A controllable honeycomb-like amorphous cobalt sulfide architecture directly grown on the reduced graphene oxide–poly(3,4-ethylenedioxythiophene) composite through electrodeposition for non-enzyme glucose sensing†
Abstract
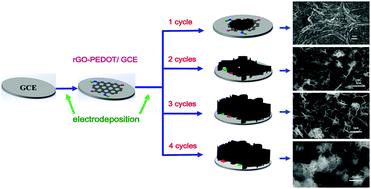
A facile, controllable two-step electrodeposition synthesis route was developed, whereby a honeycomb-like amorphous cobalt sulfide architecture was obtained via direct growth on a glassy carbon electrode (GCE) functionalized by a reduced graphene oxide–poly(3,4-ethylenedioxythiophene) (rGO–PEDOT) composite film as an electrode for glucose detection. This electrodeposition method is binder-free, rapid, low-cost and preparation-controlled. The effects of the concentration ratio between CoCl2·6H2O and thiourea, deposition scanning rate and deposition cycles on glucose detection were investigated, and the optimum preparation conditions were determined. The characterization results indicated that the honeycomb-like cobalt sulfide architecture was formed by growing vertically amorphous CoxSy nanosheets with a thickness of about 20–50 nm on the rGO–PEDOT surface, and the morphology of cobalt sulfide could be controlled by regulating the deposition cycles. Under optimal conditions, the sensor exhibited a wide linear range from 0.2 to 1380 μM (R2 = 0.9976), a sensitivity of 113.46 μA mM−1 cm−2, a low detection limit of 0.079 μM and a response time of 3 s. This sensor also displayed good selectivity, reproducibility and repeatability for non-enzyme glucose sensing. More importantly, the sensor was successfully used to determine glucose in human blood serum samples, and the results were consistent with hospital test results.


 Please wait while we load your content...
Please wait while we load your content...