Development of benzylidene-methyloxazolone based AIEgens and decipherment of their working mechanism†
Abstract
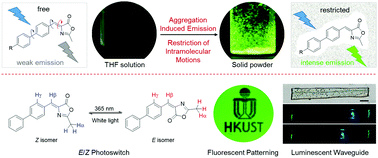
Based on an analogue of green fluorescent protein chromophore benzylidene-methyloxazolone (BMO), a series of fluorophores with an additional phenyl group, BMO-PH, BMO-PF, BMO-PM and BMO-PC, have been prepared and are found to be AIE-active. Their solutions are weakly emissive and their aggregation or solid states are highly emissive. Although these compounds readily undergo efficient E/Z isomerization (EZI) upon UV irradiation in solution, the intramolecular rotation around the double bond and phenyl rotation around the single bond serve as the key non-radiative decay channels to dissipate the excited-state energies. The EZI is only the phenomenal result. In aggregates, these intramolecular motions are greatly restricted by multiple intermolecular interactions, resulting in the AIE effect. To ensure a high solid-state quantum yield, prevention of detrimental π–π stacking is of essence. An additional phenyl group to BMO is found to increase the π–π distance and weaken the π–π interaction. Thus, the quantum yields are increased. Strong electron-donating groups and extended conjugation are effective at tuning the emission color bathochromically. Based on these principles, we succeeded in increasing the solid-state quantum yield up to 50% and obtaining a red emission maximum of 635 nm. Moreover, these compounds are promising for applications in photoswitches and fluorescent patterns, and their crystals are good candidates for luminescent waveguides with low light loss efficiency.
- This article is part of the themed collection: 2017 Journal of Materials Chemistry C HOT Papers


 Please wait while we load your content...
Please wait while we load your content...