Variation of the bone forming ability with the physicochemical properties of calcium phosphate bone substitutes
Rongquan
Duan
 abc,
Davide
Barbieri
b,
Xiaoman
Luo
b,
Jie
Weng
c,
Chongyun
Bao
d,
Joost D.
de Bruijn
abe and
Huipin
Yuan
*bfg
abc,
Davide
Barbieri
b,
Xiaoman
Luo
b,
Jie
Weng
c,
Chongyun
Bao
d,
Joost D.
de Bruijn
abe and
Huipin
Yuan
*bfg
aBiomaterials Science and Technology, MIRA Institute, University of Twente, 7500 AE, Enschede, the Netherlands
bKuros Biosciences BV, 3723 MB, Bilthoven, the Netherlands
cKey Laboratory of Advanced Technologies of Materials, Ministry of Education, School of Materials Science and Engineering, Southwest Jiaotong University, Chengdu 610031, China
dState Key Laboratory of Oral Diseases, West China School of Stomatology, Sichuan University, Chengdu 610041, China
eSchool of Engineering and Materials Science, Queen Marry University of London, E14RD, London, UK
fComplex Tissue Regeneration department, MERLN Institute for Technology Inspired Regenerative Medicine, Maastricht University, 6229 ER, Maastricht, the Netherlands. E-mail: h.yuan@maastrichtuniversity.nl; Fax: +31-30-2297299; Tel: +31-30-2297292
gCollege of Physical Science and Technology, Sichuan University, Chengdu, China
First published on 25th October 2017
Abstract
Because of their bioactive properties and chemical similarity to the inorganic component of bone, calcium phosphate (CaP) materials are widely used for bone regeneration. Six commercially available CaP bone substitutes (Bio-Oss, Actifuse, Bi-Ostetic, MBCP, Vitoss and chronOs) as well as two tricalcium phosphate (TCP) ceramics with either a micron-scale (TCP-B) or submicron-scale (TCP-S) surface structure are characterized and their bone forming potential is evaluated in a canine ectopic implantation model. After 12 weeks of implantation in the paraspinal muscle of four beagles, sporadic bone (0.1 ± 0.1%) is observed in two Actifuse implants (2/4), limited bone (2.1 ± 1.4%) in four MBCP implants (4/4) and abundant bone (21.6 ± 4.5%) is formed in all TCP-S implants (4/4). Bone is not observed in any of the Bio-Oss, Bi-Ostetic, Vitoss, chronOs and TCP-B implants (0/4). When correlating the bone forming potential with the physicochemical properties of each material, we observe that the physical characteristics (e.g. grain size and micropore size at the submicron scale) might be the dominant trigger of material directed bone formation via specific mechanotransduction, instead of protein adsorption, surface mineralization and calcium ion release.
1. Introduction
Calcium phosphate (CaP) ceramics, such as beta-tricalcium phosphate (β-TCP), hydroxyapatite (HA) and biphasic calcium phosphate (BCP), have been developed to aid the healing of bone defects.1,2 Next to their biocompatibility, bioactivity and osteoconductivity, CaP ceramics with specific physicochemical properties have also been shown to initiate bone formation on their surface after ectopic implantation (e.g. intramuscular, and subcutaneous). As bone cells are not present at these locations, this phenomenon is described as material directed bone formation (i.e. osteoinduction).3–5With the ability to induce bone formation, osteoinductive CaP bone substitutes do not only trigger bone formation faster than those lacking osteoinductivity,6 but also allow the repair of critical-sized bone defects.7,8 Moreover, an osteoinductive CaP bone substitute showed an equal bone regeneration capacity to autograft and collagen load with recombinant human bone morphogenetic protein 2 (rhBMP-2) in critical bone defects.9 The osteoinductive potential of CaP materials is largely dependent on their physicochemical properties.10–18 The chemical composition plays a role in inductive bone formation. For instance, BCP (i.e. biphasic ceramic of HA and TCP) has a higher osteoinductive potential than HA,6,10 indicating a role of the faster resorbing TCP. However, a negative influence of the TCP content was reported in a study where an increase of TCP in CaPs adversely affected the osteoinductive ability and no bone formation was detected in pure TCP ceramics.11 Next to the chemistry, the presence of micropores in the material has proven to be crucial for osteoinduction.12 For example, Ariizumi et al. found that TCP with interconnected macropores and micropores gave rise to inductive bone formation while in the absence of micropores it did not.13 Furthermore, it was shown that the osteoinductive potential increased with microporosity.14 Moreover, the size of micropores has also been reported to greatly affect inductive bone formation by CaP ceramics. For instance, a study where two BCP ceramics were compared in the muscle of goats showed that only the BCP with a smaller pore size and crystal size induced bone formation.15
Given the fact that osteoinduction of CaP materials is material-dependent, it is likely that the bone forming potential of commercially available CaP bone substitutes varies due to differences in their physicochemical properties. In this study, six commercially available CaP bone substitutes (Bio-Oss, Actifuse, Bi-Ostetic, MBCP, Vitoss and chronOs) as well as two β-TCP ceramics with either a micron-scale (TCP-B) or submicron-scale (TCP-S) surface architecture were characterized and their intramuscular bone forming potential was evaluated using a well-established canine osteoinduction study model.8,16
2. Experimental section
2.1. Calcium phosphate materials and characterization
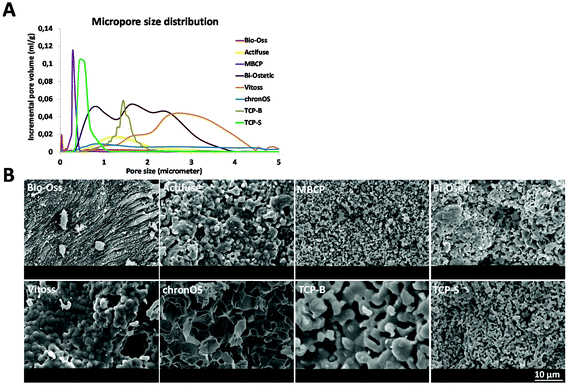
| Material | Supplier | Chemistrya | Micropore sizeb | Grain sizec [μm] | Surface structure dimension | Micro porosityb [%] | Specific surface area by weightb [m2 g−1] | Specific surface area by volumeb [m2 ml−1] |
|---|---|---|---|---|---|---|---|---|
| a As evaluated by X-ray diffractometry. b Obtained from mercury intrusion. c As confirmed by quantitative measurements from scanning microscopy images (5000×). | ||||||||
| Bio-Oss | Geistlich Pharma AG | HA | Nano | Nano-scale | Nano | 43.01 | 28.46 | 18.01 |
| Actifuse | Apatech Baxter | Si-HA | Micron | 1.02 ± 0.43 | Micron | 22.05 | 0.33 | 0.32 |
| MBCP | Biomatlante | 55HA/45TCP | Submicron | 0.71 ± 0.20 | Submicron | 40.53 | 2.93 | 2.09 |
| Bi-Ostetic | Berkeley Advanced Biomaterials | 45HA/55TCP | Micron | 0.91 ± 0.24 | Micron | 53.74 | 1.42 | 1.10 |
| Vitoss | Orthovita Stryker | TCP | Micron | 1.93 ± 0.70 | Micron | 42.74 | 0.36 | 0.30 |
| ChronOs | Synthes | TCP | Micron | 5.03 ± 1.90 | Micron | 31.52 | 0.51 | 0.59 |
| TCP-B | Home made | TCP | Micron | 2.52 ± 0.34 | Micron | 46.32 | 0.83 | 0.73 |
| TCP-S | Home made | TCP | Submicron | 0.81 ± 0.21 | Submicron | 45.14 | 1.85 | 1.59 |
2.2. Ectopic bone formation
2.3. Statistics
Statistical analysis was carried out using one-way analysis of variance (ANOVA) with Tukey's test. Differences were considered statistically significant at p < 0.05.3. Results
3.1. Calcium phosphate materials and characterization
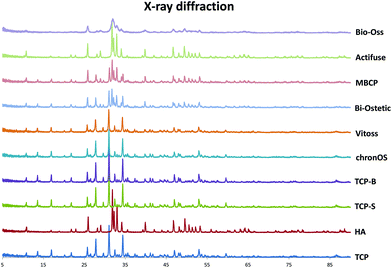 | ||
| Fig. 1 XRD pattern showing the chemistry of the eight CaP materials compared with standard HA and β-TCP. | ||
3.2. Tissue response after intramuscular implantation of the CaP materials
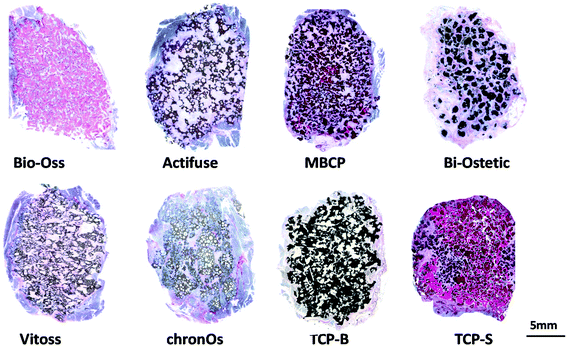
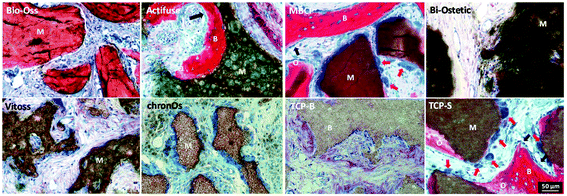
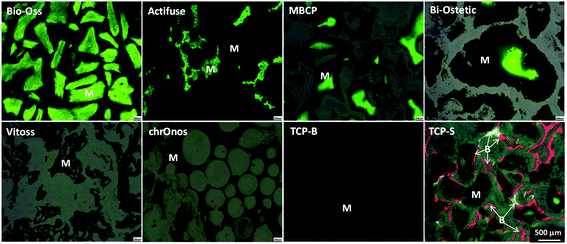
The newly formed bone was observed in TCP-S (4 out of 4), MBCP (4 out of 4) and Actifuse implants (2 out of 4), while no bone formation was seen in any of Bio-Oss, Bi-Ostetic, Vitoss, chronOs and TCP-B (Fig. 4).Normal bone structures with osteocytes embedded in the lacunae and osteoblasts laid on de novo bone were often observed in TCP-S and MBCP implants, while sporadically in Actifuse implants (Fig. 5). In addition, macrophage-like cells engulfing fine ceramic particles were mainly observed in TCP-S and MBCP samples (Fig. 5).
Under fluorescence microscopy, three colors (green, calcein, 3 weeks; red, xylenol orange, 6 weeks; and yellow, tetracycline, 9 weeks) were seen in TCP-S implants, while only green color was observed in MBCP, Actifuse, Bio-Oss and Bi-Ostetic implants, and no color was detected in Vitoss, chronOs and TCP-B implants (Fig. 6). Since the green color was seen in the CaP materials, it suggests mineralization in vivo. The red and yellow colors stained the bone tissues in TCP-S implants, indicating that the bone formation started between 3 and 6 weeks after implantation, and bone was still actively formed at week 9. Red and yellow colors were not seen in MBCP and Actifuse implants, suggesting that the bone formation process in these two materials started 9 weeks after implantation.
Quantitatively TCP-S gave rise to significantly more bone formation in the available space (21.6 ± 4.5%) than MBCP (2.1 ± 1.4%). The bone formed in Actifuse (0.1 ± 0.1%) was significantly less than that in TCP-S and MBCP (Fig. 7).
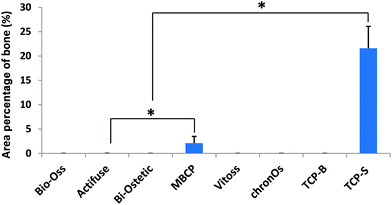 | ||
| Fig. 7 The area percentage of bone in the available spaces of the CaP material implants (*p < 0.05). | ||
4. Discussion
4.1. Which material factors are triggers for ectopic bone formation?
In the current study, Actifuse, MBCP and TCP-S implants triggered bone formation after 12 weeks, with different incidences, onset times and amounts of bone. No bone formation was detected in Bio-Oss, Bi-Ostetic, Vitoss, chronOs and TCP-B. The ectopic bone forming potential among the eight CaP materials is related to their physicochemical properties.We have previously said that the chemistry may not be a crucial trigger of material-driven bone formation. However, once the bone formation process is triggered, TCP-S having more TCP content induced more bone compared with MBCP. Therefore, the influence of the chemistry on inductive bone formation is still important for the quantity of new bone tissue formation.
4.2. How do material factors trigger bone formation?
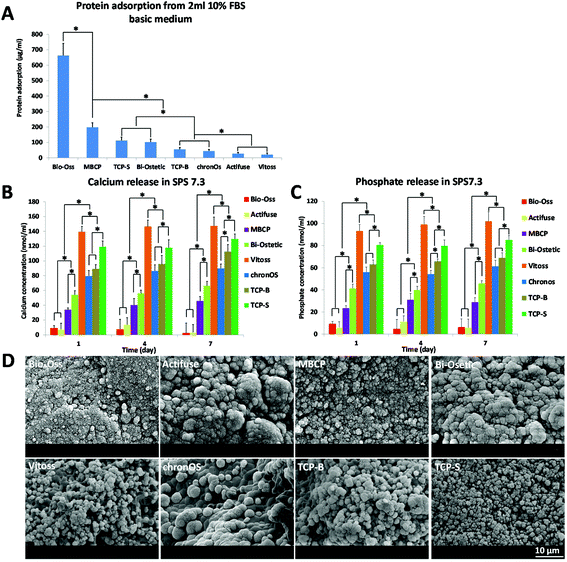
As described above, the surface structure at the submicron-scale may be the essential trigger for osteoinduction by CaP materials, but how it triggers CaP-induced bone formation is not clear as yet. It is generally thought that bone induction of CaP materials is the result of protein adsorption,3,5,9,13,22,23 surface mineralization10,24,25 and calcium and phosphate ions release.26–28In addition to modulating stem cells and osteoblasts, giant cells (e.g. macrophages) are sensitive to the surface topography. For instance, Fellah and colleagues observed that the amount of macrophages and giant cells varied according to the surface grain size of CaP ceramic implants.51 Furthermore Davison et al. showed that CaP ceramics with submicron sized surface architecture could induce an inflammatory response of macrophages, and their subsequent secretion of cytokines induced stem cell differentiation.16,52 In line with the findings by Kondo et al., who observed plenty of active multinucleated giant cells prior to osteoinductive bone formation in CaP implants,53 we observed in this study macrophage-like cells on the submicron structured surface of osteoinductive TCP-S and MBCP. It is possible that the crosstalk between macrophages and mesenchymal stem cells on the submicron scaled surface topography initiated the CaP-directed osteoinduction through mechanotransduction.
Osteoinductive CaP materials are useful for bone regeneration,6–9 and identifying the crucial material factors would be helpful for further optimization of CaP materials with respect to the bone forming ability. The submicron dimension of the surface structure of CaP materials was clearly shown to be the stronger trigger of ectopic bone formation. However, more studies are necessary to further understand the material-driven osteoinduction from a biological perspective.
5. Conclusion
We compared the bone forming potential of eight synthetic CaP bone substitutes and found that the bone forming ability varied with materials. It was observed that the specific topography engineered by submicropores and submicron crystal grains appear to be the necessary trigger for osteoinduction, and that ceramics releasing ions could enhance the inductive bone formation. The submicron-scale surface topography, via specific mechanotransduction, might be the direct trigger of osteoinduction in CaP bone substitutes rather than the theories already in the literature such as protein adsorption, surface mineralization and calcium ion release.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors would like to thank the Netherlands Institute for Regenerative Medicine (NIRM), Rapid Prototyping of Custom-Made Bone-Forming Tissue Engineering Constructs (RAPIDOS Project, Ref. NMP-2013-EU-China 604517), the Seventh Framework Programme of the European Union (REBORNE under grant agreement no. 241879) and the Horizon 2020 Framework Programme for the financial support to this research project (CHARME, grant agreement no. 674282).References
- S. Samavedi, A. R. Whittington and A. S. Goldstein, Acta Biomater., 2013, 9, 8037–8045 CrossRef CAS PubMed.
- B. Susmita and T. Solaiman, Acta Biomater., 2013, 8, 1401–1421 Search PubMed.
- H. Yamasaki, Jpn. J. Oral Biol., 1990, 32, 190–192 CrossRef CAS.
- X. Zhang, P. Zhou, J. Zhang, W. Chen and C. Wu, in Bioceramics and the Human Body, ed. A. Ravaglioli and A. Krajewski, Elsevier Applied Science, London and New York, 1991, pp. 408–415 Search PubMed.
- U. Ripamonti, Clin. Orthop. Relat. Res., 1991, 269, 284–294 Search PubMed.
- H. Yuan, C. A. van Blitterswijk, K. de Groot and J. D. de Bruijn, J. Biomed. Mater. Res., Part A, 2006, 78, 139–147 CrossRef CAS PubMed.
- P. Habibovic, H. Yuan, M. van den Doel, T. M. Sees, C. A. van Blitterswijk and K. de Groot, J. Orthop. Res., 2006, 24, 867–876 CrossRef CAS PubMed.
- R. Duan, D. Barbieri, X. Luo, J. Weng, J. D. de Bruijn and H. Yuan, J. Orthop. Res., 2016, 34, 1865–1873 CrossRef CAS PubMed.
- H. Yuan, H. Fernandes, P. Habibovic, J. de Boer, A. M. Barradas, A. de Ruiter, W. R. Walsh, C. A. van Blitterswijk and J. D. de Bruijn, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 13614–13619 CrossRef CAS PubMed.
- P. Habibovic, H. Yuan, C. M. van der Valk, G. Meijer, C. A. van Blitterswijk and K. de Groot, Biomaterials, 2005, 26, 3565–3575 CrossRef CAS PubMed.
- K. Kurashina, H. Kurita, Q. Wu and A. Ohtsuka, Biomaterials, 2002, 23, 407–412 CrossRef CAS PubMed.
- B. H. Fellah, O. Gauthier, P. Weiss, D. Chappard and P. Layrolle, Biomaterials, 2008, 29, 1177–1188 CrossRef CAS PubMed.
- T. Ariizumi, A. Ogose, N. Kondo, H. Kawashima, T. Hotta, N. Kudo, M. Hoshino, H. Inoue, H. Irie and N. Endo, J. Biomater. Nanobiotechnol., 2013, 4, 189–193 CrossRef.
- O. Chan, M. J. Coathup, A. Nesbitt, C. Y. Ho, K. A. Hing, T. Buckland, C. Campion and G. W. Blunn, Acta Biomater., 2012, 8, 2788–2794 CrossRef CAS PubMed.
- P. Habibovic, T. M. Sees, M. A. van den Doel, C. A. van Blitterswijk and K. de Groot, J. Biomed. Mater. Res., Part A, 2006, 77, 747–762 CrossRef PubMed.
- N. L. Davison, X. Luo, T. Schoenmaker, V. Everts, H. Yuan, F. Barrère-de Groot and J. D. de Bruijn, Eur. Cells Mater., 2014, 15, 281–297 CrossRef.
- T. Kokubo and H. Takadama, Biomaterials, 2006, 27, 2907–2915 CrossRef CAS PubMed.
- H. Fischer, C. Niedhart, N. Kaltenborn, A. Prange, R. Marx, F. U. Niethard and R. Telle, Biomaterials, 2005, 26, 6151–6157 CrossRef CAS PubMed.
- H. Yuan, J. D. de Bruijn, X. Zhang, C. A. van Blitterswijk and K. de Groot, presented in part at Eur. Conf. Biomater, Osteoinduction by porous alumina ceramic, London, UK, 09, 2001.
- G. D. Winter and B. J. Simpson, Nature, 1969, 223, 88–90 CrossRef CAS PubMed.
- L. C. Gerhardt and A. R. Boccaccini, Materials, 2010, 3, 3867–3910 CrossRef CAS PubMed.
- T. Boix, J. Gómez-Morales, J. Torrent-Burgués, A. Monfort, P. Puigdomènech and R. Rodríguez-Clemente, J. Inorg. Biochem., 2005, 99, 1043–1050 CrossRef CAS PubMed.
- T. J. Webster, L. S. Schadler, R. W. Siegel and R. Bizios, Tissue Eng., 2001, 7, 291–301 CrossRef CAS PubMed.
- R. Z. LeGeros, Chem. Rev., 2008, 108, 4742–4753 CrossRef PubMed.
- S. Fujibayashi, M. Neo, H. M. Kim, T. Kokubo and T. Nakamura, Biomaterials, 2004, 25, 443–450 CrossRef CAS PubMed.
- A. M. Barradas, H. A. Fernandes, N. Groen, Y. C. Chai, J. Schrooten, J. van de Peppel, J. P. van Leeuwen, C. A. van Blitterswijk and J. de Boer, Biomaterials, 2012, 33, 3205–3215 CrossRef CAS PubMed.
- S. Nakamura, T. Matsumoto, J. Sasaki, H. Egusa, K. Y. Lee, T. Nakano, T. Sohmura and A. Nakahira, Tissue Eng., Part A, 2010, 16, 2467–2473 CrossRef CAS PubMed.
- Y. R. Shih, Y. Hwang, A. Phadke, H. Kang, N. S. Hwang, E. J. Caro, S. Nguyen, M. Siu, E. A. Theodorakis, N. C. Gianneschi, K. S. Vecchio, S. Chien, O. K. Lee and S. Varghese, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 990–995 CrossRef CAS PubMed.
- M. Takemoto, S. Fujibayashi, M. Neo, J. Suzuki, T. Matsushita, T. Kokubo and T. Nakamura, Biomaterials, 2006, 27, 2682–2691 CrossRef CAS PubMed.
- T. C. Lee, S. Mohsin, D. Taylor, R. Parkesh, T. Gunnlaugsson, F. J. O'Brien, M. Giehl and W. Gowin, J. Anat., 2003, 203, 161–172 CrossRef CAS PubMed.
- S. M. van Gaalen, M. C. Kruyt, R. E. Geuze, J. D. de Bruijn, J. Alblas and W. J. Dhert, Tissue Eng., Part B, 2010, 16, 209–217 CrossRef CAS PubMed.
- M. Cerruti, Philos. Trans. R. Soc., A, 2012, 370, 1281–1312 CrossRef CAS PubMed.
- S. Mitragotr and J. Lahann, Nat. Mater., 2009, 8, 15–23 CrossRef PubMed.
- J. Fu, Y. K. Wang, M. T. Yang, R. A. Desai, X. Yu, Z. Liu and C. S. Chen, Nat. Methods, 2010, 7, 733–736 CrossRef CAS PubMed.
- H. J. Jeon, C. G. Simon and G. H. Kim, J. Biomed. Mater. Res., Part B, 2014, 102, 1580–1594 CrossRef PubMed.
- T. Steinberg, S. Schulz, J. P. Spatz, N. Grabe, E. Mussig, A. Kohl, G. Komposch and P. Tomakidi, Nano Lett., 2007, 7, 287–294 CrossRef CAS PubMed.
- K. R. Milner and C. A. Siedlecki, J. Biomed. Mater. Res., Part A, 2007, 82, 80–91 CrossRef PubMed.
- A. Verma, O. Uzun, Y. Hu, Y. Hu, H. S. Han, N. Watson, S. Chen, D. J. Irvine and F. Stellacci, Nat. Mater., 2008, 7, 588–595 CrossRef CAS PubMed.
- S. Oh, K. S. Brammer, Y. S. Li, D. Teng, A. J. Engler, S. Chien and S. Jin, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 130–135 CrossRef.
- C. H. Choi, S. H. Hagvall, B. M. Wu, J. C. Y. Dunn, R. E. Beygui and C. J. Kim, Biomaterials, 2007, 28, 1672–1679 CrossRef CAS PubMed.
- M. J. Dalby, N. Gadegaard, R. Tare, A. Andar, M. O. Riehle, P. Herzyk, C. D. Wilkinson and R. O. Oreffo, Nat. Mater., 2007, 6, 997–1003 CrossRef CAS PubMed.
- E. K. Yim, R. M. Reano, S. W. Pang, A. F. Yee, C. S. Chen and K. W. Leong, Biomaterials, 2005, 26, 5405–5413 CrossRef CAS PubMed.
- J. Lovmand, J. Justesen, M. Foss, R. H. Lauridsen, M. Lovmand, C. Modin, F. Besenbacher, F. S. Pedersen and M. Duch, Biomaterials, 2009, 30, 2015–2522 CrossRef CAS PubMed.
- K. Kolind, D. Kraft, T. Bøggild, M. Duch, J. Lovmand, F. S. Pedersen, D. A. Bindslev, C. E. Bünger, M. Foss and F. Besenbacher, Acta Biomater., 2014, 10, 641–650 CrossRef CAS PubMed.
- G. Abagnale, M. Steger, V. H. Nguyen, N. Hersch, A. Sechi, S. Joussen, B. Denecke, R. Merkel, B. Hoffmann, A. Dreser, U. Schnakenberg, A. Gillner and W. Wagner, Biomaterials, 2015, 61, 316–326 CrossRef CAS PubMed.
- K. Hatano, H. Inoue, T. Kojo, T. Matsunaga, T. Tsujisawa, C. Uchiyama and Y. Uchida, Bone, 1999, 25, 439–445 CrossRef CAS PubMed.
- S. Dupont, L. Morsut, M. Aragona, E. Enzo, S. Giulitti, M. Cordenonsi, F. Zanconato, J. Le Digabel, M. Forcato, S. Bicciato, N. Elvassore and S. Piccolo, Nature, 2011, 474, 179–183 CrossRef CAS PubMed.
- F. Gattazzo, A. Urciuolo and P. Bonaldo, Biochim. Biophys. Acta, 2014, 1840, 2506–2519 CrossRef CAS PubMed.
- L. E. McNamara, R. J. McMurray, M. J. Biggs, F. Kantawong, R. O. Oreffo and M. J. Dalby, J. Tissue Eng., 2010, 18, 120623 CrossRef PubMed.
- J. Zhang, M. T. Dalbay, X. Luo, E. Vrij, D. Barbieri, L. Moroni, J. D. de Bruijn, C. A. van Blitterswijk, J. P. Chapple, M. M. Knight and H. Yuan, Acta Biomater., 2017, 57, 487–497 CrossRef CAS PubMed.
- B. H. Fellah, B. Delorme, J. Sohier, D. Magne, P. Hardouin and P. Layrolle, J. Biomed. Mater. Res., Part A, 2010, 93, 1588–1595 Search PubMed.
- N. L. Davison, A. L. Gamblin, P. Layrolle, H. Yuan, J. D. de Bruijn and F. Barrère-de Groot, Biomaterials, 2014, 35, 5088–5088 CrossRef CAS PubMed.
- N. Kondo, A. Ogose, K. Tokunaga, H. Umezu, K. Arai, N. Kudo, M. Hoshino, H. Inoue, H. Irie, K. Kuroda, H. Mera and N. Endo, Biomaterials, 2006, 27, 4419–4419 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |