Thermally activated delayed fluorescence dendrimers with exciplex-forming dendrons for low-voltage-driving and power-efficient solution-processed OLEDs†
Kaiyong
Sun
a,
Yibai
Sun
b,
Wenwen
Tian
a,
Dan
Liu
a,
Yingli
Feng
a,
Yueming
Sun
*a and
Wei
Jiang
 *a
*a
aJiangsu Engineering Laboratory of Smart Carbon-Rich Materials and Device, Jiangsu Province Hi-Tech Key Laboratory for Bio-Medical Research, School of Chemistry and Chemical Engineering, Southeast University, Nanjing, Jiangsu 211189, P. R. China. E-mail: sun@seu.edu.cn; jiangw@seu.edu.cn
bDepartment of Chemical and Pharmaceutical Engineering of Southeast University Chengxian College, Nanjing, Jiangsu 210088, P. R. China
First published on 23rd November 2017
Abstract
Exciplex-forming hosts have the excellent function of reducing driving voltages by eliminating the injection barrier, while thermally activated delayed fluorescence (TADF) emitters are able to realize 100% internal quantum efficiency. The marriage of exciplex and TADF should be a rational strategy to design ideal luminescent materials. In this study, two new dendrimers G-TCTA and G-mCP with a non-conjugated connection of the well-known efficient TADF core and different exciplex-forming dendrons were designed and synthesized. Both dendrimers possess excellent thermal stability, good solution processability, and an visible TADF feature. The non-conjugated connection can make the photophysical properties of TADF core and the exciplex-forming function of the dendrons independent. Consequently, a nondoped device of G-mCP achieves an extremely low driving voltage of 2.7 V and a high power efficiency of 46.6 lm W−1, which is among the highest power efficiency of the reported TADF s-OLED. We believe that this study would provide a simple and practical method to design novel power-efficient TADF dendrimers used for energy-saving displays and solid-state lightings.
1. Introduction
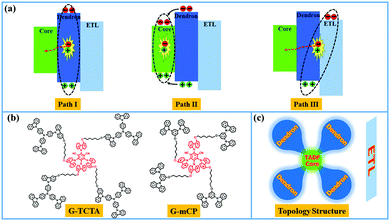
Solution-processed organic light-emitting diodes (s-OLEDs) based on TADF emitters have been one of the central research targets to realize low cost, large area, and 100% internal quantum efficiency for display and lighting applications.1–11 Phosphorescent dendrimers have been widely used for nondoped s-OLEDs because of their well-defined structures and excellent solution processability, particularly for the encapsulation of peripheral dendrons.12–19 Nevertheless, the use of rare noble metals in phosphorescent dendrimers is still a primary obstacle for the commercial application of OLEDs. Actually, this strategy is likewise suitable for TADF dendrimers and some relevant studies have been reported.20–24 Recently, our group designed some TADF dendrimers, in which the functional moieties are bound together into one TADF molecule through flexible alkyl chains so as to restrain the concentration quenching and balance the carriers.25,26In general, when a TADF dendrimer is employed as the emitting layer (EML) in a nondoped device, there may exist two possible pathways for exciton generation (Fig. 1a): (I) the injected holes and electrons on the dendron are directly combined to produce excitons, and subsequently, the energy is transferred to the emissive core; (II) the injected holes and electrons on the dendron are transported to the core, in sequence, then the excitons are formed on the core. Since the above paths initial carrier injection into the dendron, the injection barriers are usually large because of the high energy levels of the outer dendrons, causing inferior power performance derived from large driving voltages. It is well-known that the utilization of exciplex-forming hosts has been considered as an ideal strategy for realizing power-efficient OLEDs, resulting from the elimination of the injection barrier in the emitting layer (EML).27–36 However, an exciplex-based EML is normally composed of multiple components, which makes solution-based processing challenging due to the inevitable phase separation. With this idea in mind, bifunctional TADF dendrimers were designed by introducing the exciplex-forming dendrons into an efficient green TADF emissive core through flexible chains. A non-conjugated connection can make the photophysical properties of the TADF core and the exciplex-forming function of dendrons independent. We carefully selected the well-known high triplet level exciplex-forming couples, TCTA/mCP and B3PYMPM, which were used as outer dendrons for TADF dendrimers and the electron-transporting layer (ETL), respectively (Fig. 1c).30,33,37 In the designed devices, the injected holes on the dendrons and the injected electrons on the ETL were combined to form an interfacial exciplex, and subsequently, the energy was transferred to the emissive core (path III). Thus, realization of power-efficient s-OLED was simply predicted by choosing suitable exciplex-forming couples for the dendrons and ETL. Therefore, we reveal a simple and practical approach for power efficient s-OLED by combining the functions of TADF and exciplex into a single molecule.
2. Results and discussion
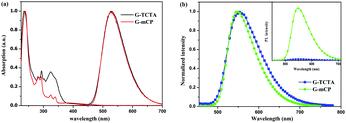
The detailed synthetic routes of the G-TCTA and G-mCP dendrimers are shown in Scheme 1. The intermediate products were coupled with 1,6-dibromohexane using cesium carbonate in N,N-dimethylformamide (DMF) to generate Br-TCTA and Br-mCP, which were reacted with 4-hydroxycarbazole in DMF to provide the key intermediates, Cz-TCTA and Cz-mCP. The emissive core G-G0, G-TCTA and G-mCP were finally synthesized through aromatic nucleophilic substitution reaction using 2,4,5,6-tetrafluoroisophthalonitrile with Cz-C6, Cz-TCTA and Cz-mCP under alkaline conditions. The chemical structures of G-G0, G-TCTA and G-mCP were characterized by 1H-NMR, 13C-NMR, mass spectrometry and elemental analysis (Fig. S1–S9, ESI†). The resultant G-TCTA and G-mCP dendrimers are thermally stable with high decomposition temperatures of 419 °C and 382 °C, and high glass transition temperatures of 135 °C and 141 °C, respectively (Fig. S10a, ESI†). In addition, the G-TCTA and G-mCP dendrimers exhibit good solubility in common organic solvents, ensuring their capability to form uniform thin films through a solution process with the root-mean-square (RMS) roughness of 0.566 nm and 0.512 nm, respectively (Fig. S10b, ESI†).The photophysical properties of the dendrimers were analyzed by recording the UV-vis absorption spectra and photoluminescence (PL) spectra. As shown in Fig. 2a, the dendrimers exhibit intense absorption bands below 350 nm and weak bands at around 400 nm, which can be attributed to the molecular backbone and intramolecular charge transition (ICT), respectively. In the solution of toluene, the dendrimers display the same PL spectra with an emission peak at 527 nm, which indicates that the photophysical properties of the TADF dendrimers are almost independent of the incorporated different dendrons. In thin films, the G-TCTA and G-mCP dendrimers show the red shift PL emission of 548 nm. It is worth noting that G-TCTA shows a broader PL emission and weaker PL intensity than the dendrimer G-mCP (Fig. 2b), which derives from the exciplex formation between the TCTA dendron and the emissive core G-G0. This behavior is similar to a reported case showing the exciplex formation between TCTA and 4CzIPN,38 indicating that the intramolecular exciplex type would not be suitable for application in TADF s-OLED. This view is confirmed from the photoluminescence quantum yields (PLQYs) of G-TCTA (0.08) and G-mCP (0.90). On the basis of the onsets of the fluorescence and phosphorescence spectra (Fig. S11, ESI†), both dendrimers exhibited smaller ΔEST values of 0.09 eV for G-TCTA and 0.08 eV for G-mCP, which are well matched with that of the 4CzIPN reported by Adachi et al.39 In the solid state, G-TCTA and G-mCP show second-order exponential decays in the film at 300 K, with prompt fluorescence lifetimes of 22 ns and 16 ns and decay fluorescence lifetimes of 1.4 μs and 1.0 μs, respectively (Fig. S12, ESI†).
Taking into account the interfacial exciplex-formation, we constructed the nondoped s-OLEDs with a configuration of ITO/PEDOT:PSS (40 nm)/G-TCTA or G-mCP (40 nm)/B3PYMPM (55 nm)/Cs2CO3 (1 nm)/Al (100 nm). Herein, PEDOT:PSS and Cs2CO3 were used as hole- and electron-injection layers, and B3PYMPM was used as the electron-transporting layer. For comparison, the control device was also prepared based on the physical blending of MO-mCP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G-G0 (molar ratio = 4
G-G0 (molar ratio = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The blended film exhibited a much coarser surface with a RMS roughness of 2.174 nm compared to that of G-mCP, which was primarily induced by the self-aggregation of the MO-mCP, leading to phase separation (Fig. S13a, ESI†). Simultaneously, the normalized PL spectrum of the MO-mCP
1). The blended film exhibited a much coarser surface with a RMS roughness of 2.174 nm compared to that of G-mCP, which was primarily induced by the self-aggregation of the MO-mCP, leading to phase separation (Fig. S13a, ESI†). Simultaneously, the normalized PL spectrum of the MO-mCP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B3PYMPM
B3PYMPM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G-G0 film (4
G-G0 film (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
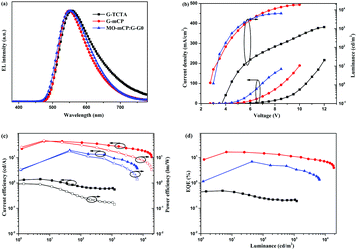
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed emission entirely from G-G0 with the peak position at 548 nm, indicating that the energy transfer from the exciplex to the fluorescent dopant is efficient (Fig. S13b, ESI†). As shown in Fig. 3, the peaks in the EL spectra of G-TCTA and G-mCP are located at 552 nm and 548 nm, respectively, which are in agreement with those of the PL spectra in the as-prepared films. The broad EL peak of G-TCTA indicates that the intramolecular exciplex also appeared under electrical stress. As a result, the performance of the G-TCTA based device considerably worsens, which is consistent with the low PLQY. In contrast, the G-mCP based device shows excellent device performance with a maximum external quantum efficiency (EQE) of 16.5%. The maximum EQE value of G-mCP is comparable to the most-efficient dendrimer-based TADF s-OLED. Expectedly, the G-mCP device achieves a low turn-on voltage of 2.7 eV, which is close to the theoretical limit of the driving voltage corresponding to the optical bandgap divided by the electron charge (Eg/e = 2.6 eV). Consequently, the G-mCP device shows a maximum power efficiency of 46.6 lm W−1 as well as a high PE of 30.9 lm W−1 at 100 cd m−2 and 19.1 lm W−1 at 1000 cd m−2, which are among the highest power efficiencies of the reported solution-processed TADF devices (Table S1, ESI†). Simultaneously, in comparison to MO-mCP:G-G0 (6.9%, 19.5 cd A−1, 17.9 lm W−1), the efficiency is improved by more than two-fold, which indicates that a single molecule system possess a better homogeneous film morphology than physical blending can efficiently attenuate the phase separation induced negative effects.
1) showed emission entirely from G-G0 with the peak position at 548 nm, indicating that the energy transfer from the exciplex to the fluorescent dopant is efficient (Fig. S13b, ESI†). As shown in Fig. 3, the peaks in the EL spectra of G-TCTA and G-mCP are located at 552 nm and 548 nm, respectively, which are in agreement with those of the PL spectra in the as-prepared films. The broad EL peak of G-TCTA indicates that the intramolecular exciplex also appeared under electrical stress. As a result, the performance of the G-TCTA based device considerably worsens, which is consistent with the low PLQY. In contrast, the G-mCP based device shows excellent device performance with a maximum external quantum efficiency (EQE) of 16.5%. The maximum EQE value of G-mCP is comparable to the most-efficient dendrimer-based TADF s-OLED. Expectedly, the G-mCP device achieves a low turn-on voltage of 2.7 eV, which is close to the theoretical limit of the driving voltage corresponding to the optical bandgap divided by the electron charge (Eg/e = 2.6 eV). Consequently, the G-mCP device shows a maximum power efficiency of 46.6 lm W−1 as well as a high PE of 30.9 lm W−1 at 100 cd m−2 and 19.1 lm W−1 at 1000 cd m−2, which are among the highest power efficiencies of the reported solution-processed TADF devices (Table S1, ESI†). Simultaneously, in comparison to MO-mCP:G-G0 (6.9%, 19.5 cd A−1, 17.9 lm W−1), the efficiency is improved by more than two-fold, which indicates that a single molecule system possess a better homogeneous film morphology than physical blending can efficiently attenuate the phase separation induced negative effects.
The exciplex-forming nature between the dendrons (MO-mCP) and ETL was investigated from the photoluminescent (PL) spectra of MO-mCP, B3PYMPM and MO-mCP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B3PYMPM (1
B3PYMPM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, mol mol−1) blended films together with the EL spectrum of the MO-mCP/B3PYMPM bilayer device. As shown in Fig. 4, in comparison to the individual PL spectra of the MO-mCP and B3PYMPM films, the MO-mCP:B3PYMPM blended film exhibits a new red-shifted broad emission with a peak at 465 nm, which is well matched with the EL of MO-mCP/B3PYMPM. The energy of the new emission (2.67 eV) is close to the energy gap between the highest occupied molecular orbital (HOMO) level of MO-mCP (−5.88 eV) (Fig. S14, ESI†) and the reported lowest unoccupied molecular orbital (LUMO) level of B3PYMPM (−3.2 eV), indicating that MO-mCP and B3PYMPM could enable exciplex excitation. The additional shoulder EL peak of 596 nm is attributable to the MO-mCP electromer, and disappears after coupling with the TADF core.
1, mol mol−1) blended films together with the EL spectrum of the MO-mCP/B3PYMPM bilayer device. As shown in Fig. 4, in comparison to the individual PL spectra of the MO-mCP and B3PYMPM films, the MO-mCP:B3PYMPM blended film exhibits a new red-shifted broad emission with a peak at 465 nm, which is well matched with the EL of MO-mCP/B3PYMPM. The energy of the new emission (2.67 eV) is close to the energy gap between the highest occupied molecular orbital (HOMO) level of MO-mCP (−5.88 eV) (Fig. S14, ESI†) and the reported lowest unoccupied molecular orbital (LUMO) level of B3PYMPM (−3.2 eV), indicating that MO-mCP and B3PYMPM could enable exciplex excitation. The additional shoulder EL peak of 596 nm is attributable to the MO-mCP electromer, and disappears after coupling with the TADF core.
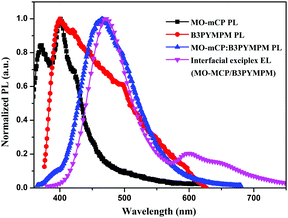 | ||
Fig. 4 Normalized PL spectra of MO-mCP, B3PYMPM, and MO-mCP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B3PYMPM (1 B3PYMPM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, mol mol−1) blended films, together with normalized EL spectrum of MO-mCP/B3PYMPM bilayer device. 1, mol mol−1) blended films, together with normalized EL spectrum of MO-mCP/B3PYMPM bilayer device. | ||
To further prove the promising power performance derived from the interfacial exciplex, we used the well-known electron-transporting material TPBi as the ETL instead of the previously used B3PYMPM. In comparison with B3PYMPM, TPBi possess a shallower HOMO level. As shown in Fig. S15 (ESI†), MO-mCP and TPBi have no exciplex nature because the MO-mCP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPBi (1
TPBi (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, mol mol−1) film does not exhibit a long wavelength emission in comparison to that of the single material. Fig. S16 (ESI†) shows all the device performances and the key parameters are summarized in Table 1. The EL peak of G-mCP is same as that of the B3PYMPM-based device. As expected, the TPBi-based device exhibited a maximum EQEs of 14.3% and a maximum PE of 31.6 lm W−1. These values are inferior to those of the B3PYMPM-based device. In particular to the power performance, the TPBi-based device achieved a higher turn-on voltage of 3.8 eV. These results strongly indicate that the carrier-recombination pathway involves interfacial exciplex formation. In addition, G-TCTA as EML is also measured with a configuration of ITO/PEDOT
1, mol mol−1) film does not exhibit a long wavelength emission in comparison to that of the single material. Fig. S16 (ESI†) shows all the device performances and the key parameters are summarized in Table 1. The EL peak of G-mCP is same as that of the B3PYMPM-based device. As expected, the TPBi-based device exhibited a maximum EQEs of 14.3% and a maximum PE of 31.6 lm W−1. These values are inferior to those of the B3PYMPM-based device. In particular to the power performance, the TPBi-based device achieved a higher turn-on voltage of 3.8 eV. These results strongly indicate that the carrier-recombination pathway involves interfacial exciplex formation. In addition, G-TCTA as EML is also measured with a configuration of ITO/PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS (40 nm)/G-TCTA (40 nm)/TPBi (40 nm)/Cs2CO3 (1 nm)/Al (100 nm), and the performance of the device is conceivably poor due to the undesirable intramolecular interaction.
PSS (40 nm)/G-TCTA (40 nm)/TPBi (40 nm)/Cs2CO3 (1 nm)/Al (100 nm), and the performance of the device is conceivably poor due to the undesirable intramolecular interaction.
| Device | Voltage (V) | LE (cd A−1) | PE (lm W−1) | EQE (%) | Luminance (cd m−2)Max | CIE (x, y) |
|---|---|---|---|---|---|---|
| 1 cd m−2 | Max/100/1000 cd m−2 | Max/100/1000 cd m−2 | Max/100/1000 cd m−2 | |||
| G-TCTA/B3PYMPM | 4.4 | 1.40/0.75/— | 0.93/0.31/— | 0.50/0.26/— | 1200 | (0.46, 0.52) |
| G-mCP/B3PYMPM | 2.7 | 44.5/39.4/30.5 | 46.6/30.9/19.1 | 16.5/14.6/11.3 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
(0.42, 0.55) |
| MO-mCP:G-G0/B3PYMPM | 3.0 | 19.5/13.9/9.4 | 17.5/11.0/5.9 | 6.9/4.9/3.3 | 6500 | (0.43, 0.55) |
| G-TCTA/TPBi | 4.5 | 0.66/0.56/— | 0.30/0.21/— | 0.22/0.20/— | 330 | (0.44, 0.53) |
| G-mCP/TPBi | 3.8 | 40.2/18.4/8.8 | 31.6/11.5/4.0 | 14.3/6.6/3.1 | 5500 | (0.41, 0.55) |
3. Conclusions
In conclusion, we propose an interfacial exciplex engineering strategy to develop TADF dendrimers, leading to a power efficient s-OLED. Utilization of the hole-transporting dendrons and the adjacent electron-transporting layer recombination for the formation of the interfacial exciplex ensures efficient charge injection and transportation and efficient singlet and triplet energy transfers from the interfacial exciplex to the TADF core. Consequently, the nondoped device of G-mCP achieves an extremely low driving voltage of 2.7 V, a high PE of 46.6 lm W−1 as well as high PE of 30.9 lm W−1 at 100 cd m−2 and 19.1 lm W−1 at 1000 cd m−2. This study presents a simple and practical strategy to design bifunctional TADF dendrimers used for power-efficient s-OLEDs.4. Experimental
General information
All solvents and materials were used as received from commercial sources without further purification. 1H NMR and 13C NMR spectra were recorded on a BRUKER AMX 300 MHz and 600 MHz instrument using Si(CH3)4 as the internal standard. Molecular masses were determined by matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF-MS) using a BRUKER DALTONICS instrument, with α-cyano-hydroxycinnamic acid as a matrix. The absorption and photoluminescence emission spectra of the target compound were measured using a SHIMADZU UV-2450 spectrophotometer and a HORIBA FLUOROMAX-4 spectrophotometer, respectively. The PL spectra of G-TCTA and G-mCP films were measured at 430 nm excitation. The solid PL quantum efficiency was measured using an integrating sphere. Cyclic voltammetry measurements were performed on a CHI750C voltammetric analyzer in CH2Cl2 solutions (10−3 M) (oxidation process) at a scan rate of 100 mV s−1 with a platinum plate as the working electrode, a silver wire as the pseudo-reference electrode, and a platinum wire as the counter electrode. The supporting electrolyte was tetrabutylammonium hexafluorophosphate (0.1 M) and ferrocene was selected as the internal standard. AFM (Seiko Instruments, SPA-400) was used to measure the film surface morphology. Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) curves were recorded with a Netzsch simultaneous thermal analyzer (STA) system (STA 409PC) and a DSC 2910 modulated calorimeter under a dry nitrogen gas flow at a heating rate of 10 °C min−1. The films were prepared as follows: in a general procedure, the quartz plate substrates were precleaned carefully and treated by UV ozone for 4 min. The solutions with the concentration of 10 mg mL−1 were spin coated onto the quartz plate substrates and dried in vacuum.Devices measurements and characterization
To fabricate the nondoped solution-processed OLEDs, a 40 nm-thick poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) film was first deposited on the pre-cleaned ITO-glass substrates and baked at 150 °C for 10 min. Then, an EML with a thickness of about 40 nm was spin-coated from a 1, 2-dichloroethane solution onto the PEDOT:PSS layer and annealed at 100 °C for 30 min to remove the residual solvent under N2 atmosphere. Subsequently, a 55 nm thick B3PYMPM layer or a 40 nm thick TPBi layer was evaporated as the hole blocking and electron transporting layer. Finally, 1 nm thick Cs2CO3 and 100 nm thick Al layers were evaporated as the cathode. The EL spectra and CIE coordinates were measured using a PR655 spectra colorimeter. The current density–voltage and brightness-voltage curves of the devices were plotted using a Keithley 2400 source meter calibrated by a silicon photodiode. All the measurements were carried out at room temperature under ambient conditions. The EQE was calculated from the brightness, current density and EL spectrum assuming a Lambertian distribution.Materials
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as an eluent to produce a white solid (3.06 g, 80%). 1H NMR (600 MHz, CDCl3, δ): 0.85–0.87 (t, J = 7.2 Hz, 3H), 1.32–1.36 (m, 4H), 1.53–1.57 (m, 2H), 1.91–1.93 (m, 2H), 6.59 (d, J = 8.4 Hz, 1H), 6.94 (d, J = 7.8 Hz, 1H), 7.15–7.18 (m, 1H), 7.23–7.26 (t, J = 7.8 Hz, 1H), 7.29–7.33 (m, 2H), 7.95 (s, 1H), 8.24 (d, J = 7.2 Hz, 1H).
4) as an eluent to produce a white solid (3.06 g, 80%). 1H NMR (600 MHz, CDCl3, δ): 0.85–0.87 (t, J = 7.2 Hz, 3H), 1.32–1.36 (m, 4H), 1.53–1.57 (m, 2H), 1.91–1.93 (m, 2H), 6.59 (d, J = 8.4 Hz, 1H), 6.94 (d, J = 7.8 Hz, 1H), 7.15–7.18 (m, 1H), 7.23–7.26 (t, J = 7.8 Hz, 1H), 7.29–7.33 (m, 2H), 7.95 (s, 1H), 8.24 (d, J = 7.2 Hz, 1H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as eluent to produce a white solid (3.70 g, 68%). 1H NMR (600 MHz, CDCl3, δ): 1.53–1.55 (m, 4H), 1.60–1.62 (m, 2H), 1.86–1.90 (m, 2H), 3.41–3.43 (t, J = 7.2 Hz, 2H), 4.06–4.08 (t, J = 6.6 Hz, 2H), 7.22 (s, 2H), 7.29–7.32 (t, J = 12 Hz, 4H), 7.40 (s, 4H), 7.43–7.45 (t, J = 7.2 Hz, 4H), 7.54 (d, J = 12 Hz, 4H), 8.02 (s, 1H), 8.14 (d, J = 7.8 Hz, 4H).
3) as eluent to produce a white solid (3.70 g, 68%). 1H NMR (600 MHz, CDCl3, δ): 1.53–1.55 (m, 4H), 1.60–1.62 (m, 2H), 1.86–1.90 (m, 2H), 3.41–3.43 (t, J = 7.2 Hz, 2H), 4.06–4.08 (t, J = 6.6 Hz, 2H), 7.22 (s, 2H), 7.29–7.32 (t, J = 12 Hz, 4H), 7.40 (s, 4H), 7.43–7.45 (t, J = 7.2 Hz, 4H), 7.54 (d, J = 12 Hz, 4H), 8.02 (s, 1H), 8.14 (d, J = 7.8 Hz, 4H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as eluent to produce a white solid (2.89 g, 70%). 1H NMR (600 MHz, CDCl3, δ): 1.60–1.67 (m, 2H), 1.68–1.74 (m, 2H), 1.90–1.93 (m, 2H), 2.01–2.04 (m, 2H), 4.03–4.05 (t, J = 6.6 Hz, 2H), 4.22–4.24 (t, J = 6.0 Hz, 2H), 6.62 (d, J = 7.8 Hz, 1H), 6.92 (d, J = 8.4 Hz, 1H). 7.15–7.16 (m, 2H), 7.23–7.31 (m, 7H), 7.37 (s, 1H), 7.42–7.44 (m, 4H), 7.55 (d, J = 7.8 Hz, 4H), 7.85 (s, 1H), 8.14 (d, J = 7.2 Hz, 4H), 8.28 (d, J = 7.2 Hz, 1H).
3) as eluent to produce a white solid (2.89 g, 70%). 1H NMR (600 MHz, CDCl3, δ): 1.60–1.67 (m, 2H), 1.68–1.74 (m, 2H), 1.90–1.93 (m, 2H), 2.01–2.04 (m, 2H), 4.03–4.05 (t, J = 6.6 Hz, 2H), 4.22–4.24 (t, J = 6.0 Hz, 2H), 6.62 (d, J = 7.8 Hz, 1H), 6.92 (d, J = 8.4 Hz, 1H). 7.15–7.16 (m, 2H), 7.23–7.31 (m, 7H), 7.37 (s, 1H), 7.42–7.44 (m, 4H), 7.55 (d, J = 7.8 Hz, 4H), 7.85 (s, 1H), 8.14 (d, J = 7.2 Hz, 4H), 8.28 (d, J = 7.2 Hz, 1H).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the grants from the National Natural Science Foundation of China (51103023), National Basic Research Program China (2013CB932902). We are also thankful for the support of the Fundamental Research Funds for the Central Universities (2242016K41082) and the Scientific Research Foundation of Graduate School of Southeast University (NO. YBJJ1631).Notes and references
- A. E. Nikolaenko, M. Cass, F. Bourcet, D. Mohamad and M. Roberts, Adv. Mater., 2015, 27, 7236 CrossRef CAS PubMed.
- Y. Zhu, Y. Zhang, B. Yao, Y. Wang, Z. Zhang, H. Zhan, B. Zhang, Z. Xie, Y. Wang and Y. Cheng, Macromolecules, 2016, 49, 4373 CrossRef CAS.
- J. Luo, G. Xie, S. Gong, T. Chen and C. Yang, Chem. Commun., 2016, 52, 2292 RSC.
- G. Xie, X. Li, D. Chen, Z. Wang, X. Cai, D. Chen, Y. Li, K. Liu, Y. Cao and S. J. Su, Adv. Mater., 2016, 28, 181 CrossRef CAS PubMed.
- Y. J. Cho, K. S. Yook and J. Y. Lee, Adv. Mater., 2014, 26, 6642 CrossRef CAS PubMed.
- C. Tang, T. Yang, X. Cao, Y. Tao, F. Wang, C. Zhong, Y. Qian, X. Zhang and W. Huang, Adv. Opt. Mater., 2015, 3, 786 CrossRef CAS.
- Y. Liu, G. Xie, K. Wu, Z. Luo, T. Zhou, X. Zeng, J. Yu, S. Gong and C. Yang, J. Mater. Chem. C, 2016, 4, 4402 RSC.
- Y. Suzuki, Q. Zhang and C. Adachi, J. Mater. Chem. C, 2015, 3, 1700 RSC.
- S. Y. Lee, T. Yasuda, H. Komiyama, J. Lee and C. Adachi, Adv. Mater., 2016, 28, 4019 CrossRef CAS PubMed.
- G. Xie, J. Luo, M. Huang, T. Chen, K. Wu, S. Gong and C. Yang, Adv. Mater., 2017, 29, 1604223 CrossRef PubMed.
- Z. Ren, R. S. Nobuyasu, F. B. Dias, A. P. Monkman, S. Yan and M. R. Bryce, Macromolecules, 2016, 49, 5452 CrossRef CAS.
- L. Chen, Z. Ma, J. Ding, L. Wang, X. Jing and F. Wang, Chem. Commun., 2011, 47, 9519 RSC.
- S. Wang, B. Zhang, Y. Wang, J. Ding, Z. Xie and L. Wang, Chem. Commun., 2017, 53, 5128 RSC.
- M. Zhu, J. Zou, X. He, C. Yang, H. Wu, C. Zhong, J. Qin and Y. Cao, Chem. Mater., 2012, 24, 174 CrossRef CAS.
- F. K. Kong, M. C. Tang, Y. C. Wong, M. Ng, M. Y. Chan and V. W. Yam, J. Am. Chem. Soc., 2017, 139, 6351 CrossRef CAS PubMed.
- L. Chen, S. Wang, Z. Yan, J. Ding and L. Wang, J. Mater. Chem. C, 2017, 5, 5749 RSC.
- D. Xia, B. Wang, B. Chen, S. Wang, B. Zhang, J. Ding, L. Wang, X. Jing and F. Wang, Angew. Chem., Int. Ed., 2014, 53, 1048 CrossRef CAS PubMed.
- Y. Wang, Y. Lu, B. Gao, S. Wang, J. Ding, L. Wang, X. Jing and F. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 29600 CAS.
- Y. Wang, S. Wang, J. Ding, L. Wang, X. Jing and F. Wang, Chem. Commun., 2016, 53, 180 RSC.
- K. Albrecht, K. Matsuoka, K. Fujita and K. Yamamoto, Angew. Chem., Int. Ed., 2015, 54, 5677 CrossRef CAS PubMed.
- Y. Li, G. Xie, S. Gong, K. Wu and C. Yang, Chem. Sci., 2016, 7, 5441 RSC.
- Y. Li, T. Chen, M. Huang, Y. Gu, S. Gong, G. Xie and C. Yang, J. Mater. Chem. C, 2017, 5, 3480 RSC.
- J. Luo, S. Gong, Y. Gu, T. Chen, Y. Li, C. Zhong, G. Xie and C. Yang, J. Mater. Chem. C, 2016, 4, 2442 RSC.
- K. Matsuoka, K. Albrecht, K. Yamamoto and K. Fujita, Sci. Rep., 2017, 7, 41780 CrossRef CAS PubMed.
- X. Ban, W. Jiang, T. Lu, X. Jing, Q. Tang, S. Huang, K. Sun, B. Huang, B. Lin and Y. Sun, J. Mater. Chem. C, 2016, 4, 8810 RSC.
- X. Ban, W. Jiang, K. Sun, B. Lin and Y. Sun, ACS Appl. Mater. Interfaces, 2017, 9, 7339 CAS.
- H. A. Al Attar and A. P. Monkman, Adv. Mater., 2016, 28, 8014 CrossRef CAS PubMed.
- C. K. Moon, K. Suzuki, K. Shizu, C. Adachi, H. Kaji and J. J. Kim, Adv. Mater., 2017, 29, 1606448 CrossRef PubMed.
- H. Shin, S. Lee, K. H. Kim, C. K. Moon, S. J. Yoo, J. H. Lee and J. J. Kim, Adv. Mater., 2014, 26, 4730 CrossRef CAS PubMed.
- W. Liu, J.-X. Chen, C.-J. Zheng, K. Wang, D.-Y. Chen, F. Li, Y.-P. Dong, C.-S. Lee, X.-M. Ou and X.-H. Zhang, Adv. Funct. Mater., 2016, 26, 2002 CrossRef CAS.
- S. Wang, X. Wang, B. Yao, B. Zhang, J. Ding, Z. Xie and L. Wang, Sci. Rep., 2015, 5, 12487 CrossRef CAS PubMed.
- Y. Seino, S. Inomata, H. Sasabe, Y. J. Pu and J. Kido, Adv. Mater., 2016, 28, 2638 CrossRef CAS PubMed.
- J. W. Sun, J. H. Lee, C. K. Moon, K. H. Kim, H. Shin and J. J. Kim, Adv. Mater., 2014, 26, 5684 CrossRef CAS PubMed.
- X. K. Liu, Z. Chen, J. Qing, W. J. Zhang, B. Wu, H. L. Tam, F. Zhu, X. H. Zhang and C. S. Lee, Adv. Mater., 2015, 27, 7079 CrossRef CAS PubMed.
- X. K. Liu, Z. Chen, C. J. Zheng, M. Chen, W. Liu, X. H. Zhang and C. S. Lee, Adv. Mater., 2015, 27, 2025 CrossRef CAS PubMed.
- X. K. Liu, Z. Chen, C. J. Zheng, C. L. Liu, C. S. Lee, F. Li, X. M. Ou and X. H. Zhang, Adv. Mater., 2015, 27, 2378 CrossRef CAS PubMed.
- S. Lee, K.-H. Kim, D. Limbach, Y.-S. Park and J.-J. Kim, Adv. Funct. Mater., 2013, 23, 4105 CrossRef CAS.
- B. S. Kim and J. Y. Lee, Adv. Funct. Mater., 2014, 24, 3970 CrossRef CAS.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR, 13C NMR, MALDI-TOF-MS, TGA, DSC, AFM and electroluminescent data. See DOI: 10.1039/c7tc04720g |
| This journal is © The Royal Society of Chemistry 2018 |