Highly phosphorescent cyclometalated platinum(ii) complexes based on 2-phenylbenzimidazole-containing ligands†
Abstract
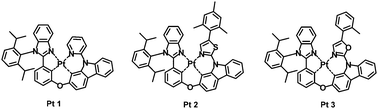
A series of tetradentate cyclometalated platinum(II) complexes (Pt 1, Pt 2, and Pt 3) based on 2-phenylbenzimidazole-containing ligands have been prepared. Their photophysical properties were investigated by absorption and emission spectroscopy as well as density functional theory calculations. Due to the robustness of their coordination frameworks, these Pt(II) complexes exhibited excellent thermal stabilities with decomposition temperatures above 400 °C. The 2-phenylbenzimidazole motif was functionalized with a twisted aryl group, which discouraged intimate intermolecular interactions, thus leading to constant CIE coordinates at different doping ratios. The effect of replacing a coordinating pyridine group by thiazole and oxazole on photophysical properties, electrochemical behaviors, and electroluminescence performance was studied. Single crystal X-ray diffraction analyses of Pt 1 revealed a weak intra-molecular interaction, particularly a negligible Pt⋯Pt interaction, in the solid state, which was consistent with its electroluminescence properties at a high doping level. Organic light emitting diodes (OLEDs) based on these Pt(II) complexes were fabricated with a typical multiple layered device configuration. Among the three Pt(II) complexes, the pyridine-based Pt 1 compound showed the highest electroluminescence performance with maximum CE, PE, and EQE of 78.5 cd A−1, 66.4 lm W−1, and 22.3%, respectively.


 Please wait while we load your content...
Please wait while we load your content...