Sequential energy transfer followed by electron transfer in a BODIPY–bisstyrylBODIPY bound to C60 triad via a ‘two-point’ binding strategy†
Shuai
Shao
a,
Michael B.
Thomas
 a,
Kyu Hyung
Park
b,
Zoe
Mahaffey
a,
Dongho
Kim
a,
Kyu Hyung
Park
b,
Zoe
Mahaffey
a,
Dongho
Kim
 *b and
Francis
D’Souza
*b and
Francis
D’Souza
 *a
*a
aDepartment of Chemistry, University of North Texas, 1155 Union Circle, #305070, Denton, TX 76203-5017, USA. E-mail: Francis.dsouza@unt.edu
bDepartment of Chemistry, Yonsei University, 50 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea. E-mail: dongho@yonsei.ac.kr
First published on 28th November 2017
Abstract
Excitation transfer from 1BODIPY* to bisstyrylBODIPY followed by electron transfer to C60 leading to a charge separated state of appreciable lifetime in a supramolecularly assembled triad is demonstrated, as a mimic of the photosynthetic ‘antenna-reaction centre’.
Solar energy harvesting is one of the most researched topics in modern science as it holds promise towards a sustainable society.1 Mimicking the early photo-initiated events of natural photosynthesis (in plants and bacteria), and utilizing that information to build energy harvesting devices is considered to be one of the avenues to tap into the vast amount of solar energy.2 Research performed on this topic by scientists across the globe has been highly promising and has strengthened our fundamental understanding of natural and artificial photosynthesis. In the process of mimicking the early events of photosynthesis, excitation transfer and electron transfer in a sequential fashion, similar to what happens in the antenna and reaction centre of natural photosynthesis,3 are deemed to be of paramount interest. Consequently, supramolecular donor–acceptor systems capable of performing these photoprocesses in a step-wise manner are considered to be very important. Although several donor–acceptor systems are known to undergo either energy transfer or electron transfer or simultaneous occurrence of these two photoprocesses in competition upon photoexcitation, there are not many systems where energy transfer and electron transfer in a sequential route have been reported.4 This becomes especially important for the maximum utilization of solar energy.
In the present study, we have accomplished this goal by designing a supramolecular triad whose structure is shown in Fig. 1. Here, BF2-chelated dipyrromethene (BODIPY) is covalently linked to another functionalized BODIPY bearing two styryl benzo-18-crown-6 entities.5 As a consequence of extended conjugation offered by the styryl entities, the bisstyrylBODIPY absorption and emission experienced redshifts of about 150–160 nm. In addition, the emission of BODIPY overlaps with the absorption of bisstyrylBODIPY, satisfying the primary requirement for excitation energy transfer. Further, the two crown ether voids were able to bind fulleropyrrolidine functionalized with two alkyl ammonium cations via cation–dipole interactions.6a As demonstrated using ultrafast transient spectroscopic techniques, selective excitation of BODIPY promoted singlet–singlet energy transfer to the neighboring bisstyrylBODIPY. Further, the intermediate photoproduct, 1bisstyrylBODIPY*, promoted electron transfer to the bound C60 entity to produce the BODIPY–bisstyrylBODIPY˙+–C60˙− charge separated state of appreciable lifetime.
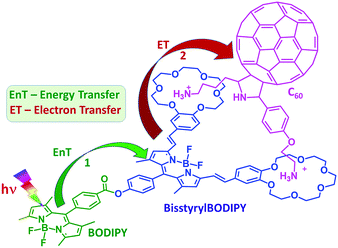 | ||
| Fig. 1 Structure of the newly synthesized BODIPY–bisstyrylBODIPY–C60 supramolecular triad, 1:C60, and the photoprocesses originated upon selective excitation of BODIPY within the triad. | ||
The synthesis of BODIPY–bisstyrylBODIPY-biscrown, 1 is shown in Scheme S1 (ESI†) while the details are given in the ESI.† This involved first the synthesis of meso-(4-hydroxyphenyl)BODIPY, 1a, by reacting 4-hydroxybenzaldehyde and 2,4-dimethylpyrrole in the presence of trifluoroacetic acid (TFA) in THF for 12 h followed by the addition of dichlorodicyanobenzoquinone (DDQ). After 4 h, the reaction mixture was treated with boron trifluoride etherate (BF3·OEt2) in the presence of triethylamine (TEA). After column purification, 1a was treated with 4′-formylbenzo-18-crown-6 with catalytic amounts of piperidine and acetic acid in benzene using the Dean–Stark apparatus to remove water from the reaction mixture and to obtain bis(benzo-18-crown-6) functionalized BODIPY, 1b. In a separate experiment, meso-(4-carboxyphenyl)BODIPY, 1c, was synthesized following a procedure similar to that used for 1a. Finally, compounds 1b and 1c were treated with EDCI in the presence of catalytic amounts of DMAP to obtain the desired BODIPY–bisstyrylBODIPY-biscrown, 1. The synthesis of bisalkylammonium functionalized fullerene, C60(NH3)22+, is given elsewhere.6c The newly synthesized compounds were characterized using 1H and 13C NMR and HR-MALDI mass techniques (see the ESI† for spectral data).
Owing to the presence of two differently functionalized BODIPY entities, the absorption spectrum of 1 revealed good spectral coverage from 300 to 700 nm. The peak maxima were located at 385, 506, 606 and 658 nm (Fig. 2) and by comparison with the absorption spectra of control compounds, 1b and 1c, the 506 nm peak was assigned to BODIPY while the 606 and 658 nm peaks were assigned to the bisstyrylBODIPY entities, respectively. The excitation of the BODIPY entity in 1 at 500 nm revealed two peaks at 525 nm corresponding to BODIPY emission; however, its intensity was quenched over 90% with the appearance of a new peak at 684 nm, corresponding to bisstyrylBODIPY. These results suggest the occurrence of singlet–singlet energy transfer in 1.7 The excitation spectrum recorded for 1 also confirmed this assignment. Additionally, the excitation of 1 at 600 nm corresponding to bisstyrylBODIPY revealed an emission at 684 nm, however, without any appreciable quenching.
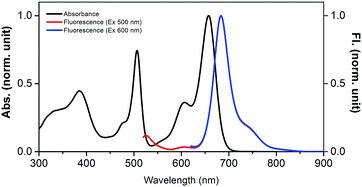 | ||
| Fig. 2 Normalized absorption (dark line) and fluorescence (red – 505 nm excitation, blue – 600 nm excitation) of BODIPY–bisstyrylBODIPY-biscrown, 1 in benzonitrile. | ||
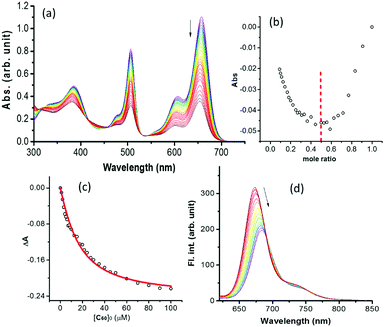
Next, the supramolecular complexation of 1 with C60(NH3)22+ was performed. Fig. 3a shows the spectral changes during the increased addition of C60(NH3)22+ to a solution of 1 in benzonitrile. The complexation was accompanied by a diminished peak intensity of 1 with isosbestic points at 415, 456 and 535 nm. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry was also established from a plot of mole ratio (Fig. 3b). The binding constant evaluated by analyzing data (Fig. 3c) was found to be 8.9 × 104 M−1, revealing stable complexation.8 Further, HR-MALDI mass and 1H NMR titrations were also performed to ascertain the 1
1 stoichiometry was also established from a plot of mole ratio (Fig. 3b). The binding constant evaluated by analyzing data (Fig. 3c) was found to be 8.9 × 104 M−1, revealing stable complexation.8 Further, HR-MALDI mass and 1H NMR titrations were also performed to ascertain the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio of the 1:C60(NH3)22+ complex (see Fig. S11–S13 in ESI†). The complexation of C60(NH3)22+ also quenched the fluorescence of 1 at 684 nm (Fig. 3d), suggesting the occurrence of excited state events from the 1BisstyrylBODIPY* to the bound C60(NH3)22+ in the supramolecular complex. The structure of the supramolecular complex was also optimized at the B3LYP/3-21G* level9 (see Fig. S1 in the ESI†). In the optimized structure, the two BODIPY entities, connected by a carboxy linker, were held at a dihedral angle of 31°, and had no steric overcrowding. The B–B, B(bisstyrylBODIPY)–C60 and B(BODIPY)–C60 distances were ∼18 Å, ∼12 Å and 30.1 Å, respectively. Hydrogen bonding between the alkyl–NH3+ and crownether oxygens was primarily responsible for the supramolecular complexation. From electrochemical studies (see Fig. S2 in the ESI†), the first oxidation of BODIPY and bisstyrylBODIPY at 0.32 and 0.76 V vs. Fc/Fc+ and the first reduction of C60(NH3)22+ at −1.10 V vs. Fc/Fc+ were recorded. Free-energy calculations10 revealed that the energy transfer from 1BODIPY* to the neighbouring bisstyrylBODIPY and the electron transfer from 1bisstyrylBODIPY* to supramolecularly bound C60(NH3)22+ to produce the BODIPY–bisstyrylBODIPY˙+–[C60(NH3)22+]˙− charge-separated state are both thermodynamically feasible with ΔG values of −0.56 and −0.42 eV, respectively.
1 stoichiometric ratio of the 1:C60(NH3)22+ complex (see Fig. S11–S13 in ESI†). The complexation of C60(NH3)22+ also quenched the fluorescence of 1 at 684 nm (Fig. 3d), suggesting the occurrence of excited state events from the 1BisstyrylBODIPY* to the bound C60(NH3)22+ in the supramolecular complex. The structure of the supramolecular complex was also optimized at the B3LYP/3-21G* level9 (see Fig. S1 in the ESI†). In the optimized structure, the two BODIPY entities, connected by a carboxy linker, were held at a dihedral angle of 31°, and had no steric overcrowding. The B–B, B(bisstyrylBODIPY)–C60 and B(BODIPY)–C60 distances were ∼18 Å, ∼12 Å and 30.1 Å, respectively. Hydrogen bonding between the alkyl–NH3+ and crownether oxygens was primarily responsible for the supramolecular complexation. From electrochemical studies (see Fig. S2 in the ESI†), the first oxidation of BODIPY and bisstyrylBODIPY at 0.32 and 0.76 V vs. Fc/Fc+ and the first reduction of C60(NH3)22+ at −1.10 V vs. Fc/Fc+ were recorded. Free-energy calculations10 revealed that the energy transfer from 1BODIPY* to the neighbouring bisstyrylBODIPY and the electron transfer from 1bisstyrylBODIPY* to supramolecularly bound C60(NH3)22+ to produce the BODIPY–bisstyrylBODIPY˙+–[C60(NH3)22+]˙− charge-separated state are both thermodynamically feasible with ΔG values of −0.56 and −0.42 eV, respectively.
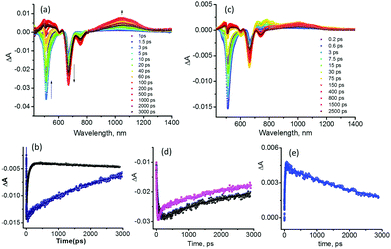
Next, the excitation transfer in 1 and the sequential energy and electron transfer in 1:C60 were probed using a femtosecond transient spectroscopic technique. Fig. 4a shows the transient spectra at the indicated delay times of 1 at the excitation wavelength of 507 nm corresponding to BODIPY in benzonitrile. Immediately after excitation, a depleted transient peak at 512 nm corresponding to ground state bleaching of 1BODIPY* was observed (see the spectrum at 1.5 ps). A rapid recovery of this peak was observed accompanied by new transient peaks corresponding to 1bisstyrylBODIPY*, confirming the occurrence of singlet–singlet energy transfer in 1.11 In the newly developed 1bisstyrylBODIPY* spectra, depleted peaks at 670 and 753 nm, and a positive peak at 1044 nm were observed. The depleted peaks were assigned to the ground state bleaching and stimulated emission, while the positive peak was assigned to the singlet–singlet transition of 1bisstyrylBODIPY*. This assignment was also confirmed by the direct excitation of 1 at 657 nm (see Fig. S3a in the ESI†). Fig. 4b shows the kinetic traces of the 512 nm peak of 1 and pristine BODIPY used as a control. The recovery lasted over 3 ns in the case of pristine BODIPY, consistent with its fluorescence lifetime of 1.9 ns in benzonitrile. However, owing to the occurrence of the energy transfer process, the recovery was rather rapid in the case of 1. By analyzing the kinetic profile of 1BODIPY* at 512 nm, a time constant of 56 ps was obtained. Using these data and those of pristine BODIPY, the energy transfer rate constant, kEnT, was calculated to be 1.73 × 1010 s−1.
Fig. 4c shows the transient spectra of 1:C60 at the excitation wavelength of 507 nm corresponding to BODIPY. Similar to the case of 1, a rapid recovery of the depleted peak of 1BODIPY* accompanied by new transient peaks related to 1bisstyrylBODIPY* was observed, indicating excitation transfer between these two entities. Additionally, the recovery/decay of the 1bisstyrylBODIPY* peaks was also rapid (compared to that observed for 1) and was accompanied by another new set of transient peaks corresponding to the formation of the BODIPY–bisstyrylBODIPY˙+–[C60(NH3)22+]˙− charge separated state.6,12 That is, the peaks at 488 and 811 nm corresponding to bisstyrylBODIPY·+ and that at 1010 nm corresponding to the [C60(NH3)22+]·− species were clear (see Fig. S4 (ESI†) for the spectrum recorded at 10 ps for 1 and 1:C60). These results conclusively prove the occurrence of sequential energy transfer followed by electron transfer in 1:C60. The kEnT value calculated using a procedure similar to that explained in the preceding paragraph was found to be 3.12 × 1010 s−1. The appreciable increase in energy transfer rate could be due to subtle changes in the geometry of 1 upon C60(NH3)22+ binding. Fig. 4d shows the kinetic traces of the 669 peak of 1 and 1:C60. The rapid recovery in the case of 1:C60 was evident (biexponential). From the fast decay component, a time constant for the charge separation process of 83 ps was arrived which yielded an electron transfer rate constant, kET, of 1.18 × 1010 s−1. The decay of [C60(NH3)22+]˙− shown in Fig. 4e lasted over 3 ns suggesting persistence of the charge separated state. The nanosecond transient spectra of 1:C60 revealed weak signals corresponding to the radical ion-pair at the earliest monitoring time window of 20 ns, suggesting this to be the upper time limit for persistence of the charge separated state. As expected, the direct excitation of bisstyrylBODIPY at 657 nm in 1:C60 also revealed charge separation (see Fig. S3b in the ESI†) with the time constants for charge separation and recombination close to those of the earlier discussed 1:C60 complex.
A novel supramolecular triad has been newly synthesized and assembled to probe sequential energy transfer followed by electron transfer events. The covalently linked BODIPY and bisstyrylBODIPY entities in 1 functioned as an energy harvester by revealing ultrafast singlet–singlet energy transfer. The two crown ether voids on 1 comfortably accommodated C60(NH3)22+via a ‘two-point’ alkyl ammonium cation–crown ether binding motif to yield the stable supramolecular 1:C60 complex. The presence of C60 in 1:C60 promoted electron transfer from the initial energy transfer product, 1bisstyrylBODIPY*, to yield the BODIPY–bisstyrylBODIPY˙+–[C60(NH3)22+]˙− charge separated state which persisted for about 20 ns. The present study brings out the importance of a BODIPY based multi-modular donor–acceptor conjugate to mimic the combined ‘antenna-reaction centre’ events of natural photosynthesis.
The authors are thankful to the National Science Foundation (Grant No. 1401188) and the National Research Foundation of Korea (NRF) funded by the Korea government (MEST) (2016R1E1A1A01943379) for support of this work. We thank Dr Guido Verbeck and the Laboratory for Imaging Mass Spectrometry at the University of North Texas for MALDI-Orbitrap Mass Spectrometry data.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) J. S. Connolly, Photochemical Conversion and Storage of Solar Energy, Academic, New York, 1981 Search PubMed; (b) W. Leibl and P. Mathis, Electron Transfer in Photosynthesis. Series on Photoconversion of Solar Energy, 2004, 2, 117 CrossRef CAS; (c) S. Lewis and D. G. Nocera, Proc. Natal. Acad. Sci. U. S. A., 2006, 103, 15729 CrossRef PubMed.
- (a) D. Gust, T. A. Moore and A. L. Moore, Acc. Chem. Res., 2009, 42, 1890 CrossRef CAS PubMed; (b) M. R. Wasielewski, Acc. Chem. Res., 2009, 42, 1910 CrossRef CAS PubMed; (c) G. Bottari, G. de la Torre, D. M. Guldi and T. Torres, Chem. Rev., 2010, 110, 6768 CrossRef CAS PubMed; (d) N. V. Tkachenko and H. Lemmetyinen, in Handbook of Carbon Nanomaterials, ed. F. D’Souza and K. M. Kadish, World Scientific Publications, Singapore, 2011, ch. 13, pp. 405–440 Search PubMed.
- (a) The Photosynthetic Reaction Center, ed. J. Deisenhofer and J. R. Norris, Academic Press, New York, 1993 Search PubMed; (b) Molecular Mechanism of Photosynthesis, ed. R. E. Blankenship, Blackwell Science, Oxford, 2002 Search PubMed.
- (a) D. Kim, Multiporphyrin Arrays: Fundamentals and Applications, Pan Stanford Publishing, Singapore, 2012 Search PubMed; (b) C. B. KC and F. D'Souza, Coord. Chem. Rev., 2016, 322, 104 CrossRef CAS.
- (a) G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184 CrossRef CAS PubMed; (b) M. E. El-Khouly, S. Fukuzumi and F. D’Souza, ChemPhysChem, 2014, 15, 30 CrossRef CAS PubMed.
- (a) L. Moreira, B. M. Illescas and N. Martin, J. Org. Chem., 2017, 82, 3347 CrossRef CAS PubMed; (b) S. Shao, H. B. Gobeze, P. A. Karr and F. D’Souza, Chem. – Asian J., 2017, 12, 2258 CrossRef CAS PubMed; (c) Y. Araki, R. Chitta, A. S. D. Sandanayaka, K. Langenwalter, S. Gadde, M. E. Zandler, O. Ito and F. D’Souza, J. Phys. Chem. C, 2008, 112, 2222 CrossRef CAS.
- Principles of Fluorescence Spectroscopy, ed. J. R. Lakowicz, Springer, Singapore, 3rd edn, 2006 Search PubMed.
- (a) P. Thordarson, Chem. Soc. Rev., 2011, 40, 1305 RSC; (b) N. L. Bill, M. Ishida, S. Bähring, J. M. Lim, S. Lee, C. M. Davis, V. M. Lynch, K. A. Nielsen, J. O. Jeppesen, K. Ohkubo, S. Fukuzumi, D. Kim and J. L. Sessler, J. Am. Chem. Soc., 2013, 135, 10852 CrossRef CAS PubMed; (c) X.-S. Ke, T. Kim, J. T. Brewster II, V. M. Lynch, D. Kim and J. L. Sessler, J. Am. Chem. Soc., 2017, 139, 4627 CrossRef CAS PubMed.
- Gaussian 09, Gaussian, Inc., Pittsburgh PA, 2009 Search PubMed.
- D. Rehm and A. Weller, Isr. J. Chem., 1970, 8, 259 CrossRef CAS.
- J. Yang, M.-C. Yoon, H. P. Kim and D. Kim, Chem. Soc. Rev., 2012, 41, 4808 RSC.
- C. O. Obondi, G. N. Lim, P. A. Karr, V. N. Nesterov and F. D’Souza, Phys. Chem. Chem. Phys., 2016, 18, 18187 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and synthetic details, additional femtosecond transient spectra, optimized geometry, 1H, and 13C NMR spectra, and HR-MALDI mass data. See DOI: 10.1039/c7cc08063h |
| This journal is © The Royal Society of Chemistry 2018 |