B. Kohler, J. Phys. Chem. B, 2015, 119, 2737–2748 CrossRef CAS PubMed.
- J. Segarra-Martí, M. Merchán and D. Roca-Sanjuán, J. Chem. Phys., 2012, 136, 244306 CrossRef PubMed.
- M. C. Ríos Rodríguez, J. C. Penedo, R. J. Willemse, M. Mosquera and F. Rodríguez-Prieto, J. Phys. Chem. A, 1999, 103, 7236–7243 CrossRef.
- J. C. Penedo, M. Mosquera and F. Rodríguez-Prieto, J. Phys. Chem. A, 2000, 104, 7429–7441 CrossRef CAS.
- S.-Y. Park, T. G. Kim, M. J. Ajitha, K. Kwac, Y. M. Lee, H. Kim, Y. Jung and O.-H. Kwon, Phys. Chem. Chem. Phys., 2016, 18, 24880–24889 RSC.
- W. Siebrand, Z. Smedarchina, E. Martínez-Núñez and A. Fernández-Ramos, Phys. Chem. Chem. Phys., 2016, 18, 22712–22718 RSC.
- G.-J. Zhao, J.-Y. Liu, L.-C. Zhou and K.-L. Han, J. Phys. Chem. B, 2007, 111, 8940–8945 CrossRef CAS PubMed.
- M. Barbatti, J. Am. Chem. Soc., 2014, 136, 10246–10249 CrossRef CAS PubMed.
- X. Liu, A. L. Sobolewski, R. Borrelli and W. Domcke, Phys. Chem. Chem. Phys., 2013, 15, 5957–5966 RSC.
- T. N. V. Karsili, D. Tuna, J. Ehrmaier and W. Domcke, Phys. Chem. Chem. Phys., 2015, 17, 32183–32193 RSC.
- R. Szabla, H. Kruse, J. Sponer and R. W. Gora, Phys. Chem. Chem. Phys., 2017, 19, 17531–17537 RSC.
- X. Wu, T. N. V. Karsili and W. Domcke, ChemPhysChem, 2016, 17, 1298–1304 CrossRef CAS PubMed.
- X. Liu, T. N. V. Karsili, A. L. Sobolewski and W. Domcke, J. Phys. Chem. B, 2015, 119, 10664–10672 CrossRef CAS PubMed.
- T. T. Eisenhart and J. L. Dempsey, J. Am. Chem. Soc., 2014, 136, 12221–12224 CrossRef CAS PubMed.
- L. Biczok, N. Gupta and H. Linschitz, J. Am. Chem. Soc., 1997, 119, 12601–12609 CrossRef CAS.
- L. Biczok, T. Berces and H. Linschitz, J. Am. Chem. Soc., 1997, 119, 11071–11077 CrossRef CAS.
- A. A. Pizano, J. L. Yang and D. G. Nocera, Chem. Sci., 2012, 3, 2457–2461 RSC.
- J. Chen, M. Kuss-Petermann and O. S. Wenger, J. Phys. Chem. B, 2015, 119, 2263–2273 CrossRef CAS.
- M.-T. Zhang, J. Nilsson and L. Hammarström, Energy Environ. Sci., 2012, 5, 7732–7736 CAS.
- W. D. Morris and J. M. Mayer, J. Am. Chem. Soc., 2017, 139, 10312–10319 CrossRef CAS PubMed.
- I. M. C. van Amsterdam, M. Ubbink, O. Einsle, A. Messerschmidt, A. Merli, D. Cavazzini, G. L. Rossi and G. W. Canters, Nat. Struct. Biol., 2002, 9, 48–52 CrossRef CAS PubMed.
- P. Ball, Chem. Rev., 2008, 108, 74–108 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Kinetic model, the supplementary experimental results and data analyses with supporting figures, and the results of the ab initio calculations. See DOI: 10.1039/c7cp07057h |
| This journal is © the Owner Societies 2018 |