Tris(2-chloroethyl) phosphate (TCEP) and tris(2-chloropropyl) phosphate (TCPP) induce locomotor deficits and dopaminergic degeneration in Caenorhabditis elegans†
Tiantian
Xu
ab,
Ping
Li
ab,
Siyu
Wu
a,
Lili
Lei
a and
Defu
He
*ab
aLab of Toxicology, School of Ecological and Environmental Sciences, East China Normal University, 500# DongChuang RD, Shanghai, 200241, China. E-mail: dfhe@des.ecnu.edu.cn; Tel: +86 189 1786 4019
bShanghai Key Laboratory for Urban Ecological Processes and Eco-Restoration, East China Normal University, Shanghai, 200241, China
First published on 26th October 2016
Abstract
Organophosphate flame retardants (PFRs) are a new class of flame retardants. The health risks of PFRs have received attention recently. However, little is known about the potential toxicity of PFRs on the nervous system. Herein, we evaluated the neurotoxic effects of two typical PFRs, tris(2-chloroethyl) phosphate (TCEP) and tris(2-chloropropyl) phosphate (TCPP), using Caenorhabditis elegans. Median lethal concentrations of chronic exposure (3 d) were 1578 and 857 mg L−1 for TCEP and TCPP, respectively. The sublethal dose of TCEP or TCPP significantly inhibited the body length and reduced the lifespans of nematodes. 500 mg L−1 and above of TCEP/TCPP led to a significant decline in the locomotor frequency of body bending and head thrashing. Furthermore, their exposure reduced the crawling speed and the frequency of bending oscillation of nematodes. This indicates that TCEP/TCPP induces locomotor deficits, along with Parkinsonian-like movement impairment including bradykinesia and hypokinesia. Using transgenic worms, we found that TCEP/TCPP could induce down-expression of Pdat-1 and resulted in the degeneration of dopaminergic neurons, especially PDE neurons. Moreover, TCEP/TCPP induced over-expression of unc-54, which indicates the aggregation of α-synuclein in the process of degeneration. These findings suggest the neurotoxicity risks of organophosphorus flame retardants, which are associated with the locomotor deficits and dopaminergic degeneration.
Introduction
Organophosphorus flame retardants (PFRs) are a new class of commonly used flame retardants. PFRs are frequently added to consumer products including furniture, textiles, cables, building materials, paints, floor polishes and electronics for fire protection.1,2 Of all the chemical flame retardants, brominated flame retardants have been the major products in the last few decades.3 However, brominated flame retardants such as polybrominated diphenyl ethers (PBDEs) have been documented for their toxicity, persistence, and presence in humans and wildlife.3,4 This led to worldwide restrictions and bans of brominated flame retardants.5 As alternative flame retardants, PFRs are considered as appropriate replacements for brominated flame retardants, and now are used in a wide range of polymers. The global production of PFRs, tris(2-chloroethyl) phosphate (TCEP), tris(1,3-dichloro-2-propyl) phosphate (TDCPP or TDCIPP) and the homologue tris(2-chloropropyl) phosphate (TCPP or TCIPP), has exceeded 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes in 2006.6
000 tonnes in 2006.6
PFRs have been frequently detected in various environmental matrices including indoor air, house dust, drinking water, wastewater and sediment.2,6–12 They have also been found in human breast milk, blood, hair, adipose tissue and various organs.13–16 Tris(2-chloropropyl) phosphate (TCPP) is a mixture of isomers. Although tris(1-chloro-2-isopropyl) phosphate (TCIPP) is the most abundant isomer, accompanying isomers include bis(1-chloro-2-propyl) (2-chloropropyl) phosphate and (1-chloro-2-propyl) bis(2-chloropropyl) phosphate. TCPP and TCEP were respectively detected at a concentration of 37–130 ng L−1 and 110–170 ng L−1 in the River Schwechat and the River Liesing.17 The highest levels of TCEP and TCPP were reported at 184 and 379 ng L−1 respectively in Lake Nidda at Oxbow, Germany.18 In a recent study, several PFRs (TBOEP, TCIPP and TCEP) were found at elevated levels in the food web, which indicated trophic magnification of PFRs within the food chain.12
The potential toxicity and adverse health risks of PFRs have received attention recently. However, toxicology studies of PFRs are currently limited, thereby hampering complete understanding of the health risks.2 For example, TCEP was reported to be carcinogenic to rats; tris(1,3-dichloro-2-propyl)phosphate (TDCIPP) can be absorbed through human skin and reduce thyroid hormone levels; while tri-n-butyl phosphate (TNBP) has been linked with sick building syndrome.19,20 In addition, TCIPP and TDCIPP have been identified as possible carcinogens.2
TCEP and triphenyl phosphate (TPHP) have been suspected to be neurotoxic.5 Dishaw et al. found that TDCPP showed concentration-dependent neurotoxicity, inhibited DNA synthesis, decreased the cell number and altered neuro-differentiation.21 Nevertheless, no adverse effect on cell viability or growth was detected, but elevated oxidative stress was shown in another in vitro study.22 To date, neuro-toxicological studies on PFRs have been scarce. Thereby the neurotoxicity and its mechanisms of PFRs remain to be elucidated.
The objective of this study was to investigate the potential neurotoxicity of typical PFRs, TCEP and TCPP, in a model organism, Caenorhabditis elegans. The nematode C. elegans has a simple and well-defined anatomy, short life cycle, and has been employed as a benchmark system for toxicological studies because of its high sensitivity to various environmental toxicants.23 In addition, C. elegans contains 302 neurons and its neuronal lineage is fully described; it is advantageous as a model organism for neurotoxicology study.24 For this purpose, chronic exposure to TCEP and TCPP was conducted in C. elegans. Behavior changes were assayed by testing the endpoints of body bends, head thrashes, pharynx pumping and crawling behavior. By using fluorescently labeled transgenic nematodes, the effects on dopaminergic neurons, GABAergic neurons and the α-synuclein protein were further investigated. These results were finally applied to assess the neurotoxicity of organophosphorus flame retardants based on deeply analyzing the relationship between behavioral toxicity and nerve damage induced by TCEP and TCPP.
Materials and methods
Chemicals
Tris(2-chloroethyl) phosphate (TCEP) and tris(2-chloropropyl) phosphate (TCPP) were purchased from Sigma Aldrich Chemicals Co. (St Louis, MO, USA). Their properties are shown in Table 1. TCPP is the homologue mixture of isomers. The main isomer is tris(1-chloro-2-propyl) phosphate (66%), while minor components are bis(1-chloro-2-propyl) (2-chloropropyl) phosphate and (1-chloro-2-propyl) bis(2-chloropropyl) phosphate. Other chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). All chemicals used in this study were of analytical grade.| Name | Abbr. | CAS number | S 5 | P 5 | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow Kow![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc Koc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
BCF2 |
|---|---|---|---|---|---|---|---|
TCPP is the homologue mixture of isomers; the main isomer is tris(1-chloro-2-propyl) phosphate. S: solubility (mg L−1) in water at 25 °C; P: vapor pressure (mmHg) at 25 °C; log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow: n-octanol/water partition coefficient; log Kow: n-octanol/water partition coefficient; log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc: soil adsorption coefficient; BCF: bioaccumulation factor.2,5 references for original data. Koc: soil adsorption coefficient; BCF: bioaccumulation factor.2,5 references for original data. |
|||||||
| Tris(2-chloroethyl) phosphate | TCEP | 115-96-8 | 7.0 × 103 | 1.1 × 10−4 | 1.44 | 2.48 | 1.37 |
| Tris(2-chloropropyl) phosphate | TCPP | 13674-84-5 | 1.6 × 103 | 0.75 | 2.59 | 2.21 | 8.51 |
C. elegans strains and exposure
All strains of C. elegans were obtained from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN, USA), and maintained in terms of standard protocols as previously described.25 Wild-type (Bristol, N2) and transgenic strains were used, including BZ555 [dat-1p::GFP; green fluorescent protein visible in dopaminergic neurons],26EG1285 [unc-47p::GFP + lin-15(+); GFP expressed in GABAergic neurons)27 and NL5901 [unc-54::alpha synuclein::yfp + unc-119; the α-synuclein protein with the yellow fluorescent protein observable in the muscles).28 Nematodes were maintained on nematode growth medium (NGM) plates seeded with Escherichia coli OP50 at 20 °C.25 Age-synchronized nematodes were produced for experiments by collecting adult nematodes into tubes by washing plates with M9 buffer, and then dissolving the adults using a bleach-containing buffer (0.45 mM NaOH, 2% HClO) to release eggs. The eggs were collected and hatched on the plates with food, then synchronized for experiments.29,30TCEP or TCPP solutions were prepared in K-medium (32 mmol L−1 KCl, 51 mmol L−1 NaCl), and the control group is K-medium. Worms were exposed to a series of concentrations of TCEP (50, 250, 500, 750, 1000 mg L−1) or TCPP (50, 250, 500, 750 mg L−1) in 24-well plates. Each well contained 50–100 age-synchronized nematodes. Four replicates were used for each exposure group. Nematodes were exposed for 3–6 d from the L1 stage.
Lethality assays
Synchronized nematodes were exposed to TCEP or TCPP of different concentrations for 6 d. The lethal experiments were performed in 24-well plates, and each well contained about 30 nematodes and 50 μL E. coli OP50 solutions for food. To prevent eggs from hatching, 0.33 μL mL−1 of fluoro-29-deoxyuridine (150 mM FUDR) was added to each well. Numbers of dead nematodes were recorded on the first, third and sixth day, respectively. Dead nematodes were identified as unresponsive to a gentle prod of the body with a needle probe. Each experiment was run in quadruplicate. The median lethal concentrations (LC50) of TCEP and TCPP were determined by linear regression analysis with Graphpad Prism.Body length and lifespan assay
After being exposed to TCEP (0, 50, 250, 500, 750, 1000 mg L−1) and TCPP (0, 50, 250, 500 and 750 mg L−1) for 3 d, worms were washed three times with M9 buffer, and then transferred to agar-padded slides and sealed with a coverslip which were immobilized with 100 mM sodium azide. Then body lengths of nematodes were measured using an imaging system.According to our previous protocol,30 lifespan assays were performed after exposure. C. elegans was transferred to fresh 6-well plates, and cultured on NGM fed with Escherichia coli OP50 at 20 °C. Worms were counted every 2 days, and transferred to fresh plates every other day until all nematodes were dead. Dead nematodes were identified as unresponsive to a gentle prod of the body with a needle probe. Each experiment was executed in quadruplicate.
Local movement
Head thrashing, body bending and pharynx pumping were utilized to evaluate the locomotion behaviors of nematodes.31 After the exposure, C. elegans was transferred to fresh (NGM) plates without food. After a minute of recovery, head thrashes and body bending were counted for 1 minute and 20 seconds, respectively.32 For the pharynx pumping assay, nematodes were transferred to another NGM plate and the number of pumps was counted in 30 s.Analysis of locomotor video
After exposure to TCEP and TCPP, C. elegans was transferred to fresh (NGM) plates. After 10 minutes of adaptation, videos of nematodes were recorded for 10 minutes using the Motic microscope and Images Advanced 3.2 software (Motic Electric Group, Xiamen, China). Locomotion videos were further analyzed by using Wormlab software (MBF Bioscience, Hong Kong, China). The crawling tracks, including forward and backward, were calculated using the midpoint of the body in terms of references.33,34 Crawling speeds were recorded in real-time during the crawling process using a bin width of 0.125 s. The body bending angle was classified as a bend angle mid-point less than 90° from the initial trajectory, following a reversal of body bends. A dorsal or ventral bend was scored if the bend angle had changed.29,35Dopamine neurons analysis
In the BZ555 strain of C. elegans, Pdat-1::gfp was co-expressed in neurons, therefore, the bright GFP was observable in dopamine neuronal soma and its axons.36 After exposure to TCEP or TCPP for 3 d, worms were washed three times with M9 buffer to remove adhering bacteria, and then transferred to agar-padded slides and sealed with a coverslip which was immobilized with 100 mM sodium azide. Images of immobilized worms were captured to assay dopamine neurons in the control and exposed groups by using a fluorescence microscope (LEICA DM400 B, Leica Microsystems Company, Shanghai, China). The fluorescence intensity of the GFP was quantified through Image-Pro Plus software. The intensities of fluorescent puncta for dopaminergic neurons were examined in at least 30 nematodes in each group.35α-Synuclein protein assay
In the NL5901 strain of C. elegans, the α-synuclein protein was assayed and the α-synuclein–YFP protein was observable in the muscles of the nematode.28,36 L1 stage nematodes (n = 30 per replicate; four replicates per group) were exposed to TCEP or TCPP for 3 d in 24-well plates. After exposure, any adhering bacteria were removed by washing the worms three times with M9 buffers, and then worms were transferred to an agar padded glass slide. Imaging of immobilized nematodes was performed by using a fluorescence microscope (LEICA DM400 B). The fluorescence intensity of YFP was quantified through Image-Pro Plus software. The intensities of fluorescent puncta were examined in at least 30 nematodes in each group.Statistical analysis
All data were expressed as mean ± Standard Deviation (SD). Mean differences between treated groups and controls were determined by one-way analysis of variance (ANOVA), followed by Dunnett's test. A p-value of less than 0.05 was considered significant.Results
Lethal effects
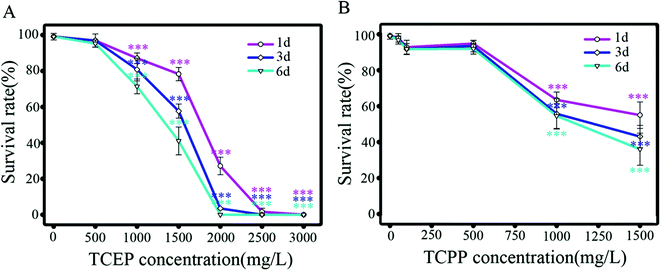
After exposure to TCEP and TCPP, survival percentages of nematodes decreased in dose- and time-dependent manners (Fig. 1). The median lethal concentration (LC50) of TCEP is 1825 mg L−1 for 1 d exposure (95% CI = 1597 to 2086; R2 = 0.99), 1578 mg L−1 for 3 d (95% CI = 1294 to 1924; R2 = 0.98) and 1381 mg L−1 for 6 d (95% CI = 909 to 2100; R2 = 0.98). LC50 of TCPP is 852 mg L−1 for 1 d exposure (95% CI = 52 to 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 897; R2 = 0.99), 857 mg L−1 for 3 d (95% CI = 164 to 4481; R2 = 0.99) and 897 mg L−1 for 6 d exposure (95% CI = 380 to 2122; R2 = 0.99). The results showed obvious lethal toxicity of TCEP and TCPP at the mg L−1 level.
897; R2 = 0.99), 857 mg L−1 for 3 d (95% CI = 164 to 4481; R2 = 0.99) and 897 mg L−1 for 6 d exposure (95% CI = 380 to 2122; R2 = 0.99). The results showed obvious lethal toxicity of TCEP and TCPP at the mg L−1 level.
Effects of TCEP and TCPP on body length and lifespans
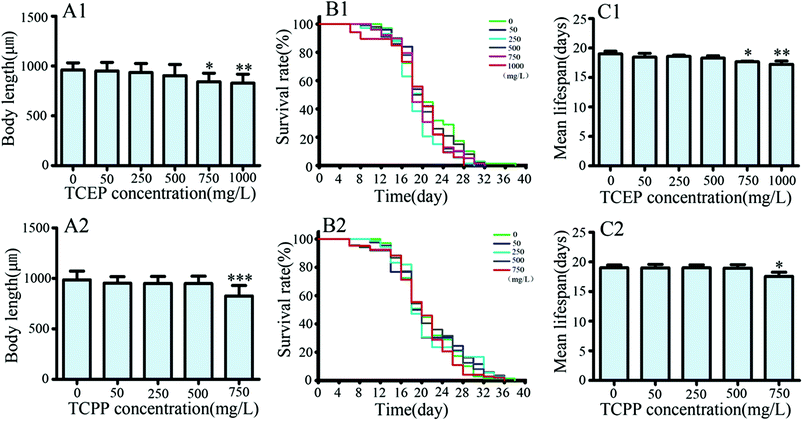
C. elegans was respectively exposed to TCEP (50, 250, 500, 750 and 1000 mg L−1) and TCPP (50, 250, 500 and 750 mg L−1) for 3 d. The body length and lifespans of nematodes were respectively assayed. The result showed that high concentrations of TCEP (750 and 1000 mg L−1) and TCPP (750 mg L−1) caused significant reductions in body length (p < 0.05, Fig. 2A1 and A2). After exposure to 750 and 1000 mg L−1 of TCEP, nematodes had significantly shorter lifespans than unexposed nematodes. Of TCEP-exposed groups, the highest concentration group (750 mg L−1) showed shorter lifespans than control worms (Fig. 2B1 and B2). 750 and 1000 mg L−1 of TCEP led to a 9.26% reduction, and 750 mg L−1 of TCPP led to a 7.5% reduction in average lifespans (Fig. 2C1 and C2).Effects of TCEP and TCPP exposure on locomotor behaviors
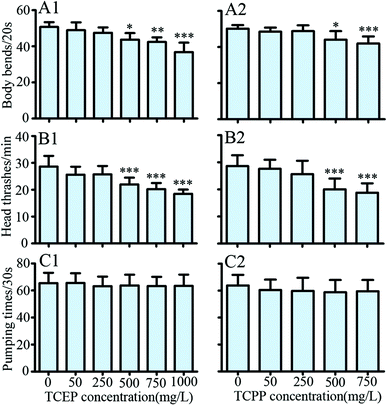
After exposure to TCEP or TCPP, the body bend, head thrash and pharynx pumping of C. elegans were respectively assayed. The results showed that 500 mg L−1 and above of TCEP or TCPP caused a significant reduction in body bends compared with the control group (p < 0.05) (Fig. 3A1 and A2). Similarly, 500 mg L−1 and above of TCEP or TCPP have caused a significant reduction in head thrashes in comparison with the control group (p < 0.001) (Fig. 3B1 and B2). However, we did not observe significant changes in the pharyngeal pump between exposed groups and the control (Fig. 3C1 and C2).Effects of TCEP and TCPP exposure on the crawling movement
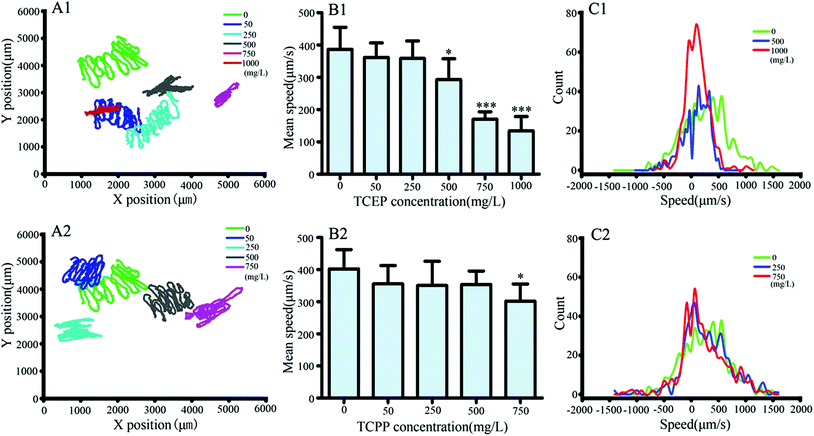
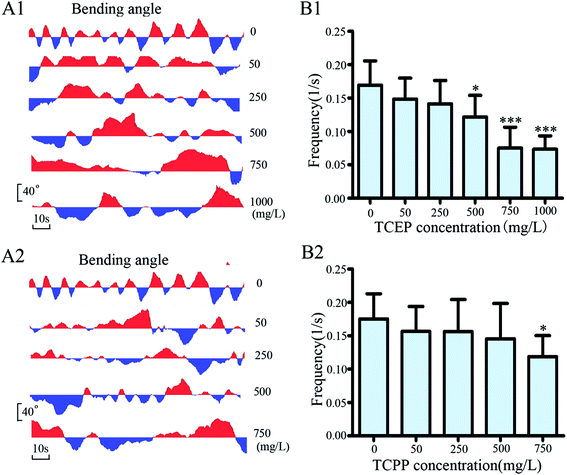
After exposure to TCEP and TCPP, crawling videos of nematodes were collected and then analyzed off-line. By analysis of videos, crawling track lines of the exposed and control nematodes were presented in the same X/Y axis system (Fig. 4A1 and A2). Compared to the control nematode, an obvious decrease in crawling distance can be found in the exposed worms, especially after being exposed to 500 mg L−1 and above of TCEP, or 750 mg L−1 of TCPP. In addition, real-time records of crawling speeds were further analyzed. The results showed that higher than 500 mg L−1 of TCEP or 750 mg L−1 of TCPP led to a significant reduction in the mean speed of crawling (Fig. 4B1 and B2). We found differences of crawling speed contribution between the exposed groups and the control. Particularly in high concentration groups of TCEP or TCPP, the distribution range of crawling speeds was relatively narrow and mainly distributed in the low speed range, but broadly distributed in the control group (Fig. 4C1 and C2).To further investigate the changes of the crawling movement after TCEP or TCPP exposure, angles of body bending were analyzed in detail. Bending angles were quantified as deviations from the midline using the midpoint of the body as a reference. A bending angle of zero indicated no directional bias, whereas a negative or positive fraction indicated a forward or backward bias, respectively. Fig. 5(A1 and A2) shows the real-time recording of the bending degree in nematodes under TCEP or TCPP exposure conditions. The frequency of bending was calculated as the frequency of bending oscillation, which indicated the locomotion balance level of worms. The results showed that 500 mg L−1 and above of TCEP or 750 mg L−1 of TCPP caused a significant decrease in body bending frequency in comparison with the control (Fig. 5B1 and B2).
Effects of TCEP and TCPP on dopaminergic neurons
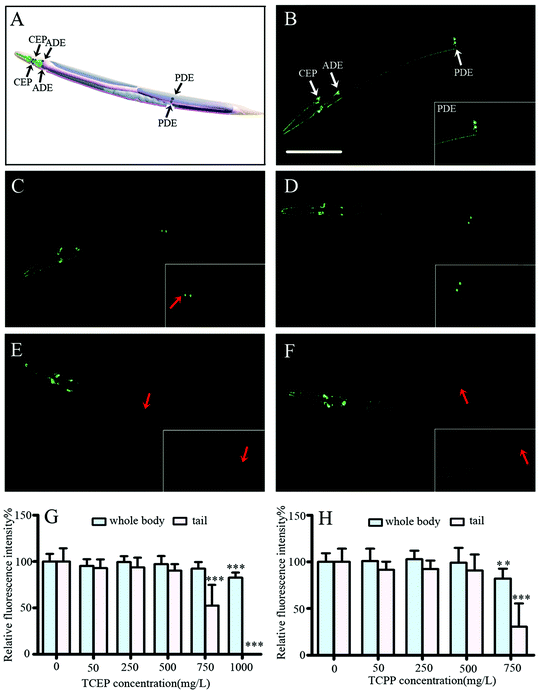
The dopamine system plays important roles in the modulation of locomotion behavior. The potential effects of TCEP and TCPP on dopaminergic neurons were investigated for exploring their neurotoxicity. In C. elegans, the dopamine system includes eight neurons, two pairs of CEP (cephalic neurons) and one pair of ADE (anterior deirid neurons) in the head, and one pair of PDE in the tail (Fig. 6A).37 In transgenic strain BZ555 nematodes, 8 dopaminergic neurons were labeled with the GFP (Fig. 6B). After exposure to 750–1000 mg L−1 of TCEP or 750 mg L−1 of TCPP, the size of fluorescent puncta was obviously decreased, especially in PDE neurons (Fig. 6C, E and F). Quantitative data showed that high concentrations (750 mg L−1 and above) of TCEP or TCPP induced a significant decrease of the fluorescence intensity of the GFP in dopaminergic neurons when compared to the control (Fig. 6G and H). Moreover, we observed a loss of the associated dendrite and soma of PDE neurons in the tail of worms (Fig. 6E and F). The fluorescence intensity of PDE neurons was very significantly reduced or even fully lost after exposure to 1000 mg L−1 of TCEP or 750 mg L−1 of TCPP, which indicates the degeneration of dopaminergic neurons (Fig. 6G and H). However, we did not observe obvious changes of GABAergic neurons in C. elegans after being exposed to TCEP and TCPP (ESI, Fig. S1†).Effects of TCEP and TCPP on the expression of α-synuclein
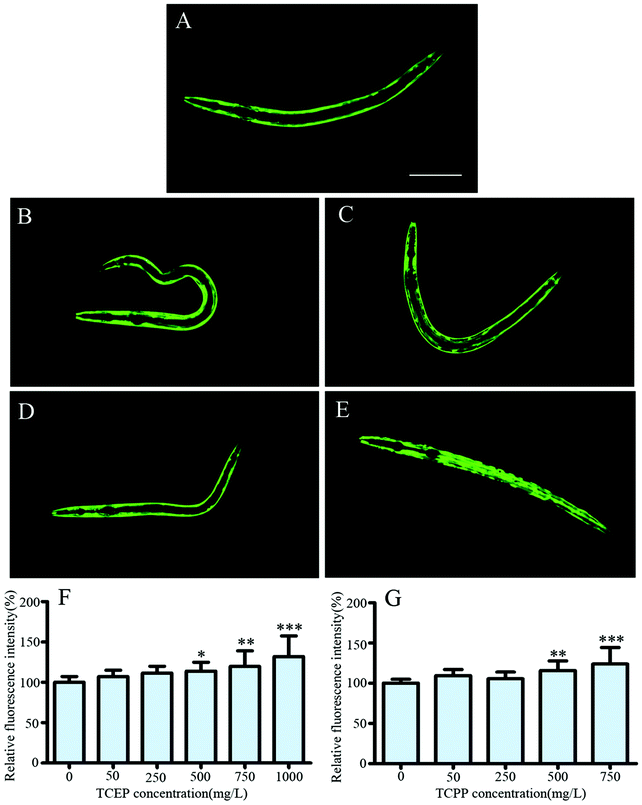
In transgenic NL5901 nematodes, the α-synuclein protein was co-expressed with the yellow fluorescent protein (YFP). Worms exhibited normal fluorescence distribution in muscle cells of the body wall, which indicated the level of α-synuclein expression (Fig. 7A). After TCEP or TCPP exposure, the distribution and intensity of the YFP were systematically analyzed. We found an observable increase of the YFP in the exposed worms (Fig. 7B–E). Statistical results showed that exposure to 500 mg L−1 and above of TCEP or TCPP caused a significant increase of YFP fluorescence intensity (Fig. 7F and G). This indicates that TCEP and TCPP induced higher levels of the α-synuclein protein.Discussion
In this study, neurotoxic effects of the typical PFRs, TCEP and TCPP, on C. elegans were fully studied. Our results showed the lethal effects of TCEP and TCPP in C. elegans, which is consistent with previous studies on other organisms.38,39 It is the first report about the lethal concentrations of TCEP and TCPP on C. elegans. The LC50s of TCEP and TCPP in this study are higher than LC50 (51 mg L−1) of TCPP in fathead minnow (Pimephales promelas), and also higher than LC50 of TCEP (6.3–250 mg L−1) in fish.38 This indicates that nematodes exhibit extensively high tolerance to PFRs. Considering that the life cycle of nematodes is about 3.5 days,25 our results demonstrate the chronic lethality of organophosphorus flame retardants on C. elegans. According to previous studies, TCEP and TCPP were toxic to aquatic organisms and caused chronic adverse effects including carcinogenic toxicity.5,40 Non-specific toxicity of PFRs has been demonstrated including growth inhibition and endocrine disrupting effects,2,19 which may contribute to the lethal toxicity. In the present study, we observed that the sublethal dose of TCEP and TCPP inhibited the body length and reduced the lifespan of worms. This indicates that exposure to PFRs can accelerate aging, shorten the longevity of animals and induce various types of adverse effects, which is consistent with other in vivo and in vitro studies.41,42To date, there have been limited studies addressing the neurotoxic effects of PFRs in vivo.2C. elegans lacks a functional blood–brain barrier. Once chemical molecules are absorbed, they may quickly diffuse into the nervous system. Additionally, protein molecules of worms can be specifically marked with the green fluorescent protein (GFP) and observed by fluorescence microscopy.43,44 In this study, multiple endpoints were utilized to evaluate the potential behavior toxicity in C. elegans. Our results showed a significant decrease in the body bend and head thrash frequency of worms after being exposed to TCEP or TCPP. This indicates the apparent locomotor deficits induced by the two PFRs. Considering that TCEP and TCPP were frequently detected in environmental matrices and biota,2,6,14 our results hint the potential chronic neurotoxicity risk of the two PFRs.
Furthermore, the crawling behavior of nematodes was analyzed. We found that TCEP or TCPP could cause significant reduction of crawling speed. After exposure, nematodes’ crawling speeds were distributed in a more narrow range, which was mostly nearly zero. Moreover, TCEP or TCPP exposure could affect the degree of body bending and reduce the frequency of bending oscillation, which hints deficit of locomotion balance. Altogether, our findings suggest that TCEP/TCPP induce locomotion behavior defects in animals. These behavior changes are similar to the observation in the Parkinson's disease (PD) model of C. elegans.45,46 It demonstrates that the two PFRs inhibit the locomotor ability and cause behavior defects, along with parkinsonian-like movement impairment, including bradykinesia and hypokinesia.
There are only 8 dopaminergic neurons in C. elegans, which are involved in some important movement functions such as swimming, crawling and feeding.46 These dopaminergic neurons and cellular parameters were further investigated after exposure to TCEP/TCPP. In the transgenic strain BZ555, PDAT-1::GFP was utilized as a fluorescent marker for dopamine neurons.35 We found that TCEP or TCPP exposure can result in progressive degeneration of dopamine neurons, especially PDE neurons in the tail of worms. For the first time, our results discover that the neurotoxicity of organophosphorus flame retardants induces the degeneration of the dopaminergic system in vivo. The dysfunction of dopaminergic neurons may be linked with locomotor defects induced by TCEP/TCPP. Nevertheless, the TCEP/TCPP exposure did not lead to obvious damage of GABAergic neurons, which hints different susceptibility of neurotoxicity at the neuron level. The previous studies have also demonstrated that TDCPP or TCEP disrupts the developing nervous system, and results in the loss of cells, an increase in cell apoptosis and damage of nerve fibers in different cell models, PC12, H295R or MVLN cells.47–49 These in vitro reports are consistent with our results, and together prove the neurotoxicity of organophosphorus flame retardants.
According to previous studies, the progressive neurodegeneration of dopaminergic neurons can affect the control function of movements, and induce parkinsonian-like movement impairment.35,50 Parkinson's disease is characterized by neuronal inclusions, Lewy bodies, which mainly contain the aggregated α-synuclein protein.50 In the C. elegans strain NL5901, α-synuclein was co-expressed with the yellow fluorescent protein under control of the unc-54 promoter in muscle cells of the body wall.28 In this transgenic nematode, expressions of α-synuclein accumulation are major in muscle cells, which are consistent with previous studies.46,51 The aggregation of α-synuclein was used as an indicator of in vivo toxicity for Parkinson-like progressive decline of motility in C. elegans.46,52 We found that the aggregation of α-synuclein was increased in a concentration dependent manner, after exposure to 500 mg L−1 and above of TCEP/TCPP. This indicates that the toxicity of TCEP/TCPP may be linked with an enhanced expression of unc-54, encoding a subunit of α-synuclein, which is a crucial protein of the Lewy body in Parkinsonian-like disorders.53,54 The previous study also showed that synaptic endocytosis could enhance α-synuclein toxicity and result in dysfunction in PD pathology in C. elegans.55 For the first time, we found the α-synuclein aggregation induced by TCEP/TCPP; however, the exact mechanisms of neurodegeneration still require further investigation.
Conclusions
In this study, we found that two types of organophosphorus flame retardants, TCEP and TCPP, caused multiple locomotor deficits in C. elegans. The behavioral alterations induced by TCEP/TCPP were characterized as Parkinsonian-like movement impairments including bradykinesia and hypokinesia. TCEP/TCPP exposure induced down-regulated expression of Pdat-1 and caused progressive degeneration in dopaminergic neurons, especially in PDE neurons. We also found the enhanced expression of unc-54 which indicates the aggregation of the α-synuclein protein in the process of degeneration of dopamine neurons. Therefore these findings suggest the neurotoxicity risks of organophosphorus flame retardants. This indicates that the TCEP/TCPP-induced locomotor deficits and dopaminergic degeneration are associated with potential risks of Parkinson's disease.Conflicts of interest
There are no conflicts of interest to declare.Funding
This study was supported by a grant from the Natural Science Foundation of Shanghai (no. 16ZR1409300) and the National Science and Technology Major Project of China (no. 2013ZX07310-001). The authors thank the Caenorhabditis Genetics Center for contributing transgenic strains.References
- N. Kajiwara, Y. Noma and H. Takigami, J. Hazard Mater., 2011, 192, 1250–1259 CrossRef CAS PubMed.
- U.S. Department of Health and Human Services, Public Health Service Agency for Toxic Substances and Disease Registry, 2012, http://atsdr.cdc.gov/toxprofiles/tp202.pdf Search PubMed.
- J. DiGangi, A. Blum, A. Bergman, C. de Wit, D. Lucas, D. Mortimer, A. Schecter, M. Scheringer, S. Shaw and T. Webster, Environ. Health Perspect., 2010, 118, A516–A518 CrossRef PubMed.
- S. Shaw, A. Blum, R. Weber, K. Kannan, D. Rich, D. Lucas, C. Koshland, D. Dobraca, S. Hanson and L. Birnbaum, Rev. Environ. Health, 2010, 25, 261–305 CAS.
- WHO, EHC 209, Geneva, Switzerland, 1998 Search PubMed.
- I. Van der Veen and J. de Boer, Chemosphere, 2012, 88, 1119–1153 CrossRef CAS PubMed.
- E. Cequier, A. Ionas, A. Covaci, R. Marcé, G. Becher and C. Thomsen, Technol. Environ. Sci., 2014, 48, 6827–6835 CrossRef CAS PubMed.
- J. W. Kim, T. Iosbe, A. Sudaryanto, G. Malarvannan, K. H. Chang, M. Prudente and S. Tanabe, Environ. Sci. Pollut. Res. Int., 2013, 20, 812–822 CrossRef CAS PubMed.
- S. Tajima, A. Araki, T. Kawai, T. Tsuboi, Y. A. Bamai, E. Yoshioka, A. Kanazawa, S. Cong and R. Kishi, Total Environ. Sci., 2014, 15, 190–199 CrossRef PubMed.
- P. E. Stackelberg, J. Gibs, E. T. Furlong, M. T. Meyer, S. D. Zaugg and R. L. Lippincott, Total Environ. Sci., 2007, 377, 255–272 CrossRef CAS PubMed.
- J. Meyer and K. Bester, J. Environ. Monit., 2004, 6, 599–605 RSC.
- S. H. Brandsma, P. E. Leonards, H. A. Leslie and J. de Boer, Total Environ. Sci., 2015, 505, 22–31 CrossRef CAS PubMed.
- A. M. Sundkvist, U. Olofsson and P. Haglund, J. Environ. Monit., 2010, 12, 943–951 RSC.
- J. W. Kim, T. Isobe, K.-H. Chang, A. Amano, R. H. Maneja, P. B. Zamora, F. P. Siringan and S. Tanabe, Environ. Pollut., 2011, 159, 3653–3659 CrossRef CAS PubMed.
- G. L. Lebel and D. T. Williams, Bull. Environ. Contam. Toxicol., 1986, 37, 41–46 CrossRef CAS PubMed.
- L.-Y. Liu, A. Salamove, K. He and R. A. Hites, J. Chromatogr., A, 2015, 1406, 251–257 CrossRef CAS PubMed.
- E. Martínez-Carballo, C. González-Barreiro, A. Sitka, S. Scharf and O. Gans, Total Environ. Sci., 2007, 388, 290–299 CrossRef PubMed.
- J. Regnery and W. Püttmann, Water Res., 2010, 44, 4097–4104 CrossRef CAS PubMed.
- A. Kanazawa, I. Saito, A. Araki, M. Takeda, M. Ma, Y. Saijo and R. Kishi, Indoor Air, 2010, 20, 72–84 CrossRef CAS PubMed.
- J. B. Meeker and H. M. Stapleton, Environ. Health Perspect, 2010, 118, 318–323 CrossRef CAS PubMed.
- L. V. Dishaw, C. M. Powers, I. T. Ryde, S. C. Roberts, F. J. Seidler, T. A. Slotkin and H. M. Stapleton, Toxicol. Appl. Pharmacol., 2011, 256, 281–289 CrossRef CAS PubMed.
- V. C. Moser, P. M. Phillips, J. M. Hedge and K. L. McDaniel, Neurotoxicol. Teratol., 2015, 52, 236–247 CrossRef CAS PubMed.
- M. C. K. Leung, P. L. Williams, A. Benedetto, C. Au, K. J. Helmcke, M. Aschner and J. N. Meyer, Toxicol. Sci., 2008, 106, 5–28 CrossRef CAS PubMed.
- I. L. Tseng, Y.-F. Yang, C.-W. Yu, W.-H. Li and V. H. C. Liao, PLoS One, 2013, 8, e82657 Search PubMed.
- S. Brenner, Genetics, 1974, 77, 71–94 CAS.
- M. Maduro and D. Pilgrim, Gene, 1996, 183, 77–85 CrossRef CAS PubMed.
- J. G. White, E. Southgate, J. N. Thomson and S. Brenner, Philos. Trans. R. Soc. London, Ser B, 1986, 314, 1–340 CrossRef CAS.
- Z. Shi, Z. Lu, Y. Zhao, Y. Wang, X.-W. Zhao, P. Guan, X. Duan, Y. Chang and B. Zhao, Neurochem. Int., 2013, 62, 940–947 CrossRef CAS PubMed.
- N. Chen, J. Li, D. Li, Y. Yang and D.-F. He, PLoS One, 2014, 9, e113453 Search PubMed.
- T.-T. Xu, P. Li, S.-Y. Wu, D. Li, J.-X. Wu, K. M. Raley-Susman and D.-F. He, Bull. Environ. Contam. Toxicol., 2016, 97, 119–123 CrossRef CAS PubMed.
- Y.-F. Zhang and D.-Y. Wang, Asian J. Ecotoxicol., 2008, 4, 313–322 Search PubMed.
- E. L. Tsalik and O. Hobert, J. Neurobiol., 2003, 56, 178–197 CrossRef PubMed.
- J. L. Donnelly, C. M. Clark, A. M. Leifer, J. K. Pirri, M. Haburcak, M. M. Francis, A. D. Samuel and M. J. Alkema, PLoS Biol., 2013, 11, e1001529 CAS.
- T. Gallagher, T. Bjorness, R. Greene, Y. J. You and L. Avery, PLoS One, 2013, 8, e59865 CAS.
- J. Li, D. Li, Y.-S. Yang, T.-T. Xu, P. Li and D.-F. He, J. Appl. Toxicol., 2016, 36, 60–67 CrossRef CAS PubMed.
- P. Jadiya, A. Khan, S. R. Sammi, S. Kaur, S. S. Mir and A. Nazir, Biochem. Biophys. Res. Commun., 2011, 413, 605–610 CrossRef CAS PubMed.
- E. R. Sawin, R. Ranganathan and H. R. Horvitz, Neuron, 2000, 26, 619–631 CrossRef CAS PubMed.
- P. R. Fisk, A. E. Girling and R. J. Wildey, Produced for Environment Agency, United Kingdom, 2003 Search PubMed.
- J. Cristale, A. G. Vázquez, C. Barata and S. Lacorte, Environ. Int., 2013, 59, 232–243 CrossRef CAS PubMed.
- Y. Ni, K. Kumagai and Y. Yanagisawa, Atmos. Environ., 2007, 41, 3235–3240 CrossRef CAS.
- S. P. McGee, E. M. Cooper, H. M. Stapleton and D. C. Volz, Environ. Health Perspect., 2012, 120, 1585–1591 CrossRef CAS PubMed.
- G.-L. Chen, S.-B. Zhang, Y.-X. Jin, Y. Wu, L. Liu and H.-F. Qian, Res. Toxcol., 2015, 57, 100–110 CAS.
- A. G. Alexander, M. Vanessa and L. Chris, Front. Genet., 2014, 5, 1–21 CAS.
- P. M. Chege and M. Gawain, Front. Aging Neurosci., 2014, 6, 1–15 Search PubMed.
- M. Dimitriadi and A. C. Hart, Neurobiol. Dis., 2010, 40, 4–11 CrossRef CAS PubMed.
- R. H. Fu, H. J. Harn, S. P. Liu, C. S. Chen, W. L. Chang, Y. M. Chen, J. E. Huang, R. J. Li, S. Y. Tsai, H. S. Hung, W. C. Shyu, S. Z. Lin and Y. C. Wang, PLoS One, 2014, 9, e85305 Search PubMed.
- H. Viberg, Int. J. Dev. Neurosci., 2009, 27, 423–429 CrossRef CAS PubMed.
- T. A. Slotkin and F. J. Seidler, Brain Res., 2010, 1353, 36–52 CrossRef CAS PubMed.
- L. V. Dishaw, C. M. Powers, I. T. Ryde, S. C. Roberts, F. J. Seidler, T. A. Slotkin and H. M. Stapleton, Toxicol. Appl. Pharmacol., 2011, 256, 281–289 CrossRef CAS PubMed.
- J. M. Shulman, P. L. De Jager and M. B. Feany, Annu. Rev. Pathol., 2011, 6, 193–222 CrossRef CAS PubMed.
- A. J. Harrington, A. L. Knight, G. A. Caldwell and K. A. Caldwell, Methods, 2011, 53, 220–225 CrossRef CAS PubMed.
- A. T. van der Goot, W. Zhu, R. P. Vazquez-Manrique, R. I. Seinstra and K. Dettmer, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 14912–14917 CrossRef CAS PubMed.
- T. J. van Ham, K. L. Thijssen, R. Breitling, R. M. W. Hofstra and R. H. A. Plasterk, PLoS Genet., 2008, 4, e1000027 Search PubMed.
- J. A. Obeso, M. C. Rodriguez-Oroz and C. G. Goetz, Nat. Med., 2010, 16, 53–61 Search PubMed.
- T. Kuwahara, A. Koyama, S. Koyama, S. Yoshina, C. H. Ren, T. Kato, S. Mitani and T. Iwatsubo, Hum. Mol. Genet., 2008, 17, 2997–3009 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6tx00306k |
| This journal is © The Royal Society of Chemistry 2017 |