Flexible electronics based on magnetic printing and the volume additive principle†
Abstract
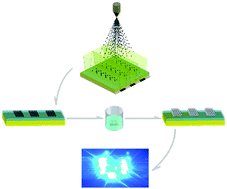
Microscale and nanoscale magnetic objects can be driven and assembled in defined locations efficiently and rapidly in magnetic fields. In this study, we present a strategy to fabricate flexible electronic circuits using non-contact magnetic printing and a volume additive substitution reaction. The process involves assembling iron nanoparticles in designated fields in the magnetic field, thereby forming patterns of iron nanoparticles. Thereafter, the nanoparticles were fused together by converting the iron nanoparticles to silver through an Fe–Ag replacement reaction. A silver flexible electronic circuit was fabricated conveniently through this process. Using this approach, flexible electronic circuits with double-sided conductive patterns could be prepared. This method provides a general and highly effective approach to fabricate various conductive silver patterns on flexible materials, such as paper, PI, PET, and others.


 Please wait while we load your content...
Please wait while we load your content...