Linking design and properties of purine-based donor–acceptor chromophores as optoelectronic materials†
Abstract
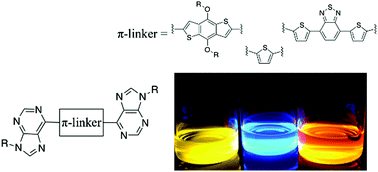
Creating new building blocks for donor–acceptor conjugated systems is an important task for continued development of materials for organic electronics. Purines were introduced into small-molecule π-conjugated systems via Stille cross-coupling using stannylated derivatives of benzodithiophene, thiophene, or dithienylbenzothiadiazole to generate a series of “purine–π–purine” chromophores having high thermal stability, long excited-state lifetimes, and high quantum yields. Photophysical and electrochemical property characterization indicate that depending on the choice of a conjugated bridging unit, purines behave as either an electron-donating or an electron-accepting unit in these small-molecule donor–acceptor chromophores. Specifically, while purine chromophores do not exhibit charge transfer character when linked to a thiophene unit, purinyl units act as a weak acceptor when coupled with benzodithiophene and as a weak donor when coupled with dithienylbenzothiadiazole. In addition to fundamental insights into the molecular design of purine-based chromophores and their charge-transfer character, the results and synthetic tailorability of purines suggest that they may be compelling building blocks in conjugated materials for optical and electronic devices and sensors.


 Please wait while we load your content...
Please wait while we load your content...