Dopant-free and low-cost molecular “bee” hole-transporting materials for efficient and stable perovskite solar cells†
Abstract
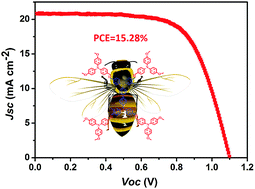
With the dramatic development of the power conversion efficiency (PCE) of perovskite solar cells (PSCs), the device lifetime has attracted extensive research interest and concern. To enhance device durability, developing dopant-free hole-transporting materials (HTMs) with a high performance is a promising strategy. Herein, three new HTMs with a N,N′-diphenyl-N,N′-di(m-tolyl)benzidine (TPD) core: TPD-4MeTPA, TPD-4MeOTPA and TPD-4EtCz are designed and synthesized, showing suitable energy levels and excellent film-formation properties. PCEs of 15.28% were achieved based on pristine TPD-4MeOTPA as the HTM, which is a little lower than that of the p-doped 2,2′,7,7′-tetrakis-(N,N-di-4-methoxyphenylamino)-9,9′-spirobifluorene (spiro-OMeTAD)-based device (17.26%). Importantly, the devices based on the new HTMs show relatively improved stability compared to devices based on spiro-OMeTAD when aged under ambient air with 30% relative humidity in the dark.


 Please wait while we load your content...
Please wait while we load your content...