A closer look into deep eutectic solvents: exploring intermolecular interactions using solvatochromic probes
C.
Florindo
 ab,
A. J. S.
McIntosh
ab,
A. J. S.
McIntosh
 c,
T.
Welton
c,
T.
Welton
 c,
L. C.
Branco
c,
L. C.
Branco
 d and
I. M.
Marrucho
d and
I. M.
Marrucho
 *ab
*ab
aInstituto de Tecnologia Química e Biológica António Xavier, Universidade Nova de Lisboa, Av. Da República, 2780-157, Oeiras, Portugal. E-mail: isabel.marrucho@tecnico.ulisboa.pt; Fax: +351 218499242; Tel: +351 218413385
bCentro de Química Estrutural, Instituto Superior Técnico, Universidade de Lisboa, Avenida Rovisco Pais, 1049-001 Lisboa, Portugal
cDepartment of Chemistry, Imperial College London, Exhibition Road, London SW7 2AZ, UK
dREQUIMTE, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, Campus da Caparica, 2829-516 Caparica, Portugal
First published on 27th November 2017
Abstract
Deep eutectic solvents (DESs) constitute a new class of ionic solvents that has been developing at a fast pace in recent years. Since these solvents are commonly suggested as green alternatives to organic solvents, it is important to understand their physical properties. In particular, polarity plays an important role in solvation phenomena. In this work, the polarity of different families of DESs was studied through solvatochromic responses of UV-vis absorption probes. Kamlet–Taft α, β, π* and ETN parameters were evaluated using different solvatochromic probes, as 2,6-dichloro-4-(2,4,6-triphenyl-N-pyridino)-phenolate (Reichardt's betaine dye 33), 4-nitroaniline, and N,N-diethyl-4-nitroaniline for several families of DESs based on cholinium chloride, DL-menthol and a quaternary ammonium salt ([N4444]Cl). In addition, a study to understand the difference in polarity properties between DESs and the corresponding ILs, namely ILs based on cholinium cation and carboxylic acids as anions ([Ch][Lev], [Ch][Gly] and [Ch][Mal]), was carried out. The chemical structure of the hydrogen bond acceptor (HBA) in a DES clearly controls the dipolarity/polarizability afforded by the DES. Moreover, Kamlet–Taft parameters do not vary much within the family, but they differ among families based on different HBA, either for DESs containing salts ([Ch]Cl or [N4444]Cl) or neutral compounds (DL-menthol). A substitution of the HBD was also found to play an important role in solvatochromic probe behaviour for all the studied systems.
Introduction
Deep eutectic solvents (DESs) have been emerging since 2004 as a new generation of solvents with great potential for a variety of applications.1 DESs can be regarded as a new class of ionic solvents, typically composed of an organic salt and at least one hydrogen bond donor, which presents a lower melting point than any of its individual components.2 The formation of a liquid compound at room temperature is due to the formation of hydrogen bonds between a hydrogen bond donor (HBD), and a hydrogen bond acceptor (HBA).3 Bearing in mind that in a salt, the cation generally acts as an HBD and the anion as HBA, the DES formation is due to the addition of an extra HBD. DESs are currently attracting widespread scientific and technological interest as alternatives to ionic liquids (ILs).4,5Although these solvents are generally compared to ILs, mainly due to their equivalently negligible vapour pressures at room temperature, they have important advantages such as their straightforward and green synthesis, that does not need any solvent and purification steps and their low toxicity and cost, since the compounds used are usually non-toxic, abundant and from renewable resources.6,7 Just like ILs, one of the most attractive aspects of these alternative solvents is the ability to fine-tune their physical–chemical properties, including hydrogen bond donating/accepting ability and polarizability, through the easy manipulation of the chemical structures of the starting compounds. However, there is still a lack in the knowledge of some important solvent properties, such as polarity.
The exact meaning of “polarity” (the sum of all possible interactions between a solvent and any potential solute8) is complex as many different intra and inter aggregate/ion pair interactions, such as hydrogen bonding, π-interactions or van der Waals forces, are involved. The molecular dynamics of DESs systems have been recently studied using 1H pulsed field gradient NMR self-diffusion coefficients. This method is very useful to detail and understand the interactions between ions, host/guest molecules and complexes formation.9–11 On the other hand, solvatochromic probes have also been used to investigate ILs and DES polarity. In particular, Kamlet–Taft parameters have been used to quantify the hydrogen-bond donating ability (α, acidity), the hydrogen bond accepting ability (β, basicity) and polarity/polarizability (π*) of ILs and DESs.5,12–14 It should be remarked that polarity is not an absolute property of the pure liquid15,16 and hence, there is no single correct value when comparing polarity scales.15,16 All polarity scales are relative and different scales give different polarities for the same solvent and even different relative polarities can arise.17
So far, only four studies18–21 on the characterization of DESs polarities have been published. All studies are mainly focused on use of solvatochromic probes to characterize the polarity of the most popular DESs, namely cholinium chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1
urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), cholinium chloride
2), cholinium chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (1
glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and cholinium chloride
2) and cholinium chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylene glycol (1
ethylene glycol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Highlighting the study developed by Pandey et al.,14,18 where they investigated the effect of temperature and the addition of water on the three DESs’ polarity was also studied. They concluded that an increase in temperature results in reduced H-bond donor acidity of the DESs, while no temperature effect was observed on the dipolarity/polarizability and H-bond accepting basicity. It was also shown that addition of water to DESs resulted in increased dipolarity/polarizability and a decrease in H-bond accepting basicity. Very recently, Teles et al.21 published a more detailed study of solvatochromic parameters of DES formed by ammonium-based salts and carboxylic acids. It was shown that the high acidity of the studied DESs was mainly due to the organic acid present in the mixture, and that an increase of the alkyl side chain of both the HBA and the HBD species leads to a lower ability of the solvent to donate protons. They concluded that DES composed of ammonium-based salts and carboxylic acids presented a higher capacity to donate and accept protons when compared to most of the ionic liquids or organic molecular solvents.
2). Highlighting the study developed by Pandey et al.,14,18 where they investigated the effect of temperature and the addition of water on the three DESs’ polarity was also studied. They concluded that an increase in temperature results in reduced H-bond donor acidity of the DESs, while no temperature effect was observed on the dipolarity/polarizability and H-bond accepting basicity. It was also shown that addition of water to DESs resulted in increased dipolarity/polarizability and a decrease in H-bond accepting basicity. Very recently, Teles et al.21 published a more detailed study of solvatochromic parameters of DES formed by ammonium-based salts and carboxylic acids. It was shown that the high acidity of the studied DESs was mainly due to the organic acid present in the mixture, and that an increase of the alkyl side chain of both the HBA and the HBD species leads to a lower ability of the solvent to donate protons. They concluded that DES composed of ammonium-based salts and carboxylic acids presented a higher capacity to donate and accept protons when compared to most of the ionic liquids or organic molecular solvents.
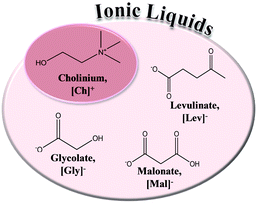
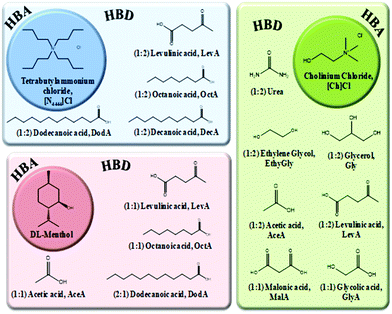
In the present work, the aim is to evaluate and discuss the polarity of DESs, which have different chemical structures, using the solvatochromic shift of different probes in the visible absorbance spectrum. Recently, we have compared the densities and viscosities of hydrophilic cholinium-based DESs and corresponding ILs.4 We have also studied highly fluid hydrophobic DESs based on DL-menthol22 while other hydrophobic DESs based on quaternary ammonium salts have also been reported in literature.23 Thus, it is now important to characterize all these different DESs from the point of view of polarity. In this way, a range of DESs, based on hydrophilic HBA (cholinium chloride) and a hydrophobic HBD (tetrabutylammonium chloride or DL-menthol), combined with several different carboxylic acids, were studied in terms of normalised polarity (ETN) and the Kamlet–Taft parameters (α, β and π*). Chemical structures and respective acronyms of the ILs and DESs used in this work are presented in Fig. 1 and 2, respectively. Additionally, a comparison of the solvatochromic parameters obtained for hydrophilic DESs and their corresponding cholinium-based ILs is also presented.
Experimental
Materials
2,6-Dichloro-4-(2,4,6-triphenyl-N-pyridino)-phenolate (Reichardt's betaine dye 33), 4-nitroaniline, and N,N-diethyl-4-nitroaniline were purchased from Fluka (≥97% mass fraction purity), Sigma Aldrich (≥99% mass fraction purity) and Frinton Laboratories, respectively, and were used as received.For the synthesis of the ILs the following reagents were used: cholinium bicarbonate solution (80% mass fraction purity in H2O), malonic acid (99% mass fraction purity), glycolic acid (99% mass fraction purity) were purchased from Sigma Aldrich and levulinic acid (98% mass fraction purity) was supplied by Acros Organics (>98% mass fraction purity). Methanol and diethyl ether were purchased as AnalaR NORMAPUR (VWR chemicals) and dichloromethane was purchased as GPR Rectapur (VWR chemicals), and were used as received. For the synthesis of the deep eutectic solvents the following reagents were used: cholinium chloride ([Ch]Cl) (≥98% mass fraction purity) and tetrabutylammonium chloride ([N4444]Cl) (>97% mass fraction purity) were purchased from Sigma-Aldrich and were dried under vacuum prior to use. Urea, ethylene glycol, glycerol, levulinic acid, glycolic acid, malonic acid, acetic acid, octanoic acid, decanoic acid and dodecanoic acid (all ≥99% mass fraction purity) were purchased from Sigma-Aldrich and used as supplied.
Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to aqueous cholinium bicarbonate, following an established procedure.24 The mixtures were stirred at ambient temperature and pressure for 12 h. The resulting products were washed with diethyl ether to remove any unreacted acid. Excess water and traces of other volatile substances were removed first by rotary evaporation, and then by stirring and heating under vacuum. The chemical structures and the purities of the synthesized cholinium-based ILs were confirmed by 1H and 13C NMR. All the IL samples were dried prior to their use by stirring and heating under vacuum at moderate temperature (40 °C, >48 h, ca. 0.01 mbar). Their water contents were determined by Karl Fischer titration (831 KF Coulometer, Metrohm) and considered in all experiments.
1) to aqueous cholinium bicarbonate, following an established procedure.24 The mixtures were stirred at ambient temperature and pressure for 12 h. The resulting products were washed with diethyl ether to remove any unreacted acid. Excess water and traces of other volatile substances were removed first by rotary evaporation, and then by stirring and heating under vacuum. The chemical structures and the purities of the synthesized cholinium-based ILs were confirmed by 1H and 13C NMR. All the IL samples were dried prior to their use by stirring and heating under vacuum at moderate temperature (40 °C, >48 h, ca. 0.01 mbar). Their water contents were determined by Karl Fischer titration (831 KF Coulometer, Metrohm) and considered in all experiments.
Results and discussion
Betaine dye scale
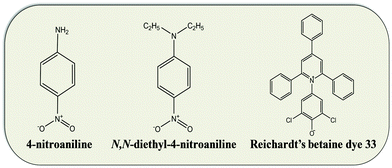
Due to the large shift in the lowest energy charge-transfer absorption band between polar and non-polar solvents, Reichardt's dye or betaine dye 30 (2,6-diphenyl-4-(2,4,6-triphenylpyridinium-1-yl)phenolate) is one of the most widely used solvatochromic probes. However, its zwitterionic nature causes its solvatochromic behaviour to be strongly affected by the HBD character of the solvent: it is well known that hydrogen-bond donating solvents stabilize the ground state more than the excited state.25 Since most of our DESs present strong acidic character (pH ≈ 1), they extensively interfere with this dye solvatochromic behaviour. To circumvent this problem, a derivative of Reichardt's dye named Reichardt's betaine dye 33 (2,6-dichloro-4-(2,4,6-triphenylpyridinium1-yl)phenolate) was used in this work. Molecular structures of the solvatochromic probes used are presented in Fig. 3.The solvatochromic parameter ET(33) was determined from the lowest energy of charge-transfer absorption band of dye in the solvent under study, and can be calculated using the following equation:
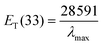 | (1) |
E T(33) is used as a measure of the solvent's overall polarity, dipolarity/polarizability and/or HBD ability, arising from interactions between the solvent and the dye.27ETN is a normalized polarity parameter and varies between 0 for TMS (extremely non-polar) and 1 for water (extremely polar). Thus, its use is recommended instead of ET(30).
Absorbance spectra of Reichardt's 33 were collected in several solvents (DESs and ILs) at room temperature, and the corresponding values of ET(33) converted into ET(30) values, through a linear regression analysis using the following eqn (2):14,28
| ET(30) = 0.9953(±0.0287) × ET(33) − 8.1132(±1.6546) | (2) |
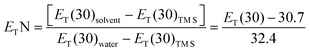 | (3) |
In Fig. 4, the absorbance spectra of Reichardt's 33 dissolved in several ILs and DESs are presented.
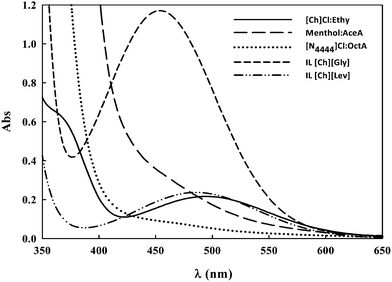 | ||
| Fig. 4 Example of the lowest energy intramolecular charge-transfer absorption band of Reichardt's betaine dye 33 for some ILs and DESs studied in this work. | ||
It can be observed that the peaks for DL-menthol:AceA and [N4444]Cl:OctA in the region between 400 nm and 600 nm are difficult to detect. To overcome this issue, first derivatives of the spectra were used to determine the λmax. The λmax value, as well as ET(33) and ETN calculated using eqn (1) and (3), are listed in Table 1 for all the ILs and DESs studied in this work.
| λ max of Reichardt's 33 (nm) | E T(33) (kcal mol−1) | E TN | ||
|---|---|---|---|---|
| Ionic liquids | [Ch][Lev] | 485.07 | 58.94 | 0.61 |
| [Ch][Mal] | 428.73 | 66.69 | 0.85 | |
| [Ch][Gly] | 453.17 | 63.09 | 0.74 | |
| Deep eutectic solvents | [Ch]Cl:LevA | 568.89 | 50.26 | 0.35 |
| [Ch]Cl:MalA | 442.53 | 64.61 | 0.79 | |
| [Ch]Cl:GlyA | 562.44 | 50.83 | 0.36 | |
| [Ch]Cl:Urea | 434.84 | 65.75 | 0.81 | |
| [Ch]Cl:Ethy | 433.82 | 65.91 | 0.83 | |
| [Ch]Cl:Gly | 429.99 | 66.49 | 0.84 | |
| DL-Menthol:AceA | 457.80 | 62.45 | 0.72 | |
| DL-Menthol:LevA | 456.56 | 62.62 | 0.73 | |
| DL-Menthol:OctA | 453.12 | 63.10 | 0.74 | |
| DL-Menthol:DodA | 454.52 | 62.90 | 0.73 | |
| [N4444]Cl:OctA | 464.71 | 61.52 | 0.69 | |
| [N4444]Cl:DecA | 474.72 | 60.23 | 0.65 | |
| [N4444]Cl:DodA | 464.36 | 61.57 | 0.69 | |
In order to validate our experimental method, the ET(33) values for [Ch]Cl:Urea, [Ch]Cl:Ethy and [Ch]Cl:Gly were also measured in this work and compared with those published in literature 65.418, 65.718 and 66.420 kcal mol−1, respectively. There is a good agreement between our results listed in Table 1 and those reported in literature, thus validating the methodology here used. High ETN values were obtained for the two families of hydrophobic DESs studied in this work, DL-menthol-based and [N4444]Cl-based, listed in Table 1, demonstrating that these solvents are hydrophobic and polar. For example, if one compares the values of DL-menthol:OctA (0.74) or DL-menthol:DodA (0.73) with those of the corresponding quaternary ammonium based DESs, [N4444]Cl:OctA (0.69) and [N4444]Cl:DodA (0.69), only small differences can be observed. Nevertheless, the ETN values obtained for the DL-menthol-based DESs are slightly higher than those obtained for [N4444]Cl-based DESs, indicating the important role of HBA in defining the polarity of hydrophobic DESs. Another observation is that ETN values are almost constant within the same HBA family. For example, the same ETN value (0.73) was obtained for DL-menthol:LevA and DL-menthol:DodA, despite the obvious chemical differences between the HBD of the two DESs. In the same way, similar ETN values were obtained for [N4444]Cl-based DESs, despite the differences in the chain length of the HBDs used. These observations corroborate the conclusion that the HBA plays a more important role in overall polarity than the HBD in hydrophobic DESs.
Focusing now on the hydrophilic [Ch]Cl-based DESs, a wider range of ETN can be observed: [Ch]Cl:MalA (0.79) has the highest ETN value, while very similar values were obtained for the other two DESs, [Ch]Cl:LevA (0.35) and [Ch]Cl:GlyA (0.36). This means that the DES based on the diacid (MalA) as HBD is more polar than those based on monoacids with different chemical groups, such as a ketone (in LevA) or an alcohol group (in Gly). In other words, the interactions between the probe and the referred DESs decrease as the groups change from acid to alcohol to ketone, as expected.
Comparing the ETN values obtained for hydrophilic and hydrophobic DESs for the same HBD, [Ch]Cl:LevA (0.35) and for DL-menthol:LevA (0.73), it can be concluded that the latter is much more polar than the former. This is somehow surprising since [Ch]Cl:LevA contains an IL as HBA, while DL-menthol:LevA contains a neutral molecule. Again, and as it was mentioned before when two hydrophobic DESs with the same HBD were compared, this can be attributed to the differences in the HBA: the chloride anion establishes a more stable hydrogen bond with the LevA than that between DL-menthol and LevA, decreasing the HBD ability of the former and thus its interaction with the probe.
The values here obtained for the three studied ILs are very similar to those found in literature for other common and well-studied ILs. In general, the ETN values for ILs vary significantly depending upon the nature of cation and anion, ranging from 0.5 to 0.7,29 in agreement with the results here obtained: [Ch][Lev] (0.61), [Ch][Gly] (0.74), [Ch][Mal] (0.85).
The explanation for this variation is essentially related to the length and the hydroxyl group functionalities of the alkyl chains of the anions and their ability to participate in hydrogen-bonding networks. Generally, for the same cation, the ETN values of ILs decrease in the following order of anions: [HCO2]− > [NO3]− > [BF4]− > [NTf2]− > [PF6]−, which is the order of decreasing basicity of the anions.30 Finally, comparing among the different ILs studied, where the anion is changed and the cation is kept constant, it can be observed that the ETN scale is particularly sensitive to the HBD ability of the anion.
Comparing the DESs based on [Ch]Cl and acids with the corresponding ILs, ILs are generally much more polar than the corresponding DESs, since the ETN for the ILs are generally higher than those of the corresponding DESs. This is in agreement with Pandey's observations, who studied DESs formed from choline chloride combined with 1,2-ethanediol, glycerol, and urea, in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratios.14,18 Another important observation is that the polarity order observed in the DESs and ILs is maintained, that is, from the most polar to the less polar the following order is attained: MalA > GlyA > LevA.
2 molar ratios.14,18 Another important observation is that the polarity order observed in the DESs and ILs is maintained, that is, from the most polar to the less polar the following order is attained: MalA > GlyA > LevA.
Kamlet–Taft scale
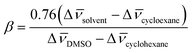 | (4) |
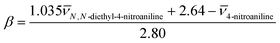 | (5) |

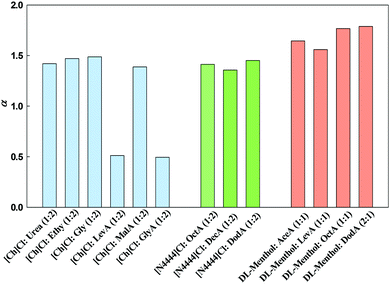
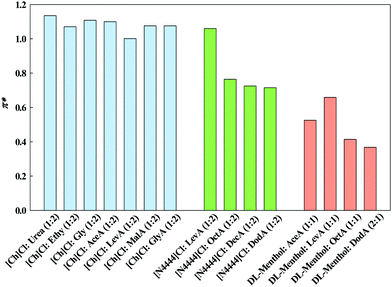
| α | β | π* | ||
|---|---|---|---|---|
| a Other ILs were synthesized in order to provide direct comparison between ILs and DESs, but solvatochromic probes measurements were not possible due their solid physical state. b No peak could be detected using the same probe for direct comparison. | ||||
| Ionic liquidsa | [Ch][Lev] | 1.07 | 1.03 | 1.00 |
| [Ch][Mal] | 1.55 | 0.62 | 1.04 | |
| [Ch][Gly] | 1.29 | 0.79 | 1.08 | |
| Deep eutectic solvents | [Ch]Cl:AceA | 0.53 | 1.10 | |
| [Ch]Cl:LevA | 0.51 | 0.57 | 1.00 | |
| [Ch]Cl:MalA | 1.39 | 0.42 | 1.08 | |
| [Ch]Cl:GlyA | 0.49 | 0.50 | 1.08 | |
| [Ch]Cl:Urea | 1.42 | 0.50 | 1.14 | |
| [Ch]Cl:Ethy | 1.47 | 0.57 | 1.07 | |
| [Ch]Cl:Gly | 1.49 | 0.52 | 1.11 | |
| DL-Menthol:AceA | 1.64 | 0.60 | 0.53 | |
| DL-Menthol: LevA | 1.56 | 0.58 | 0.66 | |
| DL-Menthol:OctA | 1.77 | 0.50 | 0.41 | |
| DL-Menthol:DodA | 1.79 | 0.57 | 0.37 | |
| [N4444]Cl:LevA | 0.82 | 1.06 | ||
| [N4444]Cl:OctA | 1.41 | 0.99 | 0.76 | |
| [N4444]Cl:DecA | 1.36 | 0.97 | 0.73 | |
| [N4444]Cl:DodA | 1.45 | 1.04 | 0.71 | |
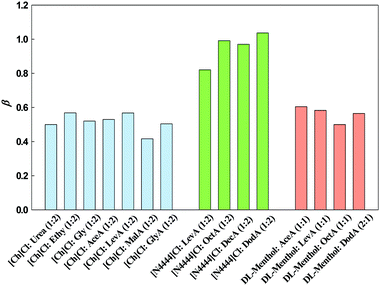
In Fig. 5, the obtained Kamlet–Taft β parameter for the studied DESs, organized by families of hydrogen bond acceptors, [Ch]Cl, [N4444]Cl and DL-menthol-based DESs, are presented.
The β values obtained for [Ch]Cl:Urea, [Ch]Cl:Ethy and [Ch]Cl:Gly are in agreement with those reported in the literature by Pandey et al.,18 where [Ch]Cl:Urea has the lowest β value, followed by [Ch]Cl:Gly and [Ch]Cl:Ethy. This can be attributed to the basicity of the HBD, since urea is by far the most basic compound, followed by glycerol and ethylene glycol, with pKa values of 0.10, 14.15 and 14.22, respectively. Regarding the β values obtained for [Ch]Cl:Urea, it is interesting to observe that the presence of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in urea does not increase the β value. In the case of [Ch]Cl:LevA, the effect of the C
O group in urea does not increase the β value. In the case of [Ch]Cl:LevA, the effect of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in levulinic acid is still there, but the effect is smaller than for the IL analogue. Nevertheless, note that levulinic acid does have a carbonyl group which can act as a HBA.
O group in levulinic acid is still there, but the effect is smaller than for the IL analogue. Nevertheless, note that levulinic acid does have a carbonyl group which can act as a HBA.
It can be seen that the values for the [Ch]Cl-based and the DL-menthol-based DESs are moderate and similar to each other, while those for [N4444]Cl-based DESs are much higher. For example, the β value of [Ch]Cl:LevA (0.58) is similar to that of DL-menthol:LevA (0.58) leading us to think that the influence of the HBA is negligible. However, comparing β values obtained for DL-menthol-based DESs with those for [N4444]Cl-based DESs for the same HBD, a large difference can be observed. For example, the β value for DL-menthol:OctA (0.50) is almost half of that of [N4444]Cl:OctA (0.97). This fact indicates that the nature of the HBA, or more precisely the interaction between the HBD and HBA which limits those with the probe, is of crucial importance in modulating the β results.
Regarding only the hydrophobic DESs studied in this work, it can be concluded that although very different values were obtained for the two families, DL-menthol- and [N4444]Cl-based DES, no large differences were obtained among the members of the same family, indicating the small effect of the HBDs on the acidity of the DESs. In particular, increasing alkyl chain of the HBD has very little effect on this descriptor in both families. Furthermore, the extra tunability of DES polarity by the easy introduction of different HBD/HBA ratios needs to be highlighted.
In Fig. 6, a comparison of the obtained Kamlet–Taft parameter for cholinium-based ILs and the corresponding DESs is presented.
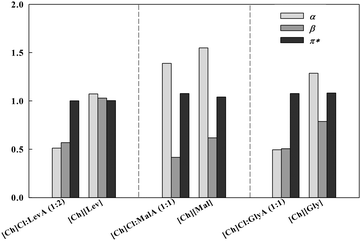 | ||
| Fig. 6 Comparison of Kamlet–Taft parameters obtained for cholinium-based ILs and corresponding DESs. | ||
The β values for the studied cholinium-based ILs are consistently higher than those of the corresponding DESs. This is due to the fact that the IL anion is the deprotonated form of the acid while the DES contains the protonated form acid, and thus the deprotonated form should be less hydrogen bonding acceptor than the protonated form. To be highlighted the very high value of β for [Ch][Lev], with a value of 1.03, while the value found for [Ch]Cl:LevA is 0.58, similar to the other cholinium-based DESs. Moreover, [Ch]Cl:LevA is also composed of two moles of levulinic acid as opposed to the IL, where only 1 mole is present. As mentioned before the large β for [Ch][Lev] can be possibly explained due to the fact that levulinic acid anion can only act as HBA, while malonic and glycolic acid anions still have one acid group and one alcohol group, respectively and thus are able to act as HBA and HBD.
The higher values of β for the studied ILs, when compared to those of the corresponding DES, lead to the conclusion that the formation of DES, through establishment of hydrogen bonds, decreases the capacity of the DES to engage in further hydrogen bonds network as HBD. It has been demonstrated that for ILs the β value is dominated by the anion while the cation just plays a secondary effect.33
| α = 0.0649ET(33) − 2.03 − 0.72π* | (6) |
For the vast majority of the studied DESs and ILs, the α values obtained are higher than unity and thus higher than the values found for other common and well-studied ILs in the literature. However, minor effects can be highlighted. For example, in the family of ILs it can be seen that [Ch][Lev] has the lowest value (1.07), which is probably justified by the levulinic acid anion has no HBD functional group. In the [Ch][Mal] IL, the value of 1.55 can be related to the acid group in malonic acid anion that can act as a HBD. Finally, for [Ch][Gly], α value of 1.29 may be due to the OH group on glycolic acid anion that can act as a HBD, but not as strong as the acid group on malonic acid anion. Regarding the family of alcohol-based DESs, it is important to refer that there is also a small effect on α values (1.42 to 1.49) essentially due to the –OH group present on the ethyleneglycol and glycerol compounds. Concerning the family of cholinium-based DESs composed of carboxylic acids, the effect of the two acid groups in the malonic acid is translated in high value of α (1.39). However, in the case of glycolic acid the presence of the –OH group has little effect, leading to a lower α value (0.49).
Comparing the α values for the tetrabutylammonium-based ILs found in the literature16 with the corresponding [N4444]Cl-based DES obtained in this work, it can be concluded that α values for the ILs are much lower than those here obtained for the DES. For example, the α value for tetrabutylammonium 2-(cyclohexylamino)ethanesulfonate is 0.56, for tetrabutylammonium 2-[bis(2-hydroxyethyl)amino]ethanesulfonate it is 0.31 and for tetrabutylammonium 2-hydroxy-4-morpholinepropanesulfate it is 0.28, which means the DES α values are 2 to 5 times higher than those observed in ILs. As already reported, and in line with the present study, there seems to be competition between the HBD and probe dye solute for the proton. The α values of the studied compounds are controlled by the ability of the compounds to act as a HBD moderated by its HBA ability.
The original π* values were an average of the values for all of the solvatochromic dyes used, with a normalisation between 0 (cyclohexane) and 1 (dimethylsulfoxide). Since in this work, we used the three most commonly used Kamlet–Taft dyes, N,N-diethyl-4-nitroaniline, 4-nitroaniline and Reichardt's dye, the π* parameter was calculated from spectroscopic data of the N,N-diethyl-4-nitroaniline probe in our solvents using the following equations:
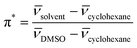 | (7) |
π* = 0.314(27.52 − ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) N,N-diethyl-4-nitroaniline) N,N-diethyl-4-nitroaniline) | (8) |
![[small nu, Greek, macron]](https://www.rsc.org/images/entities/char_e0ce.gif) (cm−1) = 107/λmax (nm). In Fig. 8, the obtained Kamlet–Taft π* parameter for all the studied DESs, organized by families of hydrogen bond acceptors, [Ch]Cl-, [N4444]Cl and DL-menthol-based DESs, are presented.
(cm−1) = 107/λmax (nm). In Fig. 8, the obtained Kamlet–Taft π* parameter for all the studied DESs, organized by families of hydrogen bond acceptors, [Ch]Cl-, [N4444]Cl and DL-menthol-based DESs, are presented.
The π* values obtained for the ILs and cholinium-based DESs are high in comparison with those of conventional organic solvents and ILs, which can be as high as 0.9.34π* values can be affected by both the cation and anion,35 and tend to be higher in ILs than most organic solvents due to the degree of delocalisation of the charge between the ions.
Major differences between the cholinium-based hydrophilic DESs and the hydrophobic DESs (either composed by DL-menthol or [N4444]Cl) were found. Both cholinium-based ILs and DESs studied in this work present similar π* values, around the unity, meaning that for these compounds the π* values are largely determined by the cholinium cation (HBA), and that chemical nature of the anion does not greatly affect the dipolarity/polarizability. On the other hand, no significant differences were observed for the π* values of cholinium-based DESs containing acids and the corresponding ILs, meaning that no difference in terms of dipolarity/polarizability is found between ILs and the corresponding hydrophilic DESs. As for the hydrophobic DESs, it can be seen that both the DL-menthol-based and the [N4444]Cl-based families have lower π* values than the [Ch]Cl-based hydrophilic DESs, indicating that the former are less dipolar and/or polarisable than the latter. For example, the π* value for [Ch]Cl:LevA (1.00) is much higher than that for DL-menthol:LevA (0.66). Comparing the two families of hydrophobic DESs, DL-menthol-based DESs have lower values of π* than the [N4444]Cl-based DESs. This can be particularly appreciated when the HBD is maintained, such as in the case of DL-menthol:OctA (0.41) and [N4444]Cl:OctA (0.76). Thus, it can concluded that the HBA plays the dominant role for this descriptor since the studied compounds can be organized according to the following π* trend: cholinium-based ILs ≈ Cholinium-based DESs > [N4444]Cl-based DESs > DL-menthol-based DESs, where the latter is the family with the lowest values of π*. This trend can be explained by the fact that the ILs and cholinium based DES are constituted by charged moieties with polar groups, thus are more polar than the [N4444]Cl-based DESs, which are constituted by charged moieties with apolar alkyl chains, which are more polar than the DL-menthol-based DESs, which are constituted by non-charged compounds. The HBD role in the π* parameter within each one of the families can also be clearly seen. The π* value decreases with the increase of the alkyl chain of the HBD, as expected. The π* values of DL-menthol-based DESs decrease as follows: DL-menthol:AceA > DL-menthol:OctA > DL-menthol:DodA, and the same trend is observed for the [N4444]Cl-based DESs. Thus, it can be concluded that the increase of the non-polar part of the HBD decreases the overall polarizability of the DESs, as expected.
Conclusions
Owing to the very large gap in understanding solvent–solute interaction in DESs, and considering that DESs and their corresponding ILs present a significant opportunity for a wide array of fields, the knowledge and understanding of their polarity in terms of their chemical structure is vital for their confident design for specific applications. To that end, solvatochromic data, polarity (ET and ETN) and Kamlet–Taft parameters (α, β, π*) were obtained for two different families of DES: those based on salts, such as cholinium chloride and tetrabutylammonium chloride, and those based on neutral compound, DL-menthol. The polarity properties of the cholinium-based DESs were studied and compared for the first time with the corresponding ILs. A structural study on the influence of the HBD or the HBA, as well as increasing the alkyl chain length in a DES on polarity and on Kamlet–Taft parameters at room temperature was carried out. It was found that all the DES investigated display high values of hydrogen bonding acidity, probably due to the organic acids presented in all systems. On the other hand, the hydrogen bonding basicity in these compounds does not vary much within the same HBA family, but substantially differs from [Ch]Cl family to [N4444]Cl family and DL-menthol family.It is also important to note that while one of the general characteristics of ILs is the dipolarity/polarizability parameter uniformly high irrespective of the chemical structures of the cations and anions, the same is not observed for DESs. In a DES the molecular structure of the hydrogen bond acceptor (HBA) clearly controls the dipolarity/polarizability afforded by the DES. Moreover, the Kamlet–Taft and polarity parameters of several families of DESs based on different acceptors, namely salts (cholinium chloride and [N4444]Cl) and a neutral compound (DL-menthol) here reported demonstrate that DES displays a high capacity to donate and accept protons when compared to common solvents and also ILs. In summary, DESs polarity can be easily designed by the convenient choice of their components.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Catarina Florindo is grateful for the grant from COST – EXIL with reference COST Action CM1206 and for the financial support of FCT/MCTES for the PhD grant SFRH/BD/102313/2014. Isabel M. Marrucho and Luis C. Branco gratefully acknowledge for the contract under Investigator FCT 2012 (IF/363/2012 and IF/0041/2013), respectively. This work was financed by CQE project (UID/QUI/00100/2013) and Research Units UID/QUI/00100/2013 (CQE) and UID/Multi/04551/2013 (GREEN-IT).Notes and references
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- Y. Dai, J. van Spronsen, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Anal. Chim. Acta, 2013, 766, 61–68 CrossRef CAS PubMed.
- Y. Hou, Y. Gu, S. Zhang, F. Yang, H. Ding and Y. Shan, J. Mol. Liq., 2008, 143, 154–159 CrossRef CAS.
- C. Florindo, F. S. Oliveira, L. P. N. Rebelo, A. M. Fernandes and I. M. Marrucho, ACS Sustainable Chem. Eng., 2014, 2, 2416–2425 CrossRef CAS.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142–9147 CrossRef CAS PubMed.
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2, 1063–1071 CrossRef CAS.
- M. Francisco, A. van den Bruinhorst and M. C. Kroon, Angew. Chem., Int. Ed., 2013, 52, 3074–3085 CrossRef CAS PubMed.
- C. Reichardt and T. Welton, Solvents and solvent effects in organic chemistry, John Wiley & Sons, 2011 Search PubMed.
- C. D'Agostino, R. C. Harris, A. P. Abbott, L. F. Gladden and M. D. Mantle, Phys. Chem. Chem. Phys., 2011, 13, 21383–21391 RSC.
- A. P. Abbott, C. D'Agostino, S. J. Davis, L. F. Gladden and M. D. Mantle, Phys. Chem. Chem. Phys., 2016, 18, 25528–25537 RSC.
- C. D'Agostino, L. F. Gladden, M. D. Mantle, A. P. Abbott, E. I. Ahmed, A. Y. M. Al-Murshedi and R. C. Harris, Phys. Chem. Chem. Phys., 2015, 17, 15297–15304 RSC.
- L. Crowhurst, P. R. Mawdsley, J. M. Perez-Arlandis, P. A. Salter and T. Welton, Phys. Chem. Chem. Phys., 2003, 5, 2790–2794 RSC.
- D. J. G. P. van Osch, L. J. B. M. Kollau, A. van den Bruinhorst, S. Asikainen, M. A. A. Rocha and M. C. Kroon, Phys. Chem. Chem. Phys., 2017, 19, 2636–2665 RSC.
- A. Pandey and S. Pandey, J. Phys. Chem. B, 2014, 118, 14652–14661 CrossRef CAS PubMed.
- S. Spange, R. Lungwitz and A. Schade, J. Mol. Liq., 2014, 192, 137–143 CrossRef CAS.
- M. A. Ab Rani, A. Brant, L. Crowhurst, A. Dolan, M. Lui, N. H. Hassan, J. P. Hallett, P. A. Hunt, H. Niedermeyer, J. M. Perez-Arlandis, M. Schrems, T. Welton and R. Wilding, Phys. Chem. Chem. Phys., 2011, 13, 16831–16840 RSC.
- A. R. Katritzky, D. C. Fara, H. Yang, K. Tämm, T. Tamm and M. Karelson, Chem. Rev., 2004, 104, 175–198 CrossRef CAS PubMed.
- A. Pandey, R. Rai, M. Pal and S. Pandey, Phys. Chem. Chem. Phys., 2014, 16, 1559–1568 RSC.
- A. R. Harifi-Mood, R. Ghobadi, S. Matić, B. Minofar and D. Řeha, J. Mol. Liq., 2016, 222, 845–853 CrossRef CAS.
- A. P. Abbott, R. C. Harris, K. S. Ryder, C. D'Agostino, L. F. Gladden and M. D. Mantle, Green Chem., 2011, 13, 82–90 RSC.
- A. R. R. Teles, E. V. Capela, R. S. Carmo, J. A. P. Coutinho, A. J. D. Silvestre and M. G. Freire, Fluid Phase Equilib., 2017, 448, 15–21 CrossRef CAS.
- B. D. Ribeiro, C. Florindo, L. C. Iff, M. A. Z. Coelho and I. M. Marrucho, ACS Sustainable Chem. Eng., 2015, 3, 2469–2477 CrossRef CAS.
- D. J. G. P. van Osch, L. F. Zubeir, A. van den Bruinhorst, M. A. A. Rocha and M. C. Kroon, Green Chem., 2015, 17, 4518–4521 RSC.
- T. Mourão, L. C. Tomé, C. Florindo, L. P. N. Rebelo and I. M. Marrucho, ACS Sustainable Chem. Eng., 2014, 2, 2426–2434 CrossRef.
- C. Reichardt and T. Welton, Solvents and Solvent Effects in Organic Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2010, pp. 7–64 Search PubMed.
- C. Reichardt, Green Chem., 2005, 7, 339–351 RSC.
- C. Reichardt and T. Welton, Solvents and Solvent Effects in Organic Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2010, pp. 425–508 Search PubMed.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- P. G. Jessop, D. A. Jessop, D. Fu and L. Phan, Green Chem., 2012, 14, 1245–1259 RSC.
- C. Reichardt and T. Welton, Solvents and Solvent Effects in Organic Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2010, pp. 509–548 Search PubMed.
- R. W. Taft and M. J. Kamlet, J. Am. Chem. Soc., 1976, 98, 2886–2894 CrossRef CAS.
- D. Fox, Molten Salts and Ionic Liquids 16, Electrochemical Society, 2009 Search PubMed.
- S. Pitula and A.-V. Mudring, Phys. Chem. Chem. Phys., 2010, 12, 7056–7063 RSC.
- K. A. Kurnia, F. Lima, A. F. M. Cláudio, J. A. P. Coutinho and M. G. Freire, Phys. Chem. Chem. Phys., 2015, 17, 18980–18990 RSC.
- C. Chiappe, C. S. Pomelli and S. Rajamani, J. Phys. Chem. B, 2011, 115, 9653–9661 CrossRef CAS PubMed.
| This journal is © the Owner Societies 2018 |